Abstract
Acetohydroxyacid synthase (AHAS), the first enzyme unique to the biosynthesis of isoleucine, leucine, and valine, is the target enzyme for several classes of herbicides. The AHAS gene from Arabidopsis thaliana, including the chloroplast transit peptide, was cloned into the bacterial expression plasmid pKK233-2. The resulting plasmid was used to transform an AHAS-deficient Escherichia coli strain MF2000. The growth of the MF2000 strain of E. coli was complemented by the functional expression of the Arabidopsis AHAS. The AHAS protein was processed to a molecular mass of 65 kilodaltons that was similar to the mature protein isolated from Arabidopsis seedlings. The AHAS activity extracted from the transformed E. coli cells was inhibited by imidazolinone and sulfonylurea herbicides. AHAS activity extracted from Arabidopsis is inhibited by valine and leucine; however, this activity was insensitive to these feedback inhibitors when extracted from the transformed E. coli.
Full text
PDF

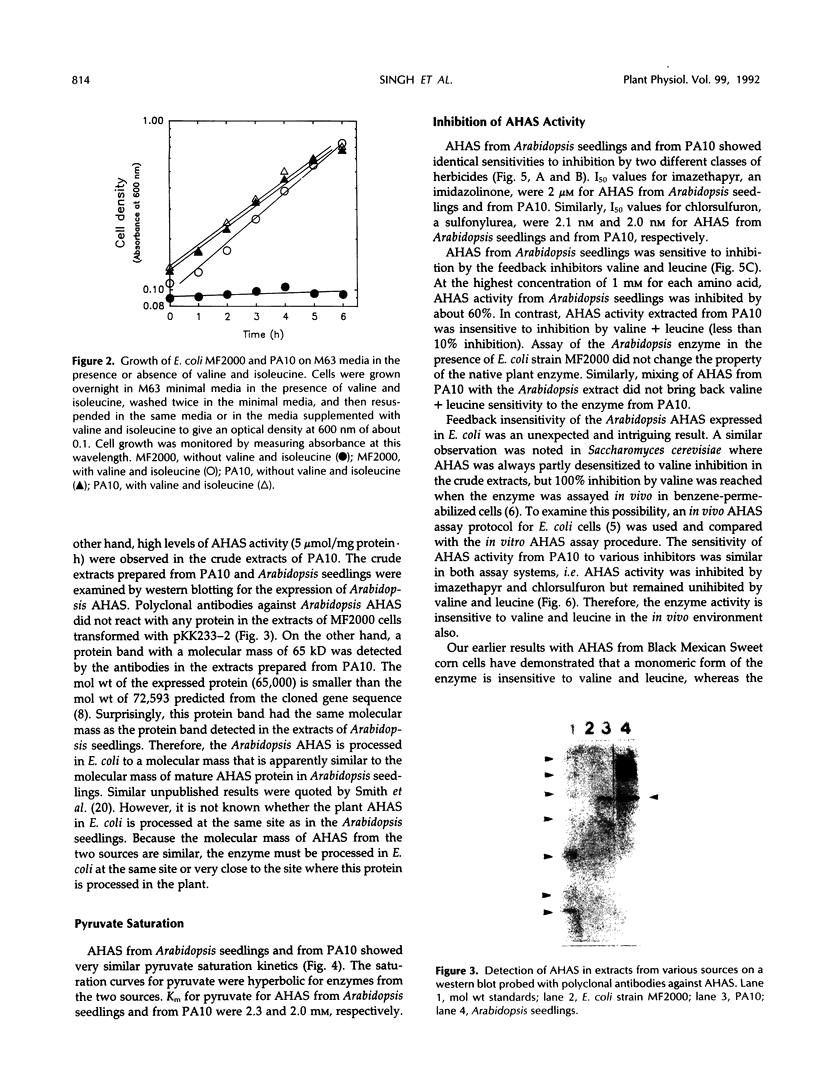
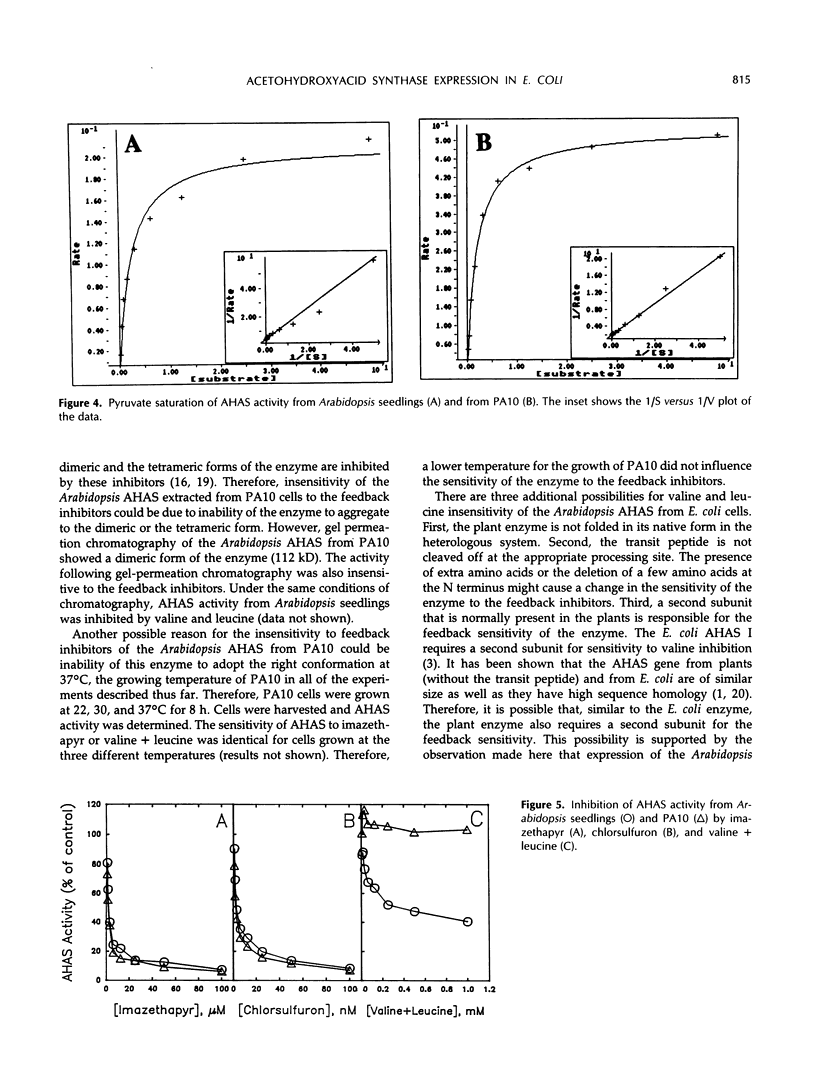

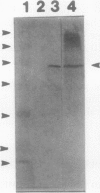
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Role of small subunit (IlvN polypeptide) of acetohydroxyacid synthase I from Escherichia coli K-12 in sensitivity of the enzyme to valine inhibition. J Bacteriol. 1986 Jun;166(3):901–904. doi: 10.1128/jb.166.3.901-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. H. Rapid assay of acetolactate synthase in permeabilized bacteria. Methods Enzymol. 1988;166:230–233. doi: 10.1016/s0076-6879(88)66030-7. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Muhitch M. J., Shaner D. L., Stidham M. A. Imidazolinones and acetohydroxyacid synthase from higher plants: properties of the enzyme from maize suspension culture cells and evidence for the binding of imazapyr to acetohydroxyacid synthase in vivo. Plant Physiol. 1987 Feb;83(2):451–456. doi: 10.1104/pp.83.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Singh B. K., Stidham M. A., Shaner D. L. Assay of acetohydroxyacid synthase. Anal Biochem. 1988 May 15;171(1):173–179. doi: 10.1016/0003-2697(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Singh B., Schmitt G., Lillis M., Hand J. M., Misra R. Overexpression of Acetohydroxyacid Synthase from Arabidopsis as an Inducible Fusion Protein in Escherichia coli: Production of Polyclonal Antibodies, and Immunological Characterization of the Enzyme. Plant Physiol. 1991 Oct;97(2):657–662. doi: 10.1104/pp.97.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. K., Schloss J. V., Mazur B. J. Functional expression of plant acetolactate synthase genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4179–4183. doi: 10.1073/pnas.86.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- Wiersma P. A., Hachey J. E., Crosby W. L., Moloney M. M. Specific truncations of an acetolactate synthase gene from Brassica napus efficiently complement ilvB/ilvG mutants of Salmonella typhimurium. Mol Gen Genet. 1990 Oct;224(1):155–159. doi: 10.1007/BF00259463. [DOI] [PubMed] [Google Scholar]