Abstract
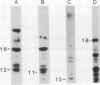
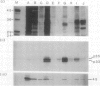
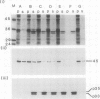
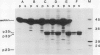
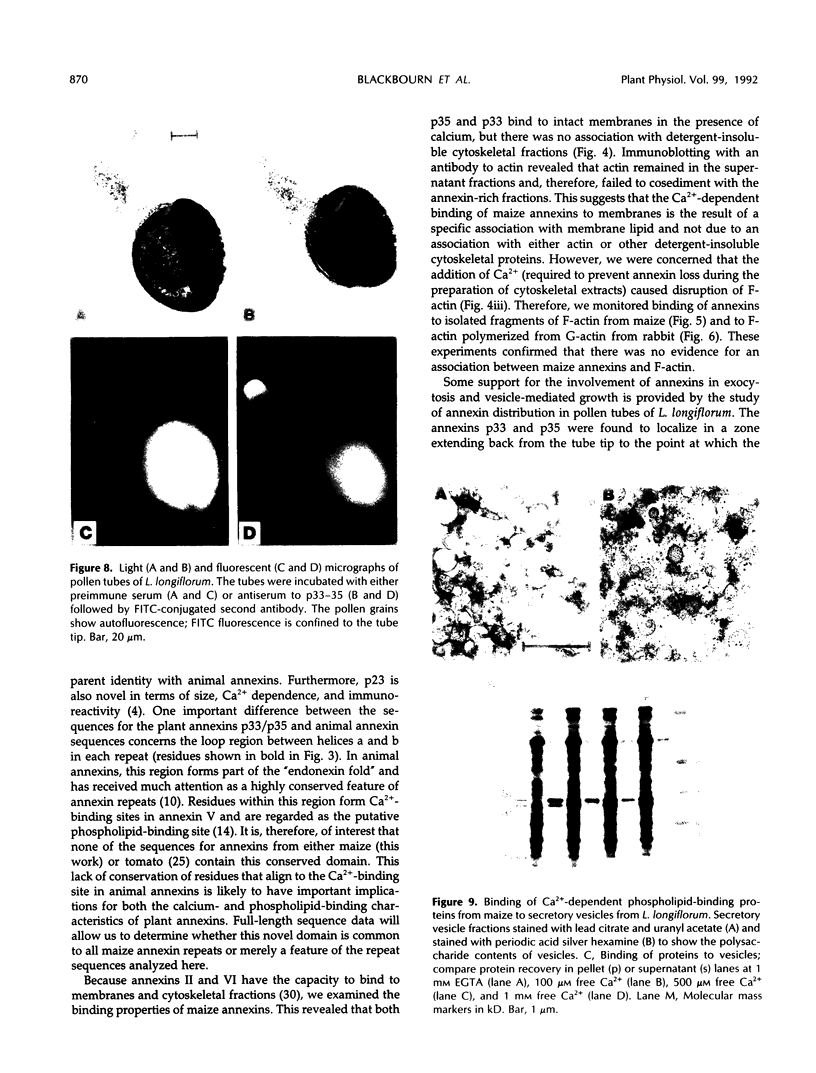
We have examined the characteristics of Ca2+-dependent phospholipid-binding proteins (annexins) in maize (Zea mays L.) coleoptiles and tip-growing pollen tubes of Lilium longiflorum. In maize, there are three such proteins, p35, p33, and p23. Partial sequence analysis reveals that peptides from p35 and p33 have identity to members of the annexin family of animal proteins and to annexins from tomato. Interestingly, multiple sequence alignments reveal that the domain responsible for Ca2+ binding in animal annexins is not conserved in these plant peptide sequences. Although p33 and p35 share the annexin characteristic of binding to membrane lipid, unlike annexins II and VI they do not associate with detergent-insoluble cytoskeletal proteins or with F-actin from either plants or animals. Immunoblotting with antiserum raised to p33/p35 from maize reveals that cross-reactive polypeptides of 33 to 35 kilodaltons are also present in protein extracts from pollen tubes of L. longiflorum. Immunolocalization at the light microscope level suggests that these proteins are predominantly confined to the nongranular zone at the tube tip, a region rich in secretory vesicles. Our hypothesis that plant annexins mediate exocytotic events is supported by the finding that p23, p33, and p35 bind to these secretory vesicles in a Ca2+-dependent manner.
Full text
PDF





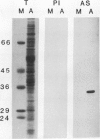
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Newman R. H., Freemont P. S., Crumpton M. J. Amino acid sequence analysis of the annexin super-gene family of proteins. Eur J Biochem. 1991 Jun 15;198(3):749–760. doi: 10.1111/j.1432-1033.1991.tb16076.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- Crompton M. R., Owens R. J., Totty N. F., Moss S. E., Waterfield M. D., Crumpton M. J. Primary structure of the human, membrane-associated Ca2+-binding protein p68 a novel member of a protein family. EMBO J. 1988 Jan;7(1):21–27. doi: 10.1002/j.1460-2075.1988.tb02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Fritsche U., Hexham J. M., Dash B., Johnson T. A consensus amino-acid sequence repeat in Torpedo and mammalian Ca2+-dependent membrane-binding proteins. Nature. 1986 Apr 17;320(6063):636–638. doi: 10.1038/320636a0. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Calcium-dependent conformational changes in the 36-kDa subunit of intestinal protein I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J Biol Chem. 1985 Feb 10;260(3):1688–1695. [PubMed] [Google Scholar]
- Huber R., Römisch J., Paques E. P. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 1990 Dec;9(12):3867–3874. doi: 10.1002/j.1460-2075.1990.tb07605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Schneider M., Mayr I., Römisch J., Paques E. P. The calcium binding sites in human annexin V by crystal structure analysis at 2.0 A resolution. Implications for membrane binding and calcium channel activity. FEBS Lett. 1990 Nov 26;275(1-2):15–21. doi: 10.1016/0014-5793(90)81428-q. [DOI] [PubMed] [Google Scholar]
- Kumar B. V., Lakshmi M. V., Atkinson J. P. Fast and efficient method for detection and estimation of proteins. Biochem Biophys Res Commun. 1985 Sep 16;131(2):883–891. doi: 10.1016/0006-291x(85)91322-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris M. R., Northcote D. H. Influence of cations at the plasma membrane in controlling polysaccharide secretion from sycamore suspension cells. Biochem J. 1977 Sep 15;166(3):603–618. doi: 10.1042/bj1660603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Smallwood M., Keen J. N., Bowles D. J. Purification and partial sequence analysis of plant annexins. Biochem J. 1990 Aug 15;270(1):157–161. doi: 10.1042/bj2700157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerWoude W. J., Morré D. J., Bracker C. E. Isolation and characterization of secretory vesicles in germinated pollen of Lilium longiflorum. J Cell Sci. 1971 Mar;8(2):331–351. doi: 10.1242/jcs.8.2.331. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Cody S. H., Gehring C. A., Parish R. W., Harris P. J. Confocal imaging of ionised calcium in living plant cells. Cell Calcium. 1990 Apr;11(4):291–297. doi: 10.1016/0143-4160(90)90006-g. [DOI] [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Evaluation of the annexins as potential mediators of membrane fusion in exocytosis. J Bioenerg Biomembr. 1990 Apr;22(2):97–120. doi: 10.1007/BF00762942. [DOI] [PubMed] [Google Scholar]