Abstract
To investigate the capacity of Toxoplasma gondii to induce cytokine-mediated toxicity, we employed a murine model of lethal shock in which hypersensitivity to microbial toxins is induced by d-galactosamine (d-Gal). Animals injected with d-Gal and tachyzoite lysate died within 12 to 24 h, whereas administration of d-Gal or lysate alone was nonlethal. Analyses of plasma cytokines revealed peaks of tumor necrosis factor (TNF) alpha and interleukin-12 (IL-12) 1 and 3 to 5 h after injection, respectively, and gradually rising levels of gamma interferon (IFN-γ) continuing until death. Nitric oxide (NO) levels in serum paralleled IFN-γ production. Transaminase assays revealed elevated levels of liver-associated enzymes in sera of lethally injected mice, indicating severe hepatic damage. Depletion of IL-12, TNF, IFN-γ, and NO rescued mice from the lethal effect of antigen (Ag) and d-Gal. T-cell-deficient animals remained sensitive to d-Gal and lysate, suggesting that T lymphocytes do not contribute to the response. Nevertheless, monoclonal antibody (MAb)-mediated granulocyte depletion completely abrogated d-Gal- and Ag-induced mortality and accompanying liver pathology. Finally, mice acutely infected with T. gondii displayed highly elevated NO and liver enzyme levels in serum immediately prior to death, and administration of anti-TNF MAb prolonged survival by approximately 24 h. Our results demonstrate that T. gondii induces lethal inflammatory cytokine shock in d-Gal-sensitized animals and suggest that a similar pathology may contribute to manifestations of acute toxoplasmosis.
Infection with the intracellular protozoan Toxoplasma gondii is characterized by an acute proliferative stage, during which infective tachyzoites invade and replicate within a wide variety of host cells, and a chronic slow growing phase consisting of parasite encystment within tissues of the brain and muscle (36). Although infection is usually innocuous, in immunocompromised hosts encysted parasites can reactivate, leading to uncontrolled tachyzoite proliferation, tissue damage, and death (42, 43, 47).
Previous studies employing cytokine repletion, monoclonal antibody (MAb)-mediated depletion, and, more recently, gene knockout mice have established the importance of type 1 cytokines, such as gamma interferon (IFN-γ), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α) in control of experimental toxoplasmosis (7, 11, 56). Absence of any one of these proinflammatory mediators results in increased mortality during infection as a result of uncontrolled tachyzoite growth. The parasite itself is remarkably effective at stimulating production of proinflammatory cytokines, mediated through its ability to trigger macrophage activation and NK cell and T-lymphocyte IFN-γ release (15, 28, 31, 59, 60).
Despite the protective role of type 1 cytokines during T. gondii infection, it is nevertheless well-known that overproduction of these same factors can underlie host pathology in certain infectious diseases. For example, much of the pathology associated with cerebral malaria is thought to be centered around parasite-induced TNF-α (18, 19). More recently, it has been shown that Schistosoma mansoni infection of IL-4 knockout mice results in lethal cachexia caused by disregulated production of TNF-α (54).
For T. gondii, infection of IL-10 knockout mice results in early mortality associated with abnormally high levels of inflammatory cytokines (16, 52). In these animals, time to death is prolonged by depletion of CD4+ cells. Similarly, oral infection of genetically susceptible C57BL/6 mice results in gut-associated, IFN-γ-mediated necrosis mediated by CD4+ T lymphocytes (40). Nevertheless, while these elegant studies establish that cytokine-mediated pathology can occur during disease progression, determination of the precise mechanisms involved has been problematic. Thus, inflammatory mediators potentially inducing detrimental host pathology are simultaneously required to halt tachyzoite growth and multiplication, preventing host death from massive parasitemia and associated tissue destruction.
We employed the hepatotoxic compound d-galactosamine (d-Gal) so that we could evaluate the parasite’s ability to induce inflammatory pathology in the absence of a host requirement to control infection. Under normal conditions, mice are relatively resistant to inflammatory cytokine-mediated toxic shock, but intraperitoneal (i.p.) administration of d-Gal induces exquisite sensitivity to overproduction of inflammatory mediators. Thus, injection of d-Gal in conjunction with microbial products such as bacterial lipopolysaccharides (LPS) and superantigens results in rapid mortality, which is believed to be the result of TNF-mediated injury to the liver (10, 25, 38, 49, 51). In the case of LPS, lethality occurs independently of T lymphocytes, but for the staphylococcal superantigens, T lymphocytes are required, since severe combined immunodeficiency (SCID) mice are resistant to d-Gal and superantigen (10, 46, 48, 49).
The precise mechanism by which d-Gal exerts its sensitizing effects is not known, but the compound specifically targets the liver, where it induces metabolic changes in hepatocytes. Thus, levels of liver UDP-galactosamine derivatives rapidly accumulate following d-Gal injection, resulting in depletion of the free nucleotide, leading in turn to widespread cessation in biosynthesis of hepatocyte macromolecules such as RNA, proteins, and glycoproteins (5, 32). Transcriptional arrest results in increased sensitivity to liver cell death mediated through TNF-α-induced apoptosis (39).
As we report here, i.p. injection of freeze-thawed tachyzoites (FTZ) of strain RH or live ts-4 (an attenuated parasite strain) triggers rapid death when coadministered with d-Gal. Lethality is a result of parasite-induced production of TNF, IFN-γ, and IL-12, as revealed by MAb depletion experiments, and is associated with catastrophic liver damage. Production of nitric oxide (NO) is also involved in the pathology of the response, since in vivo inhibition of NO with aminoguanidine (AG) renders mice resistant to d-Gal plus parasite antigen (Ag) toxicity. Finally, while the response occurs independently of T lymphocytes, antibody-mediated depletion of cells bearing the granulocyte-associated marker GR-1 rescues animals from the lethal effect of d-Gal and Ag coadministration. Our results provide a striking demonstration that T. gondii possesses the capability of inducing granulocyte-dependent inflammatory cytokine pathology, and they provide a convenient experimental framework for dissection of the response.
MATERIALS AND METHODS
Mice.
C57BL/6 and C57BL/6.scid female mice (6 to 8 weeks of age) were obtained from Taconic Farms Inc. (Germantown, N.Y.). C3H/HeJ (LPSd; hyporesponsive) and C3H/HeOuJ (LPSn; responsive) female mice (6 to 8 weeks old) were obtained from Jackson Laboratory (Bar Harbor, Maine). C57BL/6 IL-5−/− mice, kindly provided by E. J. Pearce, Cornell University, were obtained from offspring of a previously described breeding colony (35). The animals were housed under specific-pathogen-free conditions in the College of Veterinary Medicine animal facility at Cornell University.
Parasites and Ag.
Tachyzoites of strain RH and attenuated mutant ts-4 (53) were maintained on human foreskin fibroblast monolayers in Dulbecco’s modified Eagle medium (GIBCO-BRL, Gaithersburg, Md.), 1% fetal calf serum (HyClone, Logan, Utah), 100 U of penicillin per ml and 0.1 mg of streptomycin per ml (Sigma Chemical Co., St. Louis, Mo.), and 2 mM glutamine (Sigma Chemical Co.).
FTZ of the RH strain were prepared by harvesting lysed fibroblast cultures, washing them in phosphate-buffered saline (PBS), counting the parasites, and storing them in aliquots at −70°C. FTZ were thawed immediately prior to experiments.
To prepare soluble tachyzoite antigen (STAg), RH strain tachyzoites were sonicated in the presence of protease inhibitors (0.2 mM phenylmethylsulfonyl fluoride, 0.2 μM aprotinin, 1 μM leupeptin, 1 mM EDTA), dialyzed into PBS, and then centrifuged for 1 h at 10,000 × g (6). The resulting supernatants were filtered through a 0.2-μm-pore-size membrane (Corning Costar Corp., Cambridge, Mass.), subjected to a Bradford protein assay (1), and stored in aliquots at −70°C.
Fibroblast extract (FBE) was prepared by scraping of uninfected monolayers, sonication, dialysis, and filtering exactly as described for STAg preparations. The LPS content of Ag preparations was determined by the Limulus amebocyte assay (Sigma Chemical Co.) to be ≤1.9 endotoxin units (EU)/mg of protein.
Cytokine measurements.
To measure plasma cytokines, heparinized blood (collected from the tail vein) was centrifuged (12,000 × g, 10 min at 4°C), and the resulting plasma was stored at −70°C until day of assay.
IL-12 was measured as described previously (64). Briefly, samples were added to triplicate wells of a 96-well tissue culture plate (Corning Costar Corp.) containing plate-bound C15.1 MAb (anti-IL-12, added at 20 μg/ml; kindly supplied by M. Wysocka and G. Trinchieri, Wistar Institute). After incubation (4 h, room temperature [RT]), sample supernatants were removed, and naive C57BL/6 splenocytes were added (5 × 105 cells/well) in the presence of 100 U of recombinant human IL-2 (Genzyme Corp., Cambridge, Mass.) per ml. The supernatants were incubated (48 h, 37°C, 5% CO2) and collected, and IFN-γ release was measured as described below. The levels of IFN-γ were proportional to the amount of IL-12 present in the supernatants and were quantified by comparison to the amount of IFN-γ produced in response to known amounts of recombinant IL-12 standard (Genzyme Corp.).
IFN-γ was measured by a two-site enzyme-linked immunosorbent assay (ELISA) as described previously (13) using plate-bound MAb HB170 (anti-IFN-γ), a rabbit polyclonal anti-mouse IFN-γ, and peroxidase-conjugated donkey anti-rabbit immunoglobulin (Ig) (Jackson Immune Research Laboratories, West Grove, Pa.). Sample absorbances (405 nm) were measured on a Microplate Bio Kinetics Reader (Bio-Tek Instruments, Inc., Winooski, Vt.) and compared to know amounts of recombinant IFN-γ standard (Genzyme Corp.).
TNF-α levels were measured by using a mouse-specific TNF-α ELISA kit according to the instructions of the manufacturer (Genzyme Corp.).
Serum nitric oxide.
NO was measured by a modified Griess reaction (17, 21). Briefly, blood was collected, allowed to clot, and centrifuged at 12,000 × g for 5 min. Serum (100 μl) was added to a suspension of Escherichia coli with 1 M HEPES (Sigma Chemical Co.), 3 M formate (Sigma Chemical Co.), and distilled H2O. Bacteria were prepared in a nitrogen-rich environment in order to induce high levels of nitrate reductase activity and were then suspended in PBS and stored at −70°C. The suspension was incubated (1 h, 37°C) and centrifuged (3 min, 12,800 × g) to pellet the bacteria. The supernatant was transferred to a 96-well plate along with 100 μl of a 1:1 mixture of sulfanilamide (1%) in 2.5% H3PO4 and napthylethylenediamine dihydrochloride (0.1%) in 2.5% H3PO4, and the absorbance at 600 nm was measured.
Serum transaminase assays.
To measure the liver-associated enzymes glutamic oxalacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), blood was collected from the tail vein and allowed to clot at RT. The serum was collected by centrifugation (12,800 × g, 4°C, 5 min) and stored at −70°C until the day of assay. GOT levels were measured by a protocol modified from a commercial transaminase kit (Sigma Chemical Co.). Briefly, 20 μl of serum was added to 100 μl of 0.2 M dl-aspartate and 1.8 mM α-ketoglutaric acid in PBS (pH 7.5), the solution was mixed and incubated (37°C, 1 h), and then 2,4-dinitrophenylhydrazine (DNP) (100 μl) was added and the mixture was incubated a further 20 min (RT). To stop the reaction, 1 ml of 0.4 N NaOH was added, and sample absorbances were measured at 490 nm after 5 min. Serum GPT levels were measured by adding 100 μl of 0.2 M dl-alanine and 1.8 mM α-ketoglutaric acid in PBS (pH 7.5) to 20 μl of serum and incubating the mixture (37°C, 30 min). A 100-μl aliquot of DNP was added to the mixture, which was then incubated (RT, 20 min), after which 1 ml of 0.4 N NaOH was added, and the absorbance (490 nm) was measured after a 5-min incubation.
Histopathology.
Tissues were fixed in 10% formalin immediately following CO2 asphyxiation of mice. For the liver, a small sample was cut in transverse section prior to fixation. The samples were embedded in paraffin, sliced into sections (approximately 2 μm thick), and stained with hematoxylin and eosin by the Cornell University Veterinary Medicine Histopathology Laboratory.
In vivo MAb-mediated depletion.
MAbs XT22.11 (anti-mouse TNF), XMG.6 (anti-mouse-IFN-γ), and C17.8 (anti-IL-12; kindly provided by M. Wysocka and G. Trinchieri) were grown as hybridoma supernatants or ascites and purified by passage over protein G-Sepharose (Pharmacia Biotech Inc., Piscataway, N.J.) (45). Each eluted MAb was dialyzed into PBS, concentrated on a CentriCell 20 centrifugal ultrafilter (Polysciences Inc., Warrington, Pa.), and filtered through a 0.2-μm-pore-size membrane (Corning Costar Corp.). Protein concentrations were measured by the Bradford method (1), and the purity of the MAbs was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie blue staining. The granulocyte-depleting MAb RB6-8C5 (63) was originally produced by R. Coffman (DNAX Research Institute) and was kindly provided in purified form by A. Sher (National Institutes of Health). MAb GK1.5 (anti-CD4) was purified by precipitation in 50% ammonium sulfate. Rabbit anti-asialo-GM-1 antiserum was purchased from Wako BioProducts (Richmond, Va.). Control rat Ig (Accurate Chemical and Scientific Corp., Westbury, N.Y.), and normal rabbit serum (GIBCO-BRL) served as controls in the depletion experiments.
Cytokine depletion was accomplished by i.p. injection of 0.5 mg of MAb or control normal rat Ig 2 h prior to injection of d-Gal and parasite Ag. Mortality and morbidity were monitored over the subsequent 24 h.
CD4+ T lymphocytes were depleted from C3H/HeJ mice by i.p. administration of 0.5 mg of MAb GK1.5 6 and 3 days prior to d-Gal and FTZ treatment. In vivo depletion of asialo-GM-1-positive cells was achieved by i.p. injection of 0.1 ml of anti-asialo-GM-1 24 and 18 h before challenge with d-Gal and T. gondii Ag. Finally, neutrophils and eosinophils bearing the GR-1 surface marker were eliminated by injection of 0.5 mg of RB6-8C5 MAb i.p. 24 and 18 h prior to d-Gal and parasite Ag administration. Examination of peritoneal exudate cells from RB6-8C5-treated animals confirmed the specificity of depletion (polymorphonuclear leukocytes [PMN], 2%; large mononuclear cells [LMC], 92.6%; small mononuclear cells [SMC], 5.4%) relative to control rat Ig-treated mice (PMN, 23%; LMC, 70.9%; SMC, 6.1%) after i.p. administration of FTZ (5 × 107).
AG treatment.
Production of inducible NO was blocked by administration of the competitive inhibitor AG (Sigma Chemical Co.) as described elsewhere (20). Briefly, AG was dissolved in water to a concentration of 100 mM, and then the solution was filtered through a 0.2-μm-pore-size membrane (Corning Costar Corp.) and continuously supplied as drinking water to mice from day 12 prior to initiation of experiments.
FACS analysis.
Fluorescence-activated cell sorter (FACS) analysis was performed on splenocytes from GK1.5-treated C3H/HeJ mice. Spleen cells were stained with CD4-fluorescein isothiocyanate (PharMingen, San Diego, Calif.) and analyzed on a FACScaliber flow cytometer (Becton-Dickson Immunocytometry Systems, San Jose, Calif.). The CD4+ T-lymphocyte population comprised less than 1.1% following GK1.5 treatment.
Statistical analysis.
A Wilcoxon signed ranked test was employed to assign statistical significance to mortality associated with groups of mice undergoing treatment with MAb. Experiments were performed on a minimum of two independent occasions.
RESULTS
Production of TNF contributes to host death during acute RH infection.
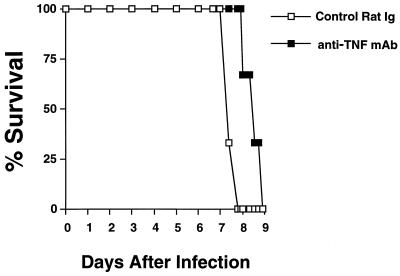
Since inflammatory cytokines contribute to pathology in genetically susceptible hosts infected with low-virulence parasite strain ME49 (16, 40, 52), we initially questioned whether similar pathology could account for some of the manifestations of illness in mice undergoing acute infection with the highly virulent parasite strain RH. As shown in Fig. 1, administration of anti-TNF MAb at day 6 postinfection (when symptoms of illness were just beginning to become apparent) resulted in a slight, but significant, delay in onset of mortality relative to that of mice receiving control rat Ig. Although this result suggested that TNF-mediated pathology may be an important component of acute infection, the simultaneous need for this cytokine to control parasite growth limited our ability to dissect the response. Therefore, we sought a surrogate experimental system to study the phenomenon further.
FIG. 1.
Administration of anti-TNF MAb during late acute T. gondii infection delays onset of death. C3H/HeJ mice were lethally infected by i.p. injection of 100 strain RH tachyzoites. On day 6 postinjection, six mice were administered a single 0.5-mg injection of either control rat IgG or MAb XT22.11 (rat anti-TNF MAb). Statistical significance was determined by using a Wilcoxon signed ranked test (P < 0.006). This experiment was repeated twice with essentially identical results.
Parasite lysate induces rapid lethality in mice coinjected with d-Gal.
Administration of the hepatotoxin d-Gal results in increased susceptibility to bacterial toxins such as LPS. This takes the form of a lethal, TNF-α-mediated shock response. Therefore, we sought to determine if T. gondii Ag displayed similar toxic properties. As shown in Table 1, while neither d-Gal nor STAg alone induced any ill effect at any point after injection, when administered together, animals became sick and died 12 to 24 h postinjection. As little as 40 μg of STAg induced 100% lethality when administered with d-Gal (Table 1). Animals became sick at approximately 8 h postinjection, with illness characterized by piloerection of the fur, hunching, and shivering. The same effect, including lethality, was observed when tachyzoites of the avirulent mutant, ts-4, were administered with d-Gal (Table 1).
TABLE 1.
T. gondii triggers rapid lethality in d-Gal-sensitized mice
| d-Gala | STAg (μg) | ts-4 tachyzoites | Mortalityb (no. dead/total) |
|---|---|---|---|
| + | 0/3 | ||
| − | 200 | 0/3 | |
| + | 200 | 3/3 | |
| + | 40 | 3/3 | |
| + | 8 | 2/3c | |
| + | 1.6 | 0/3 | |
| + | 0.3 | 0/3 | |
| − | 2 × 107 | 0/3 | |
| + | 2 × 107 | 3/3 |
d-Gal (20 mg) was injected (+) into groups of three C57BL/6 animals with or without simultaneous i.p. administration of either STAg or tachyzoites of the attenuated strain ts-4, or was not administered (−).
Lethality measured 12 h postinjection.
The final mouse succumbed between 12 and 24 h postinjection.
Lethality of parasite lysate cannot be attributed to endotoxin contamination.
Since lethality induced by parasite Ag and d-Gal grossly resembled that induced by LPS, we were concerned that our extracts may have contained bacterial endotoxin, which could be responsible for the profound effects shown in Table 1. Accordingly, extracts were assayed for bacterial endotoxin by the highly sensitive Limulus amebocyte assay. The results of the test revealed background levels of endotoxin in our Ag preparations (≤1.9 EU/mg of protein). In addition, FBE prepared in exactly the same manner as STAg was completely nontoxic when administered with d-Gal (Table 2).
TABLE 2.
Lethal effect of T. gondii in d-Gal-sensitized mice is not attributable to a factor present in FBE
| d-Gala | FBE (μg) | ts-4 tachyzoites | Mortalityb (no. dead/total) |
|---|---|---|---|
| + | 0/3 | ||
| − | 2 × 107 | 0/3 | |
| + | 2 × 107 | 2/3c | |
| − | 200 | 0/3 | |
| + | 200 | 0/3 | |
| + | 40 | 0/3 | |
| + | 8 | 0/3 |
d-Gal (20 mg) was injected i.p. into groups of three C57BL/6 mice in the presence or absence of FBE or ts-4.
Mortality at 12 h.
The final mouse displayed gross morbidity at 12 h and was subsequently euthanized.
More importantly, we compared LPS-responsive (C3H/HeOuJ) and LPS-hyporesponsive (C3H/HeJ) mouse strains for sensitivity to parasite Ag (both FTZ and STAg) and d-Gal. As shown in Table 3, the T. gondii Ag preparations were lethal in both LPS-responsive and LPS-nonresponsive mouse strains. This contrasted with administration of LPS plus d-Gal, which was lethal only in C3H/HeOuJ animals. Together, our data strongly suggest that the lethal effects observed stem from the activity of a parasite molecule(s), rather than being attributable to endotoxin contamination. Nevertheless, to avoid artifacts resulting from potential LPS contamination, the experiments described below were performed with C3H/HeJ mice, except where explicitly stated otherwise.
TABLE 3.
Both C3H/HeJ (LPSd) and C3H/HeOuJ (LPSn) mouse strains are susceptible to the lethal effect of T. gondii Ag after d-Gal sensitization
| d-Gala | FTZb | STAgc | LPSd | Mortality (no. dead/total)e
|
|
|---|---|---|---|---|---|
| C3H/HeJ | C3H/HeOuJ | ||||
| + | − | − | + | 0/3 | 3/3 |
| + | − | + | − | 3/3 | 3/3 |
| + | + | − | − | 3/3 | 3/3 |
| − | − | + | − | 0/3 | 0/3 |
20 mg of d-Gal with or without the indicated Ag coinjected i.p.
3 × 107 freeze-thawed strain RH tachyzoites.
150 μg.
1 μg.
Mortality 24 h after injection.
d-Gal plus FTZ injection, as well as acute RH infection, results in major liver damage and high levels of NO in serum.
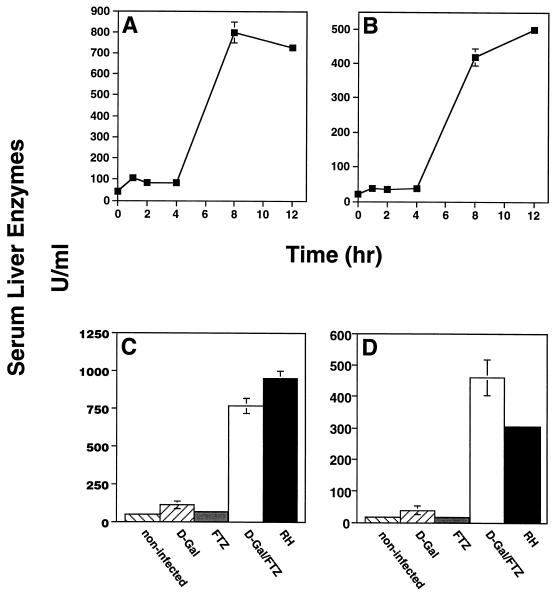
Administration of d-Gal and FTZ resulted in damage to the liver, as measured by appearance of the liver-associated enzymes GOT and GPT in the sera 4 to 8 h following injection (Fig. 2A and B). Damage to the liver was dependent upon coinjection of d-Gal and FTZ, since neither one alone induced the response (Fig. 2C and D). Notably, we also found high levels of GOT and GPT in animals undergoing acute RH infection, suggesting that liver disruption contributes to the pathology of this disease stage (Fig. 2C and D).
FIG. 2.
Appearance of the liver-associated enzymes GOT and GPT in the sera of C3H/HeJ mice injected with d-Gal plus FTZ, as well as animals undergoing acute infection. (A and B) Mice were administered d-Gal (20 mg) plus FTZ (5 × 107), and at the indicated times, sera were collected and GOT (A) and GPT (B) levels were measured. In this experiment, each point is represented by an independent group of animals (n = 3). (C and D) A group of mice was lethally infected (100 RH tachyzoites i.p.), and then, on day 6, further experimental groups were administered d-Gal alone or in combination with FTZ. On day 7, serum was collected from all of the animals, and GOT (C) and GPT (D) levels were determined. See Materials and Methods for details of enzyme measurements.
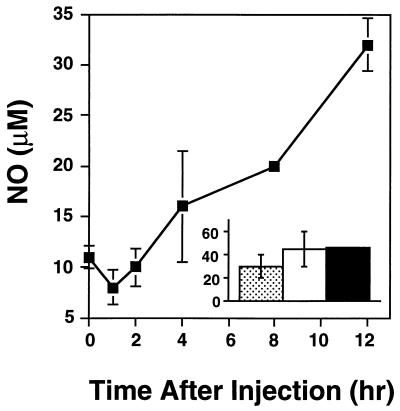
Injection of d-Gal and FTZ also induced the appearance of NO in serum. This response was characterized by steadily rising NO levels, first measurable at 4 h postinjection and continuing until death (Fig. 3). In addition, animals with lethal acute toxoplasmosis possessed high levels of NO circulating in the serum (Fig. 3).
FIG. 3.
Increased serum NO levels are associated with progression of d-Gal-plus FTZ-induced lethality and acute infection. C3H/HeJ mice were injected with d-Gal (20 mg) plus FTZ (5 × 107), and serum NO levels were monitored at the indicated times following injection (squares). In a parallel experiment (inset), three mice were infected with 100 strain RH tachyzoites, and serum NO levels were determined 7 days postinfection. The 0-h time point (large graph) shows the uninfected control levels, since these experiments were set up concurrently. Each bar in the graph represents an individual animal. A modified Griess assay was employed to measure NO (see Materials and Methods for details). These results are representative of two experiments performed.
Kinetics of the plasma cytokine response in mice injected with d-Gal plus FTZ.
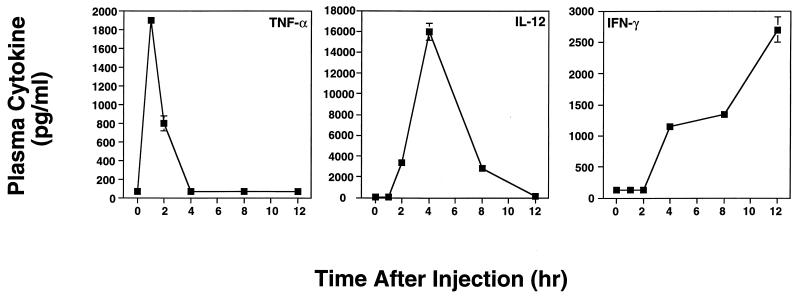
The rising levels of NO and parallel appearance of liver enzymes in the sera of lethally injected mice were suggestive of an uncontrolled inflammatory cytokine response. Accordingly, we measured TNF-α, IL-12, and IFN-γ levels in plasma of animals injected with d-Gal plus FTZ (Fig. 4). As shown, a distinct and highly reproducible pattern of cytokine production occurred following injection. Plasma TNF-α levels peaked 60 min following injection, followed by a peak IL-12 response occurring at 4 h and steadily rising levels of IFN-γ, continuing until the death of the animals. Similar TNF-α kinetics have been reported after administration of d-Gal and bacterial toxins (25, 48, 49). In addition, we found that administration of FTZ alone resulted in cytokine profiles virtually identical to those shown in Fig. 4 and that d-Gal in the absence of parasite Ag failed to induce cytokine production (data not shown). This is consistent with models of low-dose endotoxin shock, which require a sensitizing agent such as d-Gal, since mice are relatively resistant to this type of toxicity (10).
FIG. 4.
Kinetics of proinflammatory cytokine production following administration of d-Gal and FTZ. C3H/HeJ mice (three or four per time point) were i.p. injected with d-Gal (20 mg) plus FTZ (5 × 107) and then were bled at the indicated time points. Blood plasma was prepared and assayed by two-site ELISA for TNF-α and IFN-γ. Biologically active IL-2 was measured by determining the ability of test plasma samples and recombinant IL-12 standard to bind plate-bound anti-IL-12 MAb and subsequently stimulate IFN-γ production by normal spleen cells. Virtually identical results were obtained in a similar experiment employing strain C57BL/6 mice.
Death induced by d-Gal plus FTZ is mediated by TNF, IL-12, IFN-γ, and NO.
We next examined whether some, or all, of the proinflammatory cytokines induced by the parasite were involved in the lethality of d-Gal and FTZ. Injection of depleting MAbs specific for TNF, IL-12, and IFN-γ rescued mice from the lethal effect of d-Gal and parasite Ag (Table 4). Animals treated in this manner not only survived, but failed to show any signs of sickness associated with the response. In contrast, mice injected with control rat Ig were susceptible to d-Gal plus FTZ lethality.
TABLE 4.
Inhibition of endogenous IL-12, TNF, IFN-γ, and NO rescues d-Gal-sensitized mice from lethality
| d-Gala | FTZb | mg of antibody injectedc
|
AGd | Mortalitye (no. dead/total) | |||
|---|---|---|---|---|---|---|---|
| Control Ig | AntiIL-12 | Anti-TNF | Anti-IFN-γ | ||||
| + | − | − | 0/3 | ||||
| − | + | − | 0/4 | ||||
| + | + | − | 3/3 | ||||
| + | + | 0.5 | − | 3/3 | |||
| + | + | 0.5 | − | 0/3 | |||
| + | + | 0.5 | − | 0/3 | |||
| + | + | 0.5 | − | 0/3 | |||
| + | + | + | 0/3 | ||||
20 mg administered i.p.
5 × 107 administered i.p.
Injected i.p. 2 h prior to administration of d-Gal plus FTZ.
AG (100 mM) was supplied in drinking water beginning 12 days prior to injection of d-Gal and Ag.
Mortality at 24 h postinjection of C3H/HeJ mice.
The high levels of NO appearing in the sera of mice treated with d-Gal plus Ag suggested that this effector molecule may contribute to the pathology of the response. To address this issue, mice were treated with the compound AG in order to block the ability to produce inducible NO. As shown in Table 4, mice subjected to this protocol were completely resistant to d-Gal plus FTZ-induced lethality. These results show that T. gondii-triggered TNF, IL-12, and IFN-γ responses result in death of the animals and suggest that the effector molecule NO plays a crucial role in the response.
Cells bearing the granulocyte-associated marker GR-1 mediate the lethal effect of d-Gal plus FTZ.
To determine which cell types were required for d-Gal and parasite Ag lethality, T- and B-lymphocyte-deficient SCID mice were initially tested for susceptibility. As shown in Table 5, the immunodeficient C57BL/6.scid strain was susceptible to T. gondii-induced lethality, a result indicating that the toxicity of d-Gal and parasite Ag is a T- and B-lymphocyte-independent effect.
TABLE 5.
C57BL/6.scid and IL-5−/− mouse strains are susceptible to d-Gal and FTZ lethality
| Mouse strain | d-Gala | Agb | Mortalityc (no. dead/total) |
|---|---|---|---|
| C57BL/6.scid | + | − | 0/3 |
| − | + | 0/3 | |
| + | + | 3/3 | |
| IL-5−/− | + | − | 0/3 |
| − | + | 0/3 | |
| + | + | 3/3 |
20 mg administered i.p.
SCID mice were i.p. challenged with 150 μg of STAg; IL-5−/− mice were treated i.p. with 5 × 107 FTZ.
Recorded 24 h postinjection.
To further investigate the cell type involved, in vivo MAb depletions were performed. Interestingly, we found that administration of a rat MAb specific for the granulocyte marker GR-1 rescued mice from the effects of d-Gal plus FTZ, as did injection of a rabbit anti-asialo-GM-1 antiserum which recognizes a glycolipid moiety expressed on NK cells and at low levels on granulocytes (Table 6). As expected, injection of control rat Ig and normal rabbit serum failed to alter the outcome of injection of d-Gal plus FTZ.
TABLE 6.
Cell subsets involved in d-Gal-plus FTZ-induced lethality
| d-Gala | FTZb | Antibody injectionc
|
Mortalityd | ||||
|---|---|---|---|---|---|---|---|
| Rat Ig | Anti-GR-1 | Anti-CD4 | Rabbit Ig | Anti-asialo-GM-1 | |||
| + | − | − | − | − | − | − | 0/3 |
| − | + | − | − | − | − | − | 0/3 |
| + | + | − | − | − | − | − | 3/3 |
| + | + | + | − | − | − | − | 3/3 |
| + | + | − | + | − | − | − | 0/3 |
| + | + | − | − | + | − | − | 3/3 |
| + | + | − | − | − | + | − | 3/3 |
| + | + | − | − | − | − | + | 0/3 |
20 mg administered i.p.
5 × 107 administered i.p.
Antibodies were administered by i.p. injection prior to initiation of the experiment. See Materials and Methods for details.
Mortality at 24 h postinjection of C3H/HeJ mice.
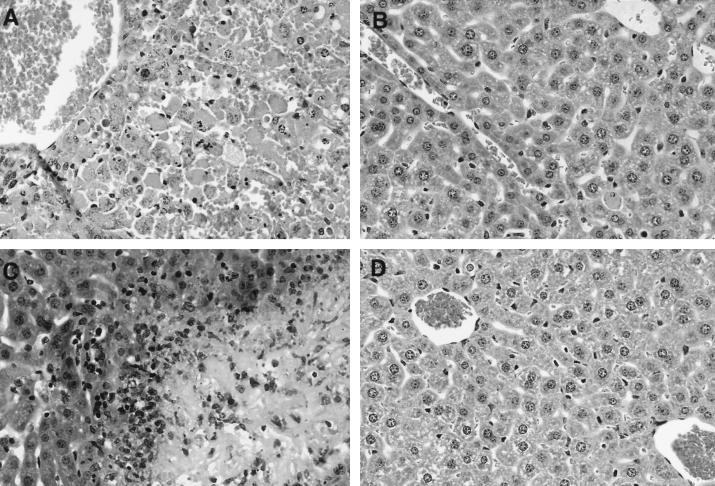
We next examined liver pathology in anti-GR-1- and control MAb-treated, as well as RH-infected, animals. As shown in Fig. 5A, injection of d-Gal plus FTZ with control Ig induced severe liver damage, consisting of severe hepatic cord dissociation, severe diffuse hepatic necroses, and hemorrhage throughout the liver. In striking contrast, animals receiving anti-GR-1 MAb displayed intact hepatic architecture and an absence of hemorrhage (Fig. 5B). Although livers from this group (Fig. 5B) appeared essentially normal (Fig. 5D), occasional necrotic hepatocytes were observed in the former. We also examined livers from mice with lethal acute infection. In this case, liver damage did not appear to extend throughout the organ as in animals receiving d-Gal plus FTZ. Nevertheless, hepatic cord dissociation and multiple foci of severe hepatic necrosis were observed (Fig. 5C). In addition, severe necrosis of the liver capsule was apparent in these sections.
FIG. 5.
Absence of liver histopathology in granulocyte-depleted animals injected with d-Gal plus FTZ. Control Ab (A) or RB6-8C5 (antigranulocyte) (B) was injected at −24 and −18 h, d-Gal (20 mg) plus FTZ (5 × 107) were administered at 0 h, and livers were removed at +12 h. Livers from RH-infected animals (day 7 postinoculation with 100 tachyzoites) were also examined (C), as were livers from healthy mice (D). Organs were fixed in 10% formalin, embedded in paraffin wax, and subjected to thin sectioning followed by hematoxylin and eosin staining. Original magnification, ×100.
Although GR-1 is expressed by both neutrophils and eosinophils, we consider it unlikely that the latter cell type is involved in the response because IL-5 knockout animals, which possess low eosinophil levels and fail to mount a blood or tissue eosinophilia (35), retain sensitivity to d-Gal plus FTZ (Table 5). Finally, elimination of CD4+ T cells had no effect on survival of the animals (Table 6), a result confirming that T lymphocytes of the CD4+ subset are not required to mediate the lethal toxicity of T. gondii Ag.
DISCUSSION
The results of this study demonstrate that T. gondii possesses the capability of inducing lethal cytokine shock in d-Gal-sensitized mice. The response is marked by a rapid burst of TNF-α in serum followed by the appearance of IL-12, IFN-γ, and NO. Blocking of any one of these mediators with MAb or, for NO, AG rescues animals from the lethal outcome of injection of d-Gal plus T. gondii Ag. Administration of the combination of d-Gal and parasite extract also induces the appearance of liver-associated enzymes in the serum, a hallmark of hepatic damage. Interestingly, while the response is not T lymphocyte dependent, cells bearing the granulocyte marker GR-1 appear to be centrally involved, since their removal with depleting MAb renders mice resistant to T. gondii-induced lethal shock. Our finding that depletion of asialo-GM-1-positive cells confers resistance also implicates NK cells in the cascade of events leading to death. Nevertheless, the latter result must be interpreted with caution, because this phenotypic marker has also been reported to be on subsets of cells of the monocyte-granulocyte lineage (50). Together, the results suggest granulocyte involvement in triggering a cascade of inflammatory cytokines, resulting in progressive NO accumulation and ultimately precipitating host death. These results indicate that Toxoplasma, like LPS, is a potent enough inflammatory stimulus to drive cytokine toxicity in the d-Gal-induced low-dose endotoxin model.
Apoptosis mediated by TNF-α has been implicated in mortality caused by d-Gal plus endotoxin, and it is possible that granulocytes serve as a source of the cytokine in this model (3, 39). Our data, which support the concept for a critical role of TNF-α, also show that depletion of IFN-γ, IL-12, and NO allows animals to survive. Therefore, lethality in the d-Gal experimental model is likely to be a complex process involving several mediators. Our laboratory is currently focusing effort on elucidating the pathways leading to death.
Perhaps the most striking aspect of our data regarding d-Gal- and parasite Ag-induced toxicity is that a GR-1-specific depleting MAb rescues animals from lethality, a result implicating granulocytes in pathogenesis of the response. Granulocytes have also been linked to LPS-induced liver damage (29, 34). Several recent reports indicate that granulocytes display protective activity during acute T. gondii infection (55, 57). Our data, in general, provide strong support for the concept that cells of this lineage play an important role during T. gondii infection and that, in the d-Gal system, they function as mediators of disease.
We do not at present know the functional role of neutrophils in our system. RB6-8C5-treated mice displayed an approximately 50% reduction in subsequent appearance of serum TNF-α (data not shown), raising the possibility that parasite Ag induces rapid release of the latter cytokine from granulocytes. Indeed, granulocytes are known to be capable of TNF-α secretion in response to stimuli such as LPS (3). An alternative model is that TNF-α induces upregulation of adhesion molecules, such as intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 in the liver and CD11b/CD18 on neutrophils. The latter molecular events would lead to neutrophil sequestration and transmigration in the liver. Subsequent organ damage could occur through production of reactive oxygen intermediates or other granulocyte mediators (8, 29).
In addition to the data presented here, several other recent reports demonstrate cytokine pathology during T. gondii infection. IL-10 knockout mice succumb during acute infection with the low-virulence ME49 strain, and death is associated with overproduction of inflammatory cytokines, presumably due to the absence of the downregulatory activity of IL-10 (16, 52). Evidence in support of this concept comes from the finding that MAb depletion of either IL-12 or IFN-γ results in delayed mortality in IL-10 knockout animals. Similarly, lethal oral infection of the C57BL/6 mouse strain results in IFN-γ-mediated gut pathology, and MAb depletion of the latter cytokine prolongs time to death in these animals (40). Interestingly, septic shock due to toxoplasmosis has also been reported for AIDS patients (41).
In each of the murine model studies described above, T or CD4+ lymphocytes were shown to play a role in mediating pathology, as shown by delayed mortality in SCID mice and in animals depleted of CD4+ cells with MAb. Our results show that T. gondii can also induce lethal pathology in the absence of the T-cell compartment, since both SCID mice and anti-CD4-treated mice remain susceptible to d-Gal plus FTZ administration. While the persistence of mortality in T-cell-deficient IL-10 knockout mice (16, 52) and orally infected C57BL/6 mice (40) may in part be attributable to uncontrolled parasite growth and dissemination, our data suggest that T-cell-independent inflammatory cytokine pathology may also contribute to death in these cases.
The role of NO in promoting endotoxemia is complex. The latter chemical mediator has been implicated in promoting lethal vasodilation and hypotension induced by LPS (22). Nevertheless, NO also possesses immunosuppressive properties and as such has been reported to play a role in downregulating overproduction of inflammatory cytokines during lethal shock induced by staphylococcal superantigens (9). In models of endotoxemia employing high doses of LPS, inducible nitric oxide synthase (iNOS) knockout mice have been reported to possess a resistant phenotype, although another report concluded that these knockout animals were indistinguishable from wild-type counterparts under the same conditions (37, 44). In low-dose models in which animals are primed with Propionibacterium acnes followed by LPS, iNOS gene inactivation has been reported to be without effect (44). These and other data suggest that low-dose and high-dose endotoxemia models are mechanistically distinct (24, 44).
For T. gondii-induced toxicity in d-Gal-sensitized mice, inhibition of NO with AG rendered mice resistant to subsequent lethality. This result was somewhat unexpected on the basis of models of low-dose endotoxemia induced by LPS, which do not appear to involve NO as a crucial factor. We are currently further exploring how T. gondii and LPS differ in activation of pathways leading to death.
Infection with a sufficiently low infectious dose of parasite strain ME49 allows survival of acute infection and establishment of chronic disease. In this case, production of NO appears to play a protective role during chronic infection, as determined by an increased cyst number in AG-treated mice (26). Furthermore, in the same model, iNOS knockout mice survive acute infection but succumb during the persistent stage of disease (59). Our data suggest that overproduction of NO may be involved in pathogenesis of lethal acute infection.
The effect of T. gondii Ag appears similar to that of LPS administration in d-Gal-sensitized mice, in that both treatments result in rapid, T-cell-independent, lethal cytokine shock (24, 49). Both LPS and T. gondii Ag induce macrophage activation in vitro, leading to inflammatory cytokine production. Therefore, it seems likely that the parasite factor(s) responsible for the in vivo effects reported here is identical to that inducing inflammatory cytokine production in cultures of murine macrophages (14, 23). While the T. gondii molecule triggering the inflammatory cytokine cascade has yet to be identified, related studies with Plasmodium falciparum and Trypanosoma cruzi suggest that specific protozoan glycolipid conjugates possess macrophage-activating capability (2, 58).
The cytokines IFN-γ, TNF, and IL-12 are crucial in the protective response to T. gondii (4, 12, 15, 27, 30, 33, 61, 62). Our data show that the parasite can stimulate production of lethally high levels of these same cytokines in d-Gal-sensitized mice. To our knowledge, this study represents the first direct demonstration that T. gondii possesses the inherent capability of inducing cytokine toxicity in a T-cell-independent, granulocyte-dependent fashion.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI 40540.
We thank A. Alcaraz for assistance with histopathology and E. Pearce and B. Butcher for helpful discussions and critical review of the manuscript.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Carmago M M, Aleida I C, Pereira M E S, Ferguson M A J, Travassos L R, Gazzinelli R T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 3.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 4.Chang H R, Grau G E, Pechere J C. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990;69:33–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Decker K, Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;71:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 6.Denkers E Y, Gazzinelli R T, Hieny S, Caspar P, Sher A. Bone marrow macrophages process exogenous Toxoplasma gondii peptides for recognition by parasite-specific cytolytic T lymphocytes. J Immunol. 1993;150:517–526. [PubMed] [Google Scholar]
- 7.Denkers E Y, Scharton-Kersten T, Gazzinelli R T, Yap G, Charest H, Sher A. Cell-mediated immunity to Toxoplasma gondii: redundant and required mechanisms as revealed by studies in gene knockout mice. In: Kaufmann S H E, editor. Medical Intelligence Unit: host response to intracellular pathogens. R. G. Austin, Tex: Landes Company; 1997. pp. 167–181. [Google Scholar]
- 8.Essani N A, Bajt M L, Farhood A, Vonderfecht S L, Jaeschke H. Transcriptional activation of vascular cell adhesion molecule-1 gene in vivo and its role in the pathophysiology of neutrophil-induced liver injury in murine endotoxic shock. J Immunol. 1997;158:5941–5948. [PubMed] [Google Scholar]
- 9.Florquin S, Amraoui Z, Goldman M. The protective role of endogenously synthesized nitric oxide in staphylococcal enterotoxin B-induced shock in mice. J Exp Med. 1994;180:1153–1158. doi: 10.1084/jem.180.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization of the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Denkers E Y, Sher A. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis. 1993;2:139–149. [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated T. gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 14.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 16.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 17.Granger D L, Hibbs J B, Jr, Perfect J R, Durack D T. Metabolic fate of l-arginine in relation to microbiostatic capability of murine macrophage. J Clin Invest. 1990;85:264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grau G E, Farjardo L F, Piguet P F, Allet B, Lambert P H, Vassali P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 19.Grau G E, Heremans H, Piguet P-F, Poiuntaire P, Lambert P-H, Billiau A, Vassalli P. Monoclonal antibody against interferon-γ can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths M J D, Messent M, MacAllister R J, Evans T W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993;110:963. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grisham M B, Johnson G G, Gautreaux M D. Measurement of nitrate and nitrite in extracellular fluids: a window to systemic nitric oxide metabolism. Methods Enzymol. 1995;7:84–90. [Google Scholar]
- 22.Gross S S, Wolin M S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 23.Grunvald E, Chiaramonte M, Hieny S, Wysocka M, Trinchieri G, Vogel S N, Gazzinelli R T, Sher A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez-Ramos J C, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 25.Harbrecht B G, Di Silvio M, Demetris A J, Simmons R L, Billiar T R. Tumor necrosis factor-α regulates in vivo nitric oxide synthesis and induces liver damage during endotoxemia. Hepatology. 1994;20:1055–1060. doi: 10.1002/hep.1840200439. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi S, Chan C C, Gazzinelli R, Roberge F G. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;154:1476–1481. [PubMed] [Google Scholar]
- 27.Hunter C A, Candolfi E, Subauste C, Van Cleave V, Remington J S. Studies on the role of IL-12 in murine toxoplasmosis. Immunology. 1995;84:16–21. [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter C A, Chizzonite R, Remington J S. IL-1β is required for IL-12 to induce production of IFN-γ by NK cells. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 29.Jaeschke H, Farhood A, Smith C W. Neutrophil-induced liver cell injury in endotoxic shock is a CD11b/CD18-dependent mechanism. Am J Physiol. 1991;261:G1051–G1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- 30.Johnson L L. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992;60:1979–1985. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson L L, Van der Vegt F P, Havell E A. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infect Immun. 1993;61:5174–5180. doi: 10.1128/iai.61.12.5174-5180.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keppler D O R, Pausch J, Decker K. Selective uridine triphosphate deficiency induced by d-galactosamine in liver and reversed by pyrimidine nucleotide precursors. J Biol Chem. 1974;249:211–216. [PubMed] [Google Scholar]
- 33.Khan I A, Matsuura T, Kasper L H. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun. 1994;62:1639–1645. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komomatsu Y, Shiratori Y, Kawase T, Hashimoto N, Han K, Shiina S, Matsumura M, Niwa Y, Kato N, Tada M, Ikeda Y, Tanaka M, Omata M. Role of polymorphonuclear leukocytes in galactosamine hepatitis: mechanism of adherence to hepatic endothelial cells. Hepatology. 1994;20:1548–1556. doi: 10.1002/hep.1840200626. [DOI] [PubMed] [Google Scholar]
- 35.Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Kohler G, Young I G, Matthaei K I. IL-5 deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 36.Krahenbul J L, Remington J S. Immunology of toxoplasma and toxoplasmosis. In: Cohen S, Warren K S, editors. Immunology of parasitic infections. London, United Kingdom: Blackwell Scientific Publications; 1982. pp. 356–421. [Google Scholar]
- 37.Laubach V E, Shesely E G, Smithies O, Sherman P A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann V, Freudenberg M A, Galanos G. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leist M, Gantner F, Bohlinger I, Germann P G, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 40.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T cell-dependent, IFN-γ-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucet J-C, Bailly M-P, Bedos J-P, Wolff M, Gachot B, Vachon F. Septic shock due to toxoplasmosis in patients with the human immunodeficiency virus. Chest. 1993;104:1054–1058. doi: 10.1378/chest.104.4.1054. [DOI] [PubMed] [Google Scholar]
- 42.Luft B, Remington J S. AIDS commentary: toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 43.Luft B J, Brooks R G, Conley F K, McCabe R E, Remington J S. Toxoplasmic encephalitis in patients with acquired immune response deficiency syndrome. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- 44.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q, Sokol K, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 45.Margulies D H. Antibodies. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1992. p. 2.7.6. [Google Scholar]
- 46.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCabe R, Remington J S. Toxoplasmosis: the time has come. N Engl J Med. 1988;380:313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- 48.Miethke T, Duschek T, Wahl C, Heeg K, Wagner H. Pathogenesis of the toxic shock syndrome: T cell mediated lethal shock caused by the superantigen TSST-1. Eur J Immunol. 1993;23:1494–1500. doi: 10.1002/eji.1830230715. [DOI] [PubMed] [Google Scholar]
- 49.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Momoi T N K, Sakakibara K, Nagai Y. Localization of a glycosphingolipid, asialo GM1, in rat immunocytes. J Biochem. 1982;91:301–310. doi: 10.1093/oxfordjournals.jbchem.a133688. [DOI] [PubMed] [Google Scholar]
- 51.Nagaki M, Muto Y, Ohnishi H, Yasuda S, Sano K, Naito T, Maeda T, Yamada T, Moriwaki H. Hepatic injury and lethal shock in galactosamine-sensitized mice induced by the superantigen staphylococcal enterotoxin B. Gastroenterology. 1994;106:450–458. doi: 10.1016/0016-5085(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 52.Neyer L E, Grunig G, Fort M, Remington J S, Rennick D, Hunter C A. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun. 1997;65:1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfefferkorn E R, Pfefferkorn L C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976;39:365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- 54.Rosa Brunet L, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. IL-4 protects against TNF-α-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 55.Sayles P C, Johnson L J. Exacerbation of toxoplasmosis in neutrophil depleted mice. Nat Immun. 1997;15:249–258. [PubMed] [Google Scholar]
- 56.Scharton-Kersten T, Denkers E Y, Gazzinelli R T, Sher A. Role of IL-12 in the induction of cell-mediated immunity to Toxoplasma gondii. Res Immunol. 1995;146:539–545. doi: 10.1016/0923-2494(96)83029-x. [DOI] [PubMed] [Google Scholar]
- 57.Scharton-Kersten T, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1–13. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sher A, Oswald I O, Hieny S, Gazzinelli R T. Toxoplasma gondii induces a T-independent IFN-γ response in NK cells which requires both adherent accessory cells and TNF-α. J Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 60.Sibley L D, Adams L B, Fukutomi A Y, Krahenbuhl J L. Tumor necrosis factor-α triggers antitoxoplasmal activity in IFN-γ primed macrophages. J Immunol. 1991;147:2340–2345. [PubMed] [Google Scholar]
- 61.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-γ for the prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 62.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 63.Tepper R I, Coffman R L, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 64.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]