Abstract
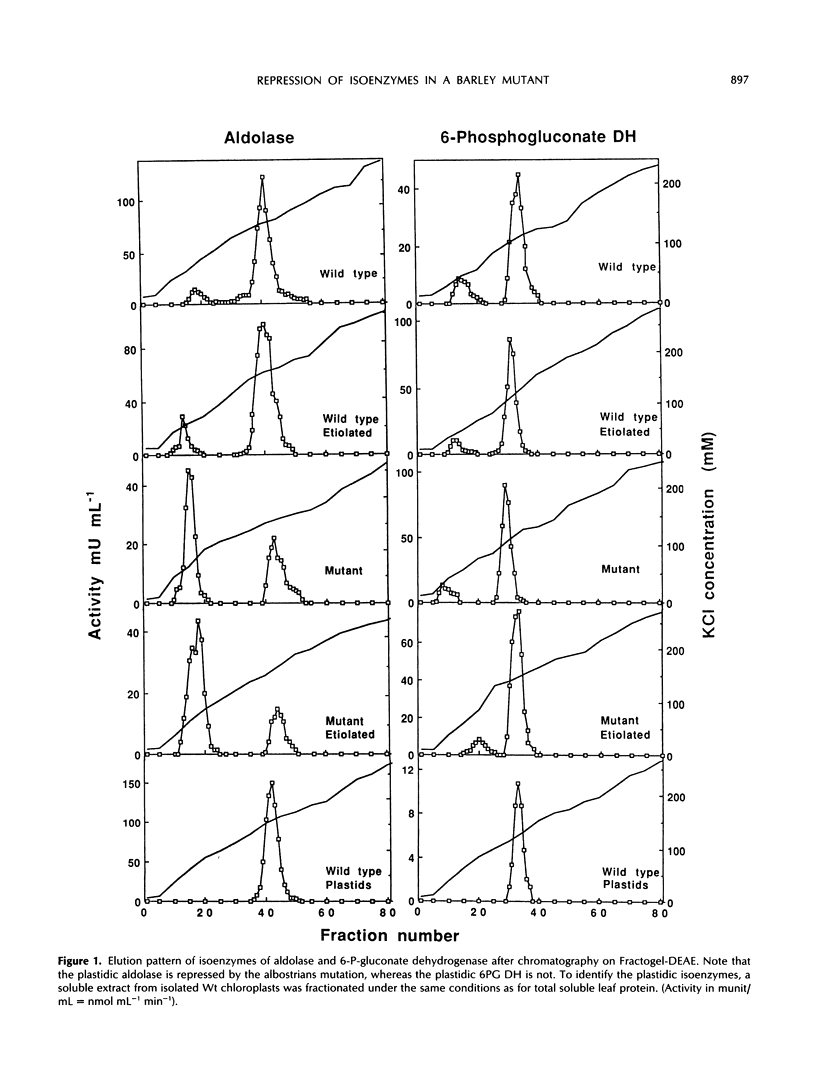
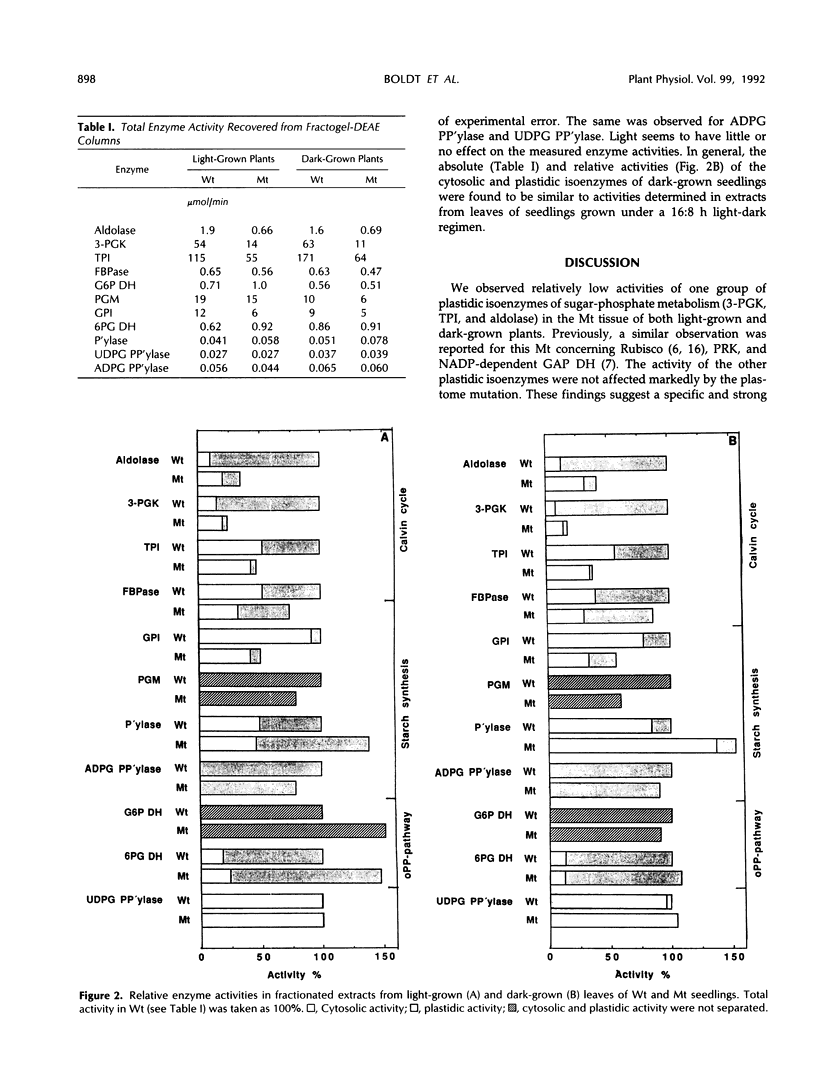
White leaves of the mutant line albostrians and green leaves of the wild-type cultivar Salome of barley (Hordeum vulgare L.) were screened for the presence of plastidic and cytosolic isoenzymes of sugar-phosphate metabolism. Isoenzyme separation was achieved by anion-exchange chromatography on Fractogel TSK DEAE-650(S). The mutant tissue had a markedly reduced level of plastidic 3-phosphoglycerate kinase, triosephosphate isomerase, and aldolase activity. In contrast, the activity of plastidic glucosephosphate isomerase, fructose 1,6-bisphosphatase, 6-phosphogluconate dehydrogenase, starch phosphorylase, and ADP-glucose pyrophosphorylase was in the same range as in wild-type leaf tissue. The activity of the corresponding cytosolic isoenzymes (including UDP-glucose pyrophosphorylase) showed essentially no differences in mutant and wild type. The same trend was observed in dark-grown mutant and wild-type leaves. Interestingly, the total activity levels of all isoenzymes were about the same when comparing dark-grown and light-grown mutant or wild-type plants. From these data, it is concluded that mutant leaves exhibit a selective decrease of a subgroup of plastidic isoenzymes associated with the Calvin cycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Wildner G. Formation of the small subunit in the absence of the large subunit of ribulose 1,5-bisphosphate carboxylase in 70 S ribosome-deficient rye leaves. Arch Biochem Biophys. 1978 Mar;186(2):283–291. doi: 10.1016/0003-9861(78)90437-x. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Flügge U. I., Borchert S. Diversity of specificity and function of phosphate translocators in various plastids. Plant Physiol. 1991 Feb;95(2):341–343. doi: 10.1104/pp.95.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger I., Schnarrenberger C. Purification, subunit structure and immunological comparison of fructose-bisphosphate aldolases from spinach and corn leaves. Eur J Biochem. 1983 Oct 17;136(1):101–106. doi: 10.1111/j.1432-1033.1983.tb07711.x. [DOI] [PubMed] [Google Scholar]
- Köpke-Secundo E., Molnar I., Schnarrenberger C. Isolation and characterization of the cytosolic and chloroplastic 3-phosphoglycerate kinase from spinach leaves. Plant Physiol. 1990 May;93(1):40–47. doi: 10.1104/pp.93.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Zimmermann G., Feller U. Evidence for a hexosediphosphatase from the cytoplasm of spinach leaves. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):321–326. doi: 10.1515/bchm2.1974.355.1.321. [DOI] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A. Two isoenzymes of glucosephosphate isomerase from spinach leaves and their intracellular compartmentation. Eur J Biochem. 1974 Jun 1;45(1):77–82. doi: 10.1111/j.1432-1033.1974.tb03531.x. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Tetour M., Herbert M. Development and intracellular distribution of enzymes of the oxidative pentose phosphate cycle in radish cotyledons. Plant Physiol. 1975 Dec;56(6):836–840. doi: 10.1104/pp.56.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R. Pyrophosphorylases in Solanum tuberosum: I. Changes in ADP-Glucose and UDP-Glucose Pyrophosphorylase Activities Associated with Starch Biosynthesis during Tuberization, Maturation, and Storage of Potatoes. Plant Physiol. 1976 Jan;57(1):63–68. doi: 10.1104/pp.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]


