Abstract
Background:
Widespread exposure to organophosphate ester (OPE) flame retardants with potential reproductive toxicity raises concern regarding the impacts of gestational exposure on birth outcomes. Previous studies of prenatal OPE exposure and birth outcomes had limited sample sizes, with inconclusive results.
Objectives:
We conducted a collaborative analysis of associations between gestational OPE exposures and adverse birth outcomes and tested whether associations were modified by sex.
Methods:
We included 6,646 pregnant participants from 16 cohorts in the Environmental influences on Child Health Outcomes (ECHO) Program. Nine OPE biomarkers were quantified in maternal urine samples collected primarily during the second and third trimester and modeled as -transformed continuous, categorized (high/low/nondetect), or dichotomous (detect/nondetect) variables depending on detection frequency. We used covariate-adjusted linear, logistic, and multinomial regression with generalized estimating equations, accounting for cohort-level clustering, to estimate associations of OPE biomarkers with gestational length and birth weight outcomes. Secondarily, we assessed effect modification by sex.
Results:
Three OPE biomarkers [diphenyl phosphate (DPHP), a composite of dibutyl phosphate and di-isobutyl phosphate (DBUP/DIBP), and bis(1,3-dichloro-2-propyl) phosphate] were detected in of participants. In adjusted models, DBUP/DIBP [odds ratio (OR) per ; 95% confidence interval (CI): 1.02, 1.12] and bis(butoxyethyl) phosphate (OR for high vs. ; 95% CI: 1.06, 1.46), but not other OPE biomarkers, were associated with higher odds of preterm birth. We observed effect modification by sex for associations of DPHP and high bis(2-chloroethyl) phosphate with completed gestational weeks and odds of preterm birth, with adverse associations among females. In addition, newborns of mothers with detectable bis(1-chloro-2-propyl) phosphate, bis(2-methylphenyl) phosphate, and dipropyl phosphate had higher birth weight-for-gestational-age -scores ( for detect vs. ); other chemicals showed null associations.
Discussion:
In the largest study to date, we find gestational exposures to several OPEs are associated with earlier timing of birth, especially among female neonates, or with greater fetal growth. https://doi.org/10.1289/EHP13182
Introduction
Organophosphate esters (OPEs) have been increasingly used as flame retardants over the last decade as polybrominated diphenyl ether (PBDE) flame retardants were phased out in the mid-2000s over concerns regarding toxicity.1 OPEs are widely applied as flame retardants and plasticizers in polyurethane foams used in furniture, baby products, electronics, textiles, and building materials.2–4 Because they are not chemically bound to the polymers, they slowly volatilize into indoor air and then partition into dust.2,5–7 Individuals are exposed to OPEs through ingestion of indoor dust, inhalation, dermal exposure, and dietary intake.3,4 OPE metabolites have been frequently detected in urine samples from the US general population.5,8 OPEs and their metabolites are expected to be less persistent in the human body, compared with PBDEs,1 with half-lives being on the order of hours to days as estimated from animal9–11 and human models.12,13 The detection of OPEs and their metabolites in pregnant people,14–16 as well as the cord blood,17 placenta,18 deciduae and chorionic villi,19 and amniotic fluid,20 indicates maternal–fetal transfer of OPEs during pregnancy.
A growing body of literature indicates that gestational exposure to environmental chemicals contributes to adverse birth outcomes.21–24 Laboratory studies suggest that OPEs have developmental and reproductive toxicity in animals.25–33 For example, parental exposure of zebrafish to environmentally relevant concentrations of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and tris(2-butoxyethyl) phosphate (TBOEP) adversely affected growth and survival of the offspring,27–29 and prenatal exposure of rats to TDCPP increased the number of noticeably smaller pups and lowered body weight in the offspring.30 In human studies, OPE levels measured prior to or during pregnancy have been associated with adverse reproductive outcomes, such as decreased fertilization, implantation, and live birth,34,35 along with pregnancy loss36 and spontaneous abortion.37 Certain OPEs were also shown to interfere with thyroid function in toxicological models38–41 and epidemiological studies.42–46 They have also been linked to changes in peroxisome proliferator-activated receptor (PPAR) activity in in vitro models47–49 and oxidative stress in animal50,51 and human studies.52,53 These biologic targets serve critical roles in multiple pathways involved in fetal growth,54–57 metabolism,58,59 and adipose tissue development.60
Adverse birth outcomes, including preterm birth and low birth weight (LBW), are risk factors for neonatal mortality and chronic morbidity, increasing risks of neurodevelopmental disabilities and respiratory and gastrointestinal complications.57,61–63 There is growing recognition of the potential adverse health outcomes with early-term birth, and those born early term experience an increased risk for infant morbidity and mortality,64 as well as for adverse cognitive and educational outcomes.65,66 Despite the potential developmental toxicity of OPEs, epidemiological evidence examining associations between maternal prenatal urinary OPE metabolites and birth outcomes, such as gestational age or birth size, is inconclusive.67–74 For example, previous studies reported adverse associations of bis(1,3-dichloro-2-propyl) phosphate (BDCPP), a composite of dibutyl phosphate and di-isobutyl phosphate (DBUP/DIBP), and isopropyl-phenyl phenyl phosphate (ip-PPP) with shorter gestational duration among females67,74 and of BDCPP with shorter gestational age among males.74 Other studies reported associations of diphenyl phosphate (DPHP) with greater risk of LBW69 and of BDCPP and bis(butoxyethyl) phosphate (BBOEP) with lower birth weight and length.70
On the other side of the spectrum, high birth weight is associated with childhood obesity.75–77 OPEs have been characterized as metabolism-disrupting compounds78 and, thus, play a role in the development of obesity.79 Childhood obesity is associated with a number of adverse health impacts, including diabetes and cardiovascular disease,80 and is a growing concern worldwide.81 One study observed a greater ponderal index, a measure of birth weight relative to birth length, associated with prenatal measurements of BDCPP.68 In contrast, another study found a reduced risk of large-for-gestational-age (LGA) infants in relation to DPHP.71 Finally, two studies found no strong associations with either gestational age or birth weight.72,73 The inconsistent results, as well as the small to moderate sample sizes of previous studies, motivated further investigation of these associations in a larger population.
The Environmental influences on Child Health Outcomes (ECHO) Program, funded by the National Institutes of Health (NIH), combines 69 cohorts across the US to understand the impact of environmental exposures on children’s health.82,83 The present analysis leverages a large, diverse sample from 16 ECHO cohorts to quantify nine OPE biomarkers in urine samples of pregnant participants. We examined associations of urinary OPE biomarker concentrations with birth outcomes related to gestational age at birth (completed gestational weeks; preterm, early-term, late/postterm birth) and birth weight [birth weight-for-gestational-age (BW-GA) -score, term LBW, small-for-gestational-age (SGA), and LGA]. As a secondary aim, we explored whether associations were modified by child’s sex.
Methods
Study Population
In 2016, the NIH established the ECHO Program, an innovative and collaborative research initiative. The overarching scientific goal of ECHO is to advance understanding of the effects of a broad array of early environmental exposures on children’s development and health outcomes with high public health impact. To achieve this goal, the ECHO Program brought together new and existing cohorts, leveraging previously collected biologic samples and other information on various environmental exposures (e.g., physical, chemical, social, behavioral). From 2017 to 2019, cohorts enrolled participants into the ECHO Program, but continued to collect data and samples under their own protocols. In late 2019, the ECHO Program initiated a common protocol that cohorts followed.
We invited all ECHO cohorts that had collected biologic samples prior to the initiation of the common protocol to participate in the present study. Sixteen ECHO cohorts that had prenatal maternal urine samples available for OPE quantification and participant-level data decided to participate in the present study. Each of these cohorts then selected participants according to their own criteria (Table S1). Data collected and submitted to ECHO prior to March 2022 were used for data analysis. Information about the participating cohorts, including their geographic locations, is provided in Table S1 and Figure S1, respectively. To maximize the sample size within budget constraints, we used a single spot or first morning urine sample per participant, primarily collected during the second and third trimesters of pregnancy. In total, urinary OPE and dilution data (described below) were available for 7,048 of 12,873 total pregnant participants enrolled in ECHO from these cohorts. We excluded pregnancies with no available child information (), multiple births (), or missing birth outcome data (gestational age, birth weight, or biological sex at birth; ). We excluded one child with a gestational age of completed weeks because the Aris et al. method for calculating birth weight -scores cannot be used for gestations of completed weeks.84 Therefore, our final analytic sample size was 6,646 mother–child dyads. A flowchart depicting inclusion/exclusion criteria is shown in Figure S2.
Institutional review boards (i.e., the ECHO single IRB or the ECHO cohorts’ local IRBs) reviewed informed consent/assent forms, Health Insurance Portability and Accountability Act (HIPAA) authorization forms, recruitment materials, and other relevant information. Each ECHO cohort obtained written informed consent or the permission of the parent/guardian. The work of the ECHO Data Analysis Center (DAC) was approved through the Johns Hopkins Bloomberg School of Public Health IRB.
OPE Biomarker Analysis
Urine samples collected from each cohort were shipped on dry ice to the Human Health Exposure Analysis Resource (HHEAR) laboratory at the New York University Grossman School of Medicine. The laboratory methods were described in a previously published study that used data from one of the included cohorts74 and are briefly summarized here. After solid-phase extraction, the identification and quantification of target compounds in urine samples were performed using high-performance liquid chromatography (HPLC; ExionLC system; SCIEX), coupled with an AB SCIEX QTRAP quadrupole mass spectrometer (Applied Biosystems). A Kinetex hydrophilic interaction liquid chromatography (HILIC) column (, particle size; Phenomenex) coupled with a Betasil C18 guard column (, particle size; Thermo Scientific) was used for the separation of nine OPE biomarkers and nine internal standards (ISs).
Quality control (QC) samples included synthetic and urine pool samples spiked with of native standard (NS) and of IS, which were analyzed with study samples. HHEAR Urine QC Pools A & B, as well as Standard Reference Materials (SRM3672 and SRM3673; National Institute of Standards and Technology), were analyzed with every sample batch. Reagent blanks demonstrated trace levels of all OPE biomarkers, thus OPE biomarker concentrations in the study samples were subtracted from the corresponding reagent blank values. Matrix-spiked samples showed average recoveries of 70.4%–133%, with coefficients of variation (CVs) of . CVs for HHEAR Urine QC Pools A & B were and , respectively. For SRM3672 and SRM3673, CVs were and , respectively. Masked duplicate samples were also analyzed with the study samples. Among 191 masked duplicates provided by seven cohorts, those in which both were quantified at or above the limit of detection () were used to calculate relative percentage differences.85
The nine OPE biomarkers analyzed include a) BBOEP, a metabolite of TBOEP; b) bis(2-chloroethyl) phosphate (BCETP), a metabolite of tris(2-chloroethyl) phosphate (TCETP); c) bis(1-chloro-2-propyl) phosphate (BCPP), a metabolite of tris(1-chloro-2-propyl) phosphate (TCPP); d) BDCPP, a metabolite of TDCPP; e) bis(2-ethylhexyl) phosphate (BEHP), a metabolite of tris(2-ethylhexyl) phosphate (TEHP); f) bis(2-methylphenyl) phosphate (BMPP), a metabolite of tris(2-methylphenyl) phosphate (TMPP); g) DBUP/DIBP, metabolites of tributyl phosphate (TBUP) and its isomer tri-isobutyl phosphate (TIBP); h) DPHP, a major metabolite of triphenyl phosphate (TPHP); and i) dipropyl phosphate (DPRP), a metabolite of tripropyl phosphate (TPRP) (Table S2). DBUP/DIBP are reported as a composite because they coeluted and could not be quantified individually; therefore, they were quantified as a composite sum of both analytes. The LOD of target analytes ranged from 0.01 to .
Birth Outcomes
ECHO cohorts ascertained birth outcomes for those children born prior to 2019 via their own protocol, with most cohorts relying on maternal or child medical record abstraction (e.g., ultrasound or last menstrual period to estimate the due date), and others using parent report or cohort-obtained data, such as staff-reported information collected at a hospital birth visit. For children born in or after 2019, the ECHO protocol specified the data source, obtained through medical record abstraction. The primary method for obtaining birth outcomes for each cohort is listed in Table S1. We assessed gestational age at birth as a continuous outcome (completed gestational weeks) and categorized as preterm ( wk), early term (37–38 wk), full term (39–40 wk), and late/postterm (41–42 wk). We calculated sex-specific BW-GA -scores based on the equations of Aris et al. and examined the continuous -scores.84 We also categorized birth weight for gestational age as binary variables corresponding to SGA and LGA ( percentile and percentile, respectively)110 and assessed term LBW (birth weight among births at wk gestation).
Covariates
We used Dagitty86 to construct a directed acyclic graph (DAG) identifying confounders, mediators, and precision variables (Figure S3). Covariate information was collected by each ECHO cohort and harmonized by the ECHO DAC. We included as potential covariates maternal race/ethnicity (as a proxy for structural inequality and racism; non-Hispanic white, non-Hispanic black, Hispanic, others), maternal age at delivery (in years), maternal education (less than high school, high school degree/general educational development, some college/associated degree/trade school, bachelor’s degree, masters/professional/doctorate degree), maternal marital status (married/living with a partner, widowed/separated/divorced, single/never married/partnered/not living together), maternal prepregnancy body mass index (BMI; in kilograms per meter squared), maternal smoking during pregnancy (yes, no), parity (0, 1, ), and child’s sex assigned at birth (male, female). We also included information related to timing of urine specimen collection including time of day, trimester, season, and year.
Statistical Analysis
We presented descriptive statistics of covariates and birth outcomes among participants included in our study sample and among participants from the 16 participating ECHO cohorts who were not included in our sample. Urinary dilution was measured as either specific gravity or creatinine in each cohort; therefore, we applied the approach described by Kuiper et al. to account for the influence of urinary dilution on biomarker concentrations.87,88 Briefly, we multiplied OPE concentrations by the ratio of the cohort-specific median dilution value to the participant’s dilution value87,88 for specific gravity, the values were first subtracted from one.89 We calculated Spearman correlation coefficients among dilution-standardized OPE biomarker concentrations and examined descriptive statistics to determine percentiles and the proportion of participants with concentrations .
For regression analyses, we modeled OPE biomarkers detected in of participants (DBUP/DIBP, DPHP, BDCPP) as dilution-standardized continuous -transformed variables based on model fit statistics (compared with untransformed concentrations) and exposure distributions.90 For OPE biomarker concentrations , we used machine-read values provided by the laboratory and replaced negative or zero values with 0.001 to facilitate transformation. Instrument-derived values were negative for some biomarkers, as the background signal for those chemicals was subtracted from those of procedural blanks. We modeled OPE biomarkers detected in 50%–80% of participants (BCPP, BCETP, BBOEP) as three-level categorical variables, with the nondetect category defined as participants with values and the remaining two categories created by dichotomizing participants at the median of dilution-adjusted values (high- and low-exposure categories). Finally, we modeled OPE biomarkers detected in of participants (BMPP, BEHP, DPRP) as binary variables dichotomized as nondetect () or detect ().
We estimated associations of each OPE biomarkers with birth outcomes in linear (continuous outcomes), logistic (binary outcomes), and multinomial logistic regression models [four-level categorized gestational age treating full term (39–40 wk) as the reference group]91 using generalized estimating equations, accounting for clustering at the cohort level. Given our large sample size, we adjusted for all measured variables on our DAG that served as confounders and risk factors of birth outcomes while excluding potential causal intermediates. We included maternal race/ethnicity, maternal age at delivery, maternal education, maternal marital status, maternal prepregnancy BMI, maternal smoking during pregnancy, parity, child’s sex, and sample collection season and year. To assess potential nonlinear associations between continuous covariates (i.e., maternal age, prepregnancy BMI, and year of specimen collection) and OPE biomarker concentrations, we fit models using restricted cubic splines to examine the shape of covariate–outcome associations. Based on visual inspection of the shape of these associations and model fit statistics, we used continuous linear terms for sample collection year and maternal prepregnancy BMI. For maternal age at delivery, we used a restricted cubic spline with 3 knots, at the 25th, 50th, and 75th percentiles, to allow for nonlinear relationships with birth outcomes. To account for covariate data with missingness, we used multiple imputation by chained equations, using all covariates, as well as the study cohort, as predictors. Because prior studies have reported sex-specific associations of some OPEs with birth outcomes,67,69,70 we explored differences in associations by child’s sex using stratified models. In addition, we tested for effect measure modification using the Wald -value for the interaction term between child’s sex and OPE biomarkers. If an interaction term was significant, we interpreted the sex-stratified estimates by comparing their magnitude and direction.
In a sensitivity analysis, we used a leave-one-cohort-out approach to evaluate the influence of each cohort on our results. For this analysis, we estimated associations of OPEs with birth outcomes as above, but we excluded one cohort at a time. For example, we ran 16 unique linear regression models of associations between DPHP and gestational age, with each model excluding participants from a different cohort. As a secondary analysis, we adjusted for potential copollutant confounding by jointly modeling all OPE biomarkers in the same regression model to examine independent effects of each compound.
We considered associations to be statistically significant if the -value was for main effects and for interaction terms in the effect measure modification analysis. We did not make adjustment for multiple comparisons because this would lead to fewer errors of interpretation in our observational setting, as recommended by Rothman.92 We conducted all analyses using SAS (version 9.4; SAS Institute, Inc.).
Results
Demographic and sample-related characteristics of 6,646 mother–child dyads from 16 cohorts are summarized in Table 1. Cohort participants were racially/ethnically diverse, with 52.5% non-Hispanic white, 19.5% non-Hispanic black, 18.9% Hispanic, and 9.0% others. The majority of the pregnant participants were married or living with a partner (76.2%); had no gestational diabetes, hypertension, or preeclampsia (84.0%); and did not smoke during pregnancy (92.8%). Prenatal maternal urine samples were collected during 2007–2020, and almost all were collected during the second or third trimester (99.6%). Approximately of newborns were born preterm, 21.6% early term, 59.4% full term, and 12.2% late/postterm, and the median gestational age was 39 wk (25th, 75th , 40 wk; Table 2). The median birth weight was (25th, 75th and ), and 6.3% of newborns were SGA and 16.0% were LGA. Among 6,197 newborns born at wk gestation, 2.4% were term LBW. There were 5,825 participants from the 16 participating ECHO cohorts who did not meet our criteria for inclusion; their demographic characteristics are presented in Table S3.
Table 1.
Demographic and sample-related characteristics among 6,646 ECHO mother–child dyads.
| Characteristics | (%)a |
|---|---|
| Maternal race/ethnicity | |
| Non-Hispanic white | 3,470 (52.5) |
| Non-Hispanic black | 1,288 (19.5) |
| Hispanic | 1,251 (18.9) |
| Other races/ethnicitiesb | 597 (9.0) |
| Missing | 40 |
| Maternal education | |
| Less than high school | 535 (8.6) |
| High school degree/GED or equivalent | 1,337 (21.4) |
| Some college, no/associate degree, trade school | 1,115 (17.9) |
| Bachelor’s degree | 1,727 (27.7) |
| Masters, professional, or doctorate degree | 1,522 (24.4) |
| Missing | 410 |
| Maternal marital status | |
| Married or living with a partner | 4,768 (76.2) |
| Widowed, separated, divorced | 280 (4.5) |
| Single, never married, partnered, not living together | 1,213 (19.4) |
| Missing | 385 |
| Maternal age at delivery (y) | |
| 209 (3.1) | |
| 20–24 | 975 (14.7) |
| 25–29 | 1,653 (24.9) |
| 30–34 | 2,218 (33.4) |
| 35–39 | 1,290 (19.4) |
| 301 (4.5) | |
| Parity | |
| 0 | 2,566 (42.8) |
| 1 | 1,994 (33.3) |
| 1,436 (23.9) | |
| Missing | 650 |
| Prepregnancy BMI () | |
| Underweight () | 177 (2.8) |
| Normal weight (18.5–24.9) | 2,866 (46) |
| Overweight (25–29.9) | 1,607 (25.8) |
| Obese () | 1,586 (25.4) |
| Missing | 410 |
| Tobacco use during pregnancy | |
| No | 5,123 (92.8) |
| Yes | 396 (7.2) |
| Missing | 1,127 |
| Child’s sex | |
| Female | 3,250 (48.9) |
| Male | 3,396 (51.1) |
| Trimester at sample collection | |
| 1 (0–13 wk) | 29 (0.4) |
| 2 (14–26 wk) | 2,928 (44.1) |
| 3 (27 wk to the end of pregnancy) | 3,689 (55.5) |
| Sample collection season | |
| Winter (December–February) | 1,481 (22.3) |
| Spring (March–May) | 1,881 (28.3) |
| Summer (June–August) | 1,736 (26.1) |
| Autumn (September–November) | 1,548 (23.3) |
| Sample collection year | |
| 2007 | 189 (2.8) |
| 2008 | 330 (5.0) |
| 2009 | 456 (6.9) |
| 2010 | 554 (8.3) |
| 2011 | 798 (12.0) |
| 2012 | 824 (12.4) |
| 2013 | 516 (7.8) |
| 2014 | 593 (8.9) |
| 2015 | 400 (6.0) |
| 2016 | 508 (7.6) |
| 2017 | 488 (7.3) |
| 2018 | 720 (10.8) |
| 2019 | 263 (4.0) |
| 2020 | 7 (0.1) |
Note: BMI, body mass index; ECHO, Environmental influences on Child Health Outcomes; GED, general educational development.
Percentage was calculated without missing observations.
Other races/ethnicities include Asian, native Hawaiian or other Pacific Islanders, American Indian or Alaska native, and multiple race.
Table 2.
Gestational length and birth weight outcomes among 6,646 ECHO mother–child dyads.
| Birth outcomes | (%)a | ||
|---|---|---|---|
| All () | Females () | Males () | |
| Gestational age at birth (completed weeks) | |||
| Preterm (20–36) | 449 (6.8) | 221 (6.8) | 228 (6.7) |
| Early term (37–38) | 1,436 (21.6) | 659 (20.3) | 777 (22.9) |
| Full term (39–40) | 3,947 (59.4) | 1,964 (60.4) | 1,983 (58.4) |
| Late/postterm (40–42) | 814 (12.2) | 406 (12.5) | 408 (12.0) |
| Birth weight (g) | |||
| 200–2,499 | 365 (5.5) | 204 (6.3) | 161 (4.8) |
| 2,500–3,999 | 5,644 (84.9) | 2,824 (86.9) | 2,820 (83.0) |
| 637 (9.6) | 222 (6.8) | 415 (12.2) | |
| Term LBWa | |||
| No | 6,047 (97.6) | 2,939 (97.0) | 3,108 (98.1) |
| Yes | 150 (2.4) | 90 (3.0) | 60 (1.9) |
| SGA ( percentile BW-GA -score) | |||
| No | 6,227 (93.7) | 3,032 (93.3) | 3,195 (94.1) |
| Yes | 419 (6.3) | 218 (6.7) | 201 (5.9) |
| LGA ( percentile BW-GA -score) | |||
| No | 5,580 (84.0) | 2,752 (84.7) | 2,828 (83.3) |
| Yes | 1,066 (16.0) | 498 (15.3) | 568 (16.7) |
Note: BW-GA, birth weight for gestational age; ECHO, Environmental influences on Child Health Outcomes; LBW, low birth weight; LGA, large for gestational age; SGA, small for gestational age.
Among 6,197 term births.
Detection frequencies and distributions of dilution-standardized urinary OPE biomarker concentrations in all participants and by cohort are presented in Tables 3 and S4 and Figure S4, respectively. DPHP, DBUP/DIBP, and BDCPP were detected in 99.5%, 95%, and 87% of the study samples. The detection frequencies of BCETP, BBOEP, and BCPP were between 50% and 80%, and those of BMPP, BEHP, and DPRP were . The highest median concentrations were observed for DPHP () and BDCPP (), followed by BCETP (), DBUP/DIBP (), BCPP (), and BBOEP (). The nine OPE biomarkers were only weakly correlated with each other (Spearman’s correlation to 0.26) (Table S5). Medians of relative percentage differences calculated from valid masked duplicate samples ranged from 6.2% to 16.9% for OPE biomarkers with detection frequencies of , 13.3% to 26.6% for those with detection frequencies between 50% and 80%, and 17.8% to 53.6% for those with detection frequencies of (Table S6).
Table 3.
Distributions of dilution-standardized urinary OPE biomarker concentrations among 6,646 ECHO pregnant participants.
| OPE biomarkers | LOD (ng/mL) | (%) | Percentile | ||||
|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||
| DPHP | 0.03 | 6,613 (99.5) | 0.26 | 0.54 | 0.92 | 1.78 | 8.33 |
| DBUP/DIBP | 0.04 | 6,343 (95) | 0.06 | 0.12 | 0.19 | 0.30 | 0.88 |
| BDCPP | 0.02 | 5,784 (87) | 0.31 | 0.86 | 1.70 | 5.02 | |
| BCETP | 0.02 | 4,589 (69) | 0.52 | 1.58 | 8.22 | ||
| BBOEP | 0.02 | 4,398 (66) | 0.05 | 0.09 | 0.25 | ||
| BCPP | 0.02 | 3,494 (53) | 0.12 | 0.75 | 3.45 | ||
| BMPP | 0.01 | 2,383 (36) | 0.03 | 0.13 | |||
| BEHP | 0.02 | 1,963 (30) | 0.04 | 0.55 | |||
| DPRP | 0.03 | 1,690 (25) | 0.03 | 0.31 | |||
Note: BBOEP, bis(butoxyethyl) phosphate; BCETP, bis(2-chloroethyl) phosphate; BCPP, bis(1-chloro-2-propyl) phosphate; BDCPP, bis(1,3-dichloro-2-propyl) phosphate; BEHP, bis(2-ethylhexyl) phosphate; BMPP, bis(2-methylphenyl) phosphate; DBUP/DIBP, composite of dibutyl phosphate and di-isobutyl phosphate; DPHP, diphenyl phosphate; DPRP, dipropyl phosphate; ECHO, Environmental influences on Child Health Outcomes; LOD, limit of detection; OPE, organophosphate ester.
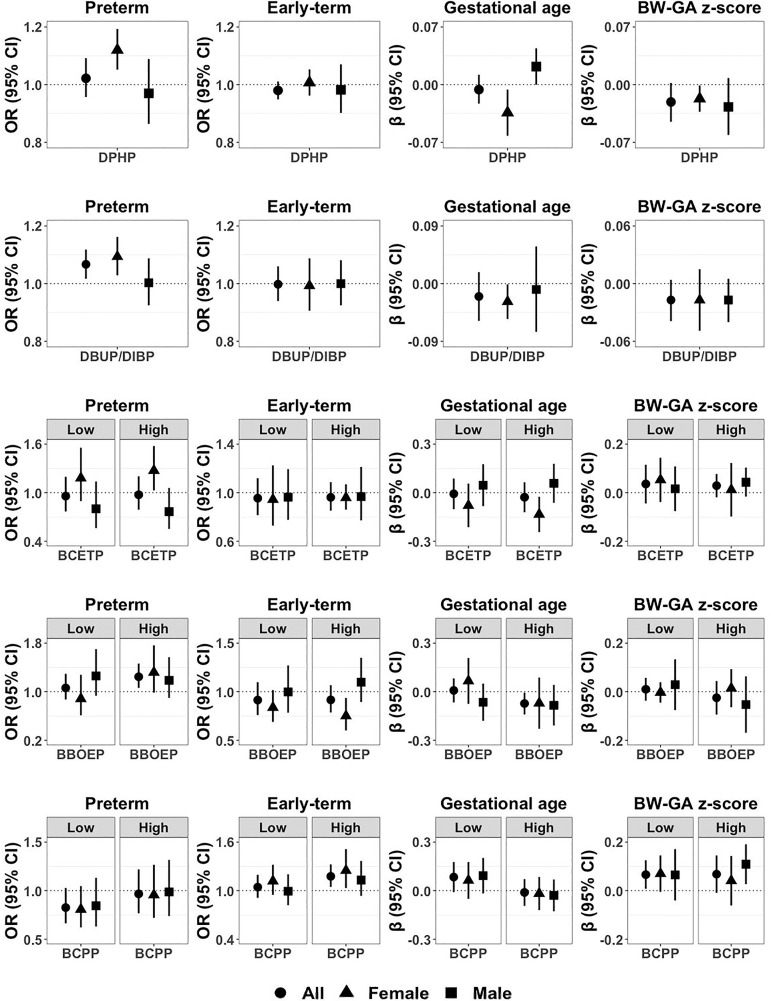
We did not observe associations between prenatal maternal urinary concentrations of DPHP or BDCPP and gestational duration in the overall study population (Table 4). However, associations of DPHP with continuous gestational age and with preterm and early-term birth differed by sex ( for interaction term between OPE and sex; ) (Figure 1 and Table S7). Among females, higher DPHP concentration was associated with shorter gestational age [regression wk; 95% confidence interval (CI): , ] and higher odds of preterm vs. full-term birth [odds ratio (OR) per doubling in ; 95% CI: 1.05, 1.19], whereas among males higher DPHP concentrations were associated with greater gestational age ( wk; 95% CI: 0.00, 0.04). DBUP/DIBP was associated with higher odds of preterm birth (; 95% CI: 1.02, 1.12) in all newborns. When stratified by child’s sex, DBUP/DIBP was associated with shorter gestational age ( wk; 95% CI: , ) and higher odds of preterm birth (; 95% CI: 1.03, 1.16) among female newborns only, although the tests for interaction were not statistically significant ().
Table 4.
Associations between prenatal maternal urinary OPE biomarkers and gestational duration in the ECHO cohorts.
| OPE biomarkers | Gestational age (wk) () | Preterm () [vs. full term ()] | Early term () [vs. full term ()] | Late/postterm () [vs. full term ()] | ||||
|---|---|---|---|---|---|---|---|---|
| (95% CI) | -Value | OR (95% CI) | -Value | OR (95% CI) | -Value | OR (95% CI) | -Value | |
| Continuous (-transformed, dilution-standardized) | ||||||||
| DPHP | (, 0.01) | 0.51 | 1.02 (0.96, 1.09) | 0.51 | 0.98 (0.95, 1.01) | 0.21 | 1.00 (0.95, 1.04) | 0.83 |
| DBUP/DIBP | (, 0.02) | 0.31 | 1.07 (1.02, 1.12) | 0.01 | 1.00 (0.94, 1.06) | 0.95 | 0.99 (0.96, 1.03) | 0.66 |
| BDCPP | (, 0.01) | 0.43 | 1.00 (0.96, 1.04) | 0.92 | 1.01 (0.99, 1.02) | 0.36 | 0.99 (0.97, 1.00) | 0.09 |
| High/low (compared with nondetect) | ||||||||
| BCETP—low | (, 0.09) | 0.88 | 0.96 (0.77, 1.20) | 0.71 | 0.96 (0.82, 1.12) | 0.57 | 0.83 (0.73, 0.95) | 0.01 |
| BCETP—high | (, 0.06) | 0.55 | 0.97 (0.79, 1.20) | 0.81 | 0.96 (0.85, 1.09) | 0.53 | 0.94 (0.81, 1.08) | 0.35 |
| BBOEP—low | 0.01 (, 0.08) | 0.84 | 1.06 (0.87, 1.29) | 0.55 | 0.91 (0.76, 1.10) | 0.34 | 1.03 (0.87, 1.21) | 0.74 |
| BBOEP—high | (, ) | 0.03 | 1.25 (1.06, 1.46) | 0.01 | 0.92 (0.79, 1.07) | 0.26 | 1.02 (0.80, 1.31) | 0.85 |
| BCPP—low | 0.08 (, 0.18) | 0.08 | 0.83 (0.66, 1.03) | 0.09 | 1.04 (0.91, 1.20) | 0.53 | 1.14 (0.97, 1.36) | 0.12 |
| BCPP—high | (, 0.07) | 0.79 | 0.97 (0.77, 1.22) | 0.78 | 1.18 (1.05, 1.32) | 0.01 | 1.07 (0.92, 1.23) | 0.39 |
| Detect (compared with nondetect) | ||||||||
| BMPP | (, 0.06) | 0.72 | 1.00 (0.86, 1.16) | 1.00 | 1.04 (0.91, 1.19) | 0.53 | 1.02 (0.86, 1.21) | 0.82 |
| BEHP | (, 0.03) | 0.18 | 1.01 (0.83, 1.22) | 0.94 | 1.03 (0.89, 1.18) | 0.73 | 0.84 (0.72, 0.97) | 0.02 |
| DPRP | 0.09 (, 0.19) | 0.10 | 0.90 (0.69, 1.18) | 0.45 | 1.02 (0.93, 1.13) | 0.63 | 1.18 (0.98, 1.43) | 0.08 |
Note: Linear or multinomial regression models, fitted using generalized estimating equations with a random effect for cohort, were used to estimate or ORs, respectively, and their corresponding 95% CIs and -values. Regression models were adjusted for maternal race/ethnicity, maternal age at delivery, maternal education, maternal marital status, maternal prepregnancy BMI, maternal smoking during pregnancy, parity, child’s sex, and sample collection season and year. BBOEP, bis(butoxyethyl) phosphate; BCETP, bis(2-chloroethyl) phosphate; BCPP, bis(1-chloro-2-propyl) phosphate; BDCPP, bis(1,3-dichloro-2-propyl) phosphate; BEHP, bis(2-ethylhexyl) phosphate; BMPP, bis(2-methylphenyl) phosphate; CI, confidence interval; DBUP/DIBP, composite of dibutyl phosphate and di-isobutyl phosphate; DPHP, diphenyl phosphate; DPRP, dipropyl phosphate; ECHO, Environmental influences on Child Health Outcomes; OPE, organophosphate ester; OR, odds ratio.
Figure 1.
Associations of DPHP, DBUP/DIBP, BCETP, BBOEP, and BCPP with preterm () and early-term (), compared with full-term birth (), gestational age (in weeks) (), and BW-GA -score () among all newborns in the ECHO cohorts, and stratified by child’s sex (females: , males: ). Point estimates indicate regression coefficients or odds ratios (ORs), and error bars indicate 95% confidence intervals (CIs). Regression models were adjusted for maternal race/ethnicity, maternal age at delivery, maternal education, maternal marital status, maternal prepregnancy BMI, maternal smoking during pregnancy, parity, child’s sex, and sample collection season and year. Sample size of each birth outcome by child’s sex is presented in Table 2, and numeric data regarding regression coefficients, ORs, 95% CIs, and -values for main effects and interaction terms between child’s sex and OPE biomarkers are presented in Tables 4, 5, S7, and S8. Note: BBOEP, bis(butoxyethyl) phosphate; BCETP, bis(2-chloroethyl) phosphate; BCPP, bis(1-chloro-2-propyl) phosphate; BMI, body mass index; BW-GA, birth weight for gestational age; DBUP/DIBP, composite of dibutyl phosphate and di-isobutyl phosphate; DPHP, diphenyl phosphate; ECHO, Environmental influences on Child Health Outcomes.
The low-exposure category of BCETP, compared with the nondetect category, was associated with lower odds of late/postterm vs. full-term birth among all births (; 95% CI: 0.73, 0.95). The associations of the high category of BCETP with preterm birth and gestational age differed by sex (), indicating higher odds of preterm birth and shorter gestational age among females [ (95% CI: 1.03, 1.58) for preterm birth; wk (95% CI: , ) for gestational age] and lower odds of preterm birth and longer gestational age among males [ (95% CI: 0.55, 1.06) for preterm birth; wk (95% CI: , 0.18) for gestational age]. The high-exposure category of BBOEP was associated with shorter gestational age ( wk; 95% CI: , ) and higher odds of preterm vs. full-term birth (; 95% CI: 1.06, 1.46) in all newborns. The association between the high category of BBOEP and early-term birth differed by sex (), with lower odds among females (; 95% CI: 0.60, 0.94) and higher odds among males (; 95% CI: 0.89, 1.35). The high category of BCPP was associated with higher odds of early-term birth compared with full-term birth (; 95% CI: 1.05, 1.32). Three binary OPE biomarkers were not associated with gestational duration, except for an association of detectable BEHP with lower odds of late/postterm vs. full-term birth (; 95% CI: 0.72, 0.97).
We did not observe evidence of associations of DPHP, DBUP/DIBP, BDCPP, BBOEP, and BEHP with fetal growth (Table 5). The low and high categories of BCPP and detectable BMPP and DPRP (compared with nondetectable) were associated with greater BW-GA -score ( for low and high BCPP categories and BMPP, 0.04 for DPRP) in the overall population. Similarly, the high category of BCPP (; 95% CI: 0.31, 0.89) and detectable DPRP (; 95% CI: 0.55, 0.94) were associated with lower odds of term LBW. There were no statistically significant associations for SGA or LGA among all births, except for an association between the high category of BCETP and lower odds of SGA (; 95% CI: 0.71, 0.96). When stratified by child’s sex, lower odds of SGA were observed among male newborns only in association with BDCPP, BCETP, BCPP, and BMPP, although most of the tests for interaction were not statistically significant (Table S8).
Table 5.
Associations between prenatal maternal urinary OPE biomarkers and fetal growth in the ECHO cohorts.
| OPE biomarkers | BW-GA -score () | Term LBW () [vs. term non-LBW ()]a | SGA () [vs. non-SGA ()] | LGA () [vs. non-LGA ()] | ||||
|---|---|---|---|---|---|---|---|---|
| (95% CI) | -Value | OR (95% CI) | -Value | OR (95% CI) | -Value | OR (95% CI) | -Value | |
| Continuous (-transformed, dilution-standardized) | ||||||||
| DPHP | (, 0.00) | 0.01 | 1.05 (0.96, 1.15) | 0.28 | 1.05 (0.99, 1.11) | 0.11 | 0.97 (0.92, 1.02) | 0.17 |
| DBUP/DIBP | (, 0.00) | 0.11 | 0.91 (0.78, 1.06) | 0.22 | 1.02 (0.96, 1.08) | 0.58 | 0.97 (0.94, 1.01) | 0.11 |
| BDCPP | 0.00 (, 0.00) | 0.33 | 0.98 (0.95, 1.01) | 0.14 | 0.99 (0.97, 1.01) | 0.18 | 0.98 (0.97, 1.00) | 0.05 |
| High/low (compared with nondetect) | ||||||||
| BCETP—low | 0.04 (, 0.12) | 0.38 | 0.98 (0.60, 1.61) | 0.94 | 0.91 (0.69, 1.19) | 0.49 | 1.13 (0.95, 1.34) | 0.18 |
| BCETP—high | 0.03 (, 0.08) | 0.24 | 0.90 (0.59, 1.39) | 0.64 | 0.83 (0.71, 0.96) | 0.01 | 1.04 (0.92, 1.18) | 0.56 |
| BBOEP—low | 0.01 (, 0.06) | 0.68 | 0.91 (0.65, 1.26) | 0.56 | 0.87 (0.74, 1.04) | 0.12 | 0.99 (0.90, 1.10) | 0.90 |
| BBOEP—high | (, 0.04) | 0.48 | 1.04 (0.74, 1.45) | 0.84 | 1.04 (0.92, 1.19) | 0.52 | 0.97 (0.84, 1.13) | 0.70 |
| BCPP—low | 0.07 (0.01, 0.13) | 0.03 | 0.76 (0.58, 1.00) | 0.05 | 0.80 (0.61, 1.04) | 0.10 | 1.06 (0.90, 1.25) | 0.46 |
| BCPP—high | 0.07 (, 0.15) | 0.09 | 0.52 (0.31, 0.89) | 0.02 | 0.84 (0.62, 1.12) | 0.23 | 1.04 (0.86, 1.25) | 0.68 |
| Detect (compared with nondetect) | ||||||||
| BMPP | 0.07 (0.02, 0.11) | 0.004 | 0.97 (0.64, 1.46) | 0.88 | 0.85 (0.72, 1.00) | 0.06 | 1.10 (0.95, 1.28) | 0.20 |
| BEHP | (, 0.02) | 0.19 | 1.29 (0.99, 1.67) | 0.06 | 1.11 (0.93, 1.32) | 0.24 | 0.95 (0.81, 1.11) | 0.50 |
| DPRP | 0.04 (0.00, 0.07) | 0.03 | 0.72 (0.55, 0.94) | 0.02 | 0.88 (0.74, 1.06) | 0.18 | 1.08 (0.93, 1.26) | 0.31 |
Note: Linear or multinomial regression models, fitted using generalized estimating equations with a random effect for cohort, were used to estimate or ORs, respectively, and their corresponding 95% CIs and -values. Regression models were adjusted for maternal race/ethnicity, maternal age at delivery, maternal education, maternal marital status, maternal prepregnancy BMI, maternal smoking during pregnancy, parity, child’s sex, and sample collection season and year. BBOEP, bis(butoxyethyl) phosphate; BCETP, bis(2-chloroethyl) phosphate; BCPP, bis(1-chloro-2-propyl) phosphate; BDCPP, bis(1,3-dichloro-2-propyl) phosphate; BEHP, bis(2-ethylhexyl) phosphate; BMI, body mass index; BMPP, bis(2-methylphenyl) phosphate; BW-GA, birth weight for gestational age; CI, confidence interval; DBUP/DIBP, composite of dibutyl phosphate and di-isobutyl phosphate; DPHP, diphenyl phosphate; DPRP, dipropyl phosphate; ECHO, Environmental influences on Child Health Outcomes; LBW, low birth weight; LGA, large for gestational age; OPE, organophosphate ester; OR, odds ratio; SGA, small for gestational age.
LBW vs. non-LBW among 6,197 non-preterm births ( wk gestation).
Leave-one-out analyses confirmed the robustness of the results to the exclusion of each cohort (Figures S5–S7 and Excel Tables S1–S3). Excluding the two largest cohorts, Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE; ) or New Hampshire Birth Cohort Study (NHBCS; ), the two largest cohorts, slightly attenuated or strengthened some associations, but the directions of the estimates were not changed. When jointly modeling all OPE biomarkers in the same regression model to adjust for potential copollutant confounding, the results were similar to the primary results (Tables S9 and S10).
Discussion
In this large, geographically and sociodemographically diverse ECHO sample that included over 6,600 participants from across the US, several OPE biomarkers were frequently detected in prenatal maternal urine samples. We observed that DBUP/DIBP and the high-exposure category of BBOEP were associated with decreased gestational duration, specifically, greater odds of preterm birth. Child’s sex appeared to modify associations of higher DPHP and the high category of BCETP both with continuous gestational age at birth and with preterm birth, with adverse findings among female newborns. On the other hand, we observed modest associations of detectable BCPP, BMPP, and DPRP with increased fetal growth, specifically greater BW-GA -score and lower odds of term LBW, although we did not observe a corresponding increased risk of LGA birth or sex-dimorphic association.
Most prior studies of urinary OPE metabolite concentrations in pregnant people have primarily quantified DPHP and BDCPP, with few studies exploring di--butyl phosphate (DNBP), BCPP, BCETP, and BBOEP (Table S11).14,16,67,68,70,71,93,94 Higher median concentrations of DPHP were observed in pregnancy cohorts in North Carolina (; 2001–2006),67 Ohio ( in different trimesters; 2003–2006),16 and Puerto Rico (; 2011–2015)93 compared with those in our ECHO participants (; 2007–2020). Other US studies conducted in California,14 Massachusetts,71 Rhode Island,94 and Maryland68 showed comparable or slightly lower median concentrations of DPHP. For BDCPP, pregnancy cohorts in North Carolina (),67 Puerto Rico (),93 and Rhode Island (; 2014)94 had higher median concentrations than the present study (), whereas other US cohorts had comparable or lower median levels. Median concentrations of BCETP were higher in the North Carolina cohort ()67 but lower in the Rhode Island cohort ()94 when compared with the present study (). Only the Ohio cohort quantified DNBP (),16 comparable with our DBUP/DIBP concentrations (). A Chinese birth cohort based in Wuhan (2014–2016) showed considerably lower median concentrations of DPHP () and BDCPP () than the US studies but found higher median concentrations of BBOEP () compared with the present study ().70 There are a multitude of possible reasons for these differences, including differences in OPE sources by geographic region, sampling year and season, and sociodemographic characteristics. Further studies on possible sources and exposure pathways could help clarify the observed differences and help determine methods for reducing exposures.
At least eight prior epidemiological studies have examined birth outcomes in association with prenatal exposure to OPEs, with most of them reporting sex-specific associations.67–73 Two studies based on the Wuhan birth cohort reported findings that were generally consistent with our study.69,70 For example, among 339 participants, prenatal urinary concentrations of DPHP and sum of OPE metabolites were associated with higher risk of LBW, especially among female newborns.69 Their later study investigating trimester-specific associations among 213 pregnant people from the same population observed that BDCPP and BBOEP in the third trimester were inversely associated with birth weight and length unadjusted for gestational age, and associations were stronger among males than females.70 They also found that DPHP in the first trimester was associated with lower birth weight, especially among females. In line with our findings, a Boston-area cohort including 90 pregnant people reported that DPHP and a mixture of DPHP and BDCPP were associated with lower odds of LGA.71 Similar to our sex-stratified results, the North Carolina cohort of 349 pregnant people observed that prenatal urinary concentrations of BDCPP and ip-PPP were associated with shorter gestational age and higher odds of preterm birth among female newborns, whereas DPHP was associated with longer gestational age and lower odds of preterm birth among males.67 On the other hand, a Baltimore-area cohort of 90 pregnant people reported that BDCPP was associated with greater ponderal index, a measure of weight-for-length similar to BMI,68 and two other studies did not observe strong associations with any of the birth outcomes studied.72,73
Some inconsistent results may be attributable to differences in population characteristics and urinary OPE metabolite levels, as well as smaller sample sizes, of prior studies. In particular, our study indicates that DBUP/DIBP is highly detected () in our large sample of US pregnant people and that it may be associated with increased risk of preterm birth. However, DBUP and DIBP have not been frequently quantified in previous US studies and were detected with low frequency in the Chinese studies (Table S11), which were rarely examined in association with adverse birth outcomes. One of the included cohorts, Maternal And Developmental Risks from Environmental and Social stressors (MADRES), conducted site-specific analyses using 421 Southern Californian pregnant people, who were also included in the present study, and reported that DBUP/DIBP was associated with shorter gestational duration,74 underscoring the necessity for further explorations into the potential role of this compound as a contributing factor to shortened gestational durations.
There are several potential mechanisms by which prenatal OPE exposure could disrupt the timing of birth and explain observed sex-specific differences. One of the underlying mechanisms that may link OPE exposure with altered birth outcomes is thyroid hormone disruption, which plays an essential role in fetal development.95,96 Several epidemiological studies suggest that prenatal OPE exposure may disrupt neonatal thyroid hormone levels in sexually dimorphic ways.43,44,46 Prenatal maternal urinary concentrations of DPHP and DBUP were associated with higher levels of thyroid stimulating hormone (TSH) in newborns, especially among females. These associations were partially mediated by the oxidative stress of DNA damage.43 Higher BBOEP in the third trimester was associated with higher neonatal TSH, especially among males, whereas higher DPHP in the third trimester was associated with lower neonatal TSH among females.44 DNBP, DPHP, and BDCPP were associated with lower levels of neonatal triiodothyronine and thyroxine.46 Prenatal OPE metabolites, such as DPHP and BDCPP, were also associated with altered thyroid hormone levels in pregnant people.43,45,46 Maternal thyroid disruption during pregnancy was further associated with higher risks of preterm birth and LBW.97,98 Oxidative stress and inflammation induced by OPEs could also provide a mechanistic link between exposures and preterm birth.43,53,55,56 Finally, similar to other endocrine disrupting chemicals, OPEs could also contribute to abnormal placental development and functions in sex-dependent manners.99,100
Disruption of maternal metabolic functions and other endocrine systems by OPEs could also alter fetal growth.57 OPEs can activate PPARs that play critical roles in energy homeostasis and lipid metabolism.47–49 PPAR activation has previously been proposed as a mechanism linking other environmental chemical exposures, such as phthalates101 and per- and polyfluoroalkyl substances,102 to weight gain. PPAR activation could therefore potentially explain the associations with higher BW-GA -scores that we observed for three of the compounds.79 The parent compound of DPHP and 2-ethylhexyl diphenyl phosphate (EHDPP) exhibited activation in human placental choriocarcinoma cells.59 Mice perinatally exposed to a mixture of parent compounds of DPHP, BDCPP, and dicresyl phosphate showed higher neonatal body weight and activation in the hypothalamus and liver, especially in female pups.103
The present study has several strengths. This is by far the largest study quantifying OPE biomarkers in the urine of pregnant people and examining their associations with birth outcomes. We used a geographically and sociodemographically diverse study population, combining 16 pregnancy/birth cohorts across the US. The quantification of urinary OPE biomarkers was performed by a single laboratory, minimizing measurement error. Furthermore, leave-one-out analyses suggested robustness of our results across the cohorts. In addition, this research considered a range of birth outcomes, enabling us to make inferences about the effect of the OPE exposures on duration of gestation and fetal growth,104 considering the full range of early and late gestational age outcomes and size-for-gestation outcomes. We used generalized estimating equations to address cohort variability in birth outcome ascertainment methods (e.g., medical record abstraction, maternal self-report), but there is a slight concern regarding the potential for decreased accuracy when using self-report methods. It should be also noted that our study population had a slightly lower prevalence of preterm birth (6.8%) and LBW (5.5%) compared with the US national statistics for singleton births (8.8% and 6.9%, respectively).105 This is attributed not only to the inclusion criteria for this analysis (i.e., the availability of a prenatal urine sample for measurement of urinary OPE biomarkers) but also to the fact that the majority of cohorts sought to recruit pregnant people without severe pregnancy conditions. There were two high-risk autism spectrum disorder cohorts [Early Autism Risk Longitudinal Investigation (EARLI) and Markers of Autism Risk in Babies-Learning Early Signs (MARBLES)] and one cohort that enrolled pregnant people who were cigarette smokers [Vitamin C to Decrease Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP)], but these three cohorts accounted for only 7% of the study population. In addition, the number of pregnancies contributed by the 16 cohorts varied from 20 to 1,453, and each cohort provided different proportions of their full study sample to this analysis (Table S1). Therefore, our study findings may not be generalizable to all the participating cohorts or to the US birthing population, which includes individuals with numerous other risk factors for birth outcomes.
Another limitation is the measurement of concentrations of OPE biomarkers in a single spot or first morning urine sample collected primarily during mid- to late-pregnancy, which reflects only recent exposure because of their short half-lives.106 Previous studies have reported intraclass correlation coefficients of urinary DPHP, DNBP, BDCPP, and BCETP ranging from 0.2 to 0.7, indicating low to moderate reproducibility over a 4–6 month period in mid- to late-pregnancy.16,68,94,107 Although OPE exposure likely stems from the home environment, which is fairly constant over time, transplacental transfer, as well as seasonal factors, such as greater air partitioning in warmer months and variations in indoor time and ventilation frequency, can influence OPE exposure levels in pregnant people across the pregnancy.15 Therefore, further epidemiological studies using repeated measurements of urinary OPE biomarkers are warranted to reduce exposure misclassification and to investigate potential periods of heightened susceptibility. It should be also noted that DPHP is a nonspecific metabolite of several OPEs, including TPHP, resorcinol bis(diphenylphosphate), and EHDPP.108,109 This implies that DPHP does not differentiate between prenatal exposure to OPEs with varying levels of toxicity. Last, we conducted neither a mixtures analysis to examine overall or joint effects of OPE mixtures nor investigations into nonlinear associations between the three continuous OPE biomarkers and birth outcomes. Future studies could address whether specific combinations of OPEs have additive or synergistic effects and explore potential nonlinear relationships to provide a more comprehensive understanding of the impact of prenatal OPE exposures on birth outcomes.
Conclusion
Our study, based on a large, diverse sample of the US population, found that greater prenatal exposure to several OPEs related to elevated risks of preterm birth and shorter gestational age, especially among female newborns. Some OPEs were modestly associated with higher BW-GA -scores, a risk factor for childhood obesity. Although the magnitudes of the associations are modest, the number of births that may be impacted by these compounds is large given the widespread exposures to emerging OPE flame retardants among US pregnant people.
Supplementary Material
Acknowledgments
We acknowledge the contribution of the following ECHO Program collaborators:
ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: P.B. Smith, L.K. Newby; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: L.P. Jacobson; Research Triangle Institute, Durham, North Carolina: D.J. Catellier; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: R. Gershon, D. Cella.
ECHO Awardees and Cohorts—Emory University, Atlanta, Georgia: A.L. Dunlop; University of Washington, Department of Environmental and Occupational Health Sciences, Seattle, Washington: C. Karr; University of California, San Francisco, San Francisco, California: T.J. Woodruff; EARLI; Columbia University Medical Center, New York, New York: J.B. Herbstman; University of Colorado Denver, Denver, Colorado: D. Dabelea; University of Illinois, Beckman Institute, Urbana, Illinois: S. Schantz; University of Southern California, Los Angeles, California: C.V. Breton; University of Rochester Medical Center, Rochester, New York: T. O’Connor; University of California, Davis, Davis, California: R.J. Schmidt; Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire: M.R. Karagas; New York University Grossman School of Medicine, New York, New York: L. Trasande; Kaiser Permanente Northern California Division of Research, Oakland, California: A. Ferrara; Oregon Health and Science University, Portland, Oregon: C.T. McEvoy.
Research reported in this publication was supported by the ECHO Program, Office of The Director, National Institutes of Health (NIH), under award nos. U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319, with co-funding from the Office of Behavioral and Social Science Research (PRO Core), U2CES026542 (K. Kannan), UH3OD023286, UH3OD023318, R01NR014800, R24ESO29490, P50ESO2607, EPA 83615301 (A.L. Dunlop), UH3OD023271 (C. Karr, S. Sathyanarayana, F.A. Tylavsky), P01ES022841, RD83543301, R01ES027051 (T.J. Woodruff), UH3OD023272 (R. Morello-Frosch, S. Schantz, T.J. Woodruff), UH3OD023342 (H.E. Volk), UH3OD023290 (J.B. Herbstman), UH3OD023248 (D. Dabelea, A.P. Starling), P30ES007048, P50ES026086, EPA 83615801, P50MD01570, UH3OD023287 (C.V. Breton, T.M. Bastain), UH30D023342 (K. Lyall, R.J. Schmidt), UH3OD023365 (D.H. Bennett, I. Hertz-Picciotto, J. Oh), UH3OD023275, NIGMS P20GM104416 (M.R. Karagas, M.E. Romano), UH3OD023305 (L. Trasande, A. Ghassabian), UH3OD023289 (A. Ferrara, L.A. Croen), UH3OD023349, R01HD083369, P30 ES005022 (T. O’Connor, E.S. Barrett), UH3OD023288 (C.T. McEvoy).
We thank our Environmental influences on Child Health Outcomes (ECHO) Program colleagues; the medical, nursing, and program staff; as well as the children and families participating in the ECHO cohorts.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Deidentified data from the ECHO Program are available through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH). DASH is a centralized resource that allows researchers to access data from various studies via a controlled-access mechanism. Researchers can request access to these data by creating a DASH account and submitting a Data Request Form. The NICHD DASH Data Access Committee will review the request and provide a response in . Once granted access, researchers will be able to use the data for 3 y. See the DASH Tutorial (https://dash.nichd.nih.gov/resource/tutorial) for more detailed information on the process.
References
- 1.Blum A, Behl M, Birnbaum LS, Diamond ML, Phillips A, Singla V, et al. . 2019. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett 6(11):638–649, PMID: , 10.1021/acs.estlett.9b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Gong S, Ye L, Li J, Liu C, Chen D, et al. . 2021. Organophosphate (OP) diesters and a review of sources, chemical properties, environmental occurrence, adverse effects, and future directions. Environ Int 155:106691, PMID: , 10.1016/j.envint.2021.106691. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Chen P, Ma S, Lu S, Yu Y, An T. 2022. A critical review of human internal exposure and the health risks of organophosphate ester flame retardants and their metabolites. Crit Rev Environ Sci Technol 52(9):1528–1560, 10.1080/10643389.2020.1859307. [DOI] [Google Scholar]
- 4.Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, et al. . 2015. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut 196:29–46, PMID: , 10.1016/j.envpol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Saillenfait AM, Ndaw S, Robert A, Sabaté JP. 2018. Recent biomonitoring reports on phosphate ester flame retardants: a short review. Arch Toxicol 92(9):2749–2778, PMID: , 10.1007/s00204-018-2275-z. [DOI] [PubMed] [Google Scholar]
- 6.Chupeau Z, Bonvallot N, Mercier F, Le Bot B, Chevrier C, Glorennec P. 2020. Organophosphorus flame retardants: a global review of indoor contamination and human exposure in Europe and epidemiological evidence. Int J Environ Res Public Health 17(18):6713, PMID: , 10.3390/ijerph17186713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty BT, Hammel SC, Daniels JL, Stapleton HM, Hoffman K. 2019. Organophosphate esters: are these flame retardants and plasticizers affecting children’s health? Curr Environ Health Rep 6(4):201–213, PMID: , 10.1007/s40572-019-00258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM. 2018. Exposure to organophosphate flame retardant chemicals in the US general population: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 110:32–41, PMID: , 10.1016/j.envint.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomeir AA, Kato S, Matthews HB. 1981. The metabolism and disposition of tris (1, 3-dichloro-2-propyl) phosphate (Fyrol FR-2) in the rat. Toxicol Appl Pharmacol 57(3):401–413, PMID: , 10.1016/0041-008x(81)90238-6. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki K, Suzuki T, Takeda M, Uchiyama M. 1984. Metabolism of phosphoric acid triesters by rat liver homogenate. Bull Environ Contam Toxicol 33(3):281–288, PMID: , 10.1007/BF01625544. [DOI] [PubMed] [Google Scholar]
- 11.Minegishi K, Kurebayashi H, Nambaru S, Morimoto K, Takahashi T, Yamaha T. 1988. Comparative studies on absorption, distribution, and excretion of flame retardants halogenated alkyl phosphate in rats. Eisei Kagaku 34(2):102–114, 10.1248/jhs1956.34.102. [DOI] [Google Scholar]
- 12.Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. 2013. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett 223(1):9–15, PMID: , 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Carignan CC, Fang M, Stapleton HM, Heiger-Bernays W, McClean MD, Webster TF. 2016. Urinary biomarkers of flame retardant exposure among collegiate US gymnasts. Environ Int 94:362–368, PMID: , 10.1016/j.envint.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castorina R, Butt C, Stapleton HM, Avery D, Harley KG, Holland N, et al. . 2017. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere 179:159–166, PMID: , 10.1016/j.chemosphere.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, et al. . 2017. Predictors of urinary flame retardant concentration among pregnant women. Environ Int 98:96–101, PMID: , 10.1016/j.envint.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percy Z, Vuong AM, Ospina M, Calafat AM, La Guardia MJ, Xu Y, et al. . 2020. Organophosphate esters in a cohort of pregnant women: variability and predictors of exposure. Environ Res 184:109255, PMID: , 10.1016/j.envres.2020.109255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Chen P, Zhao L, Zhu L, Wu F. 2021. Transplacental behaviors of organophosphate tri-and diesters based on paired human maternal and cord whole blood: efficiencies and impact factors. Environ Sci Technol 55(5):3091–3100, PMID: , 10.1021/acs.est.0c06095. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Xu Z, Huang W, Feng L, Yang F. 2016. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ 554–555:211–217, PMID: , 10.1016/j.scitotenv.2016.02.171. [DOI] [PubMed] [Google Scholar]
- 19.Zhao F, Chen M, Gao F, Shen H, Hu J. 2017. Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi. Environ Sci Technol 51(11):6489–6497, PMID: , 10.1021/acs.est.7b01122. [DOI] [PubMed] [Google Scholar]
- 20.Bai XY, Lu SY, Xie L, Zhang B, Song SM, He Y, et al. . 2019. A pilot study of metabolites of organophosphorus flame retardants in paired maternal urine and amniotic fluid samples: potential exposure risks of tributyl phosphate to pregnant women. Environ Sci Process Impacts 21(1):124–132, PMID: , 10.1039/c8em00389k. [DOI] [PubMed] [Google Scholar]
- 21.Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, McEvoy CT, et al. . 2020. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—implications for research on perinatal outcomes in the ECHO program. J Perinatol 40(1):10–24, PMID: , 10.1038/s41372-019-0510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Population Health and Public Health Practice; Board on Environmental Studies and Toxicology; Committee on the Guidance on PFAS Testing and Health Outcomes. 2022. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 23.Ferguson KK, McElrath TF, Meeker JD. 2014. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168(1):61–67, PMID: , 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardi-Bee J, Smyth A, Britton J, Coleman T. 2008. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 93(5):F351–F361, PMID: , 10.1136/adc.2007.133553. [DOI] [PubMed] [Google Scholar]
- 25.Yuan S, Li H, Dang Y, Liu C. 2018. Effects of triphenyl phosphate on growth, reproduction and transcription of genes of Daphnia magna. Aquat Toxicol 195:58–66, PMID: , 10.1016/j.aquatox.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Su G, Zou M, Yu L, Letcher RJ, Yu H, et al. . 2015. Effects of tris (1,3-dichloro-2-propyl) phosphate on growth, reproduction, and gene transcription of Daphnia magna at environmentally relevant concentrations. Environ Sci Technol 49(21):12975–12983, PMID: , 10.1021/acs.est.5b03294. [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Jia Y, Su G, Sun Y, Letcher RJ, Giesy JP, et al. . 2017. Parental transfer of tris (1,3-dichloro-2-propyl) phosphate and transgenerational inhibition of growth of zebrafish exposed to environmentally relevant concentrations. Environ Pollut 220(pt A):196–203, PMID: , 10.1016/j.envpol.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Liu J, Yu L, Liu C, Wang J. 2019. Gonadal impairment and parental transfer of tris (2-butoxyethyl) phosphate in zebrafish after long-term exposure to environmentally relevant concentrations. Chemosphere 218:449–457, PMID: , 10.1016/j.chemosphere.2018.11.139. [DOI] [PubMed] [Google Scholar]
- 29.Ren X, Wang W, Zhao X, Ren B, Chang L. 2019. Parental exposure to tris (1,3-dichloro-2-propyl) phosphate results in thyroid endocrine disruption and inhibition of growth in zebrafish offspring. Aquat Toxicol 209:132–141, PMID: , 10.1016/j.aquatox.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Moser VC, Phillips PM, Hedge JM, McDaniel KL. 2015. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris (1,3-dichloro-2-propyl) phosphate (TDCIPP) and tris (2-chloro-2-ethyl) phosphate (TCEP). Neurotoxicol Teratol 52(pt B):236–247, PMID: , 10.1016/j.ntt.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Zeng X, Sun H, Huang Y, Liu J, Yu L, Liu C, et al. . 2018. Effects of environmentally relevant concentrations of tris (2-butoxyethyl) phosphate on growth and transcription of genes involved in the GH/IGF and HPT axes in zebrafish (Danio rerio). Chemosphere 212:376–384, PMID: , 10.1016/j.chemosphere.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Ma X, Su G, Yu L, Letcher RJ, Hou J, et al. . 2015. Environmentally relevant concentrations of the flame retardant tris (1,3-dichloro-2-propyl) phosphate inhibit growth of female zebrafish and decrease fecundity. Environ Sci Technol 49(24):14579–14587, PMID: , 10.1021/acs.est.5b03849. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Wu D, Xu Q, Yu L, Liu C, Wang J. 2017. Acute exposure to tris (2-butoxyethyl) phosphate (TBOEP) affects growth and development of embryo-larval zebrafish. Aquat Toxicol 191:17–24, PMID: , 10.1016/j.aquatox.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Carignan CC, Mínguez-Alarcón L, Butt CM, Williams PL, Meeker JD, Stapleton HM, et al. . 2017. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ Health Perspect 125(8):087018, PMID: , 10.1289/EHP1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carignan CC, Mínguez-Alarcón L, Williams PL, Meeker JD, Stapleton HM, Butt CM, et al. . 2018. Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environ Int 111:232–238, PMID: , 10.1016/j.envint.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messerlian C, Williams PL, Mínguez-Alarcón L, Carignan CC, Ford JB, Butt CM, et al. . 2018. Organophosphate flame-retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertil Steril 110(6):1137–1144.e1, PMID: , 10.1016/j.fertnstert.2018.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Ding J, Lv L, Zhang H. 2021. Exposure to organophosphate flame esters during early pregnancy and risk of spontaneous abortion: a case-control study. Chemosphere 268:129375, PMID: , 10.1016/j.chemosphere.2020.129375. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Ji C, Yin X, Yan L, Lu M, Zhao M. 2016. Thyroid hormone-disrupting activity and ecological risk assessment of phosphorus-containing flame retardants by in vitro, in vivo and in silico approaches. Environ Pollut 210:27–33, PMID: , 10.1016/j.envpol.2015.11.051. [DOI] [PubMed] [Google Scholar]
- 39.Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, et al. . 2013. In ovo effects of two organophosphate flame retardants—TCPP and TDCPP—on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci 134(1):92–102, PMID: , 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, et al. . 2013. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic–pituitary–thyroid axis. Aquat Toxicol 126:207–213, PMID: , 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Jung J, Lee I, Jung D, Youn H, Choi K. 2015. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol 160:188–196, PMID: , 10.1016/j.aquatox.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN, et al. . 2017. Associations between urinary diphenyl phosphate and thyroid function. Environ Int 101:158–164, PMID: , 10.1016/j.envint.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Y, Li M, Pan L, Duan Y, Duan X, Li Y, et al. . 2021. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: thyroid endocrine disruption and mediation role of oxidative stress. Environ Int 146:106215, PMID: , 10.1016/j.envint.2020.106215. [DOI] [PubMed] [Google Scholar]
- 44.Tao Y, Hu L, Liu L, Yu M, Li Y, Li X, et al. . 2021. Prenatal exposure to organophosphate esters and neonatal thyroid-stimulating hormone levels: a birth cohort study in Wuhan, China. Environ Int 156:106640, PMID: , 10.1016/j.envint.2021.106640. [DOI] [PubMed] [Google Scholar]
- 45.Choi G, Keil AP, Villanger GD, Richardson DB, Daniels JL, Hoffman K, et al. . 2021. Pregnancy exposure to common-detect organophosphate esters and phthalates and maternal thyroid function. Sci Total Environ 782:146709, PMID: , 10.1016/j.scitotenv.2021.146709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Percy Z, Vuong AM, Xu Y, Xie C, Ospina M, Calafat AM, et al. . 2021. Maternal urinary organophosphate esters and alterations in maternal and neonatal thyroid hormones. Am J Epidemiol 190(9):1793–1802, PMID: , 10.1093/aje/kwab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. 2014. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett 228(2):93–102, PMID: , 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang M, Webster TF, Stapleton HM. 2015. Activation of human peroxisome proliferator-activated nuclear receptors (PPARγ1) by semi-volatile compounds (SVOCs) and chemical mixtures in indoor dust. Environ Sci Technol 49(16):10057–10064, PMID: , 10.1021/acs.est.5b01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, et al. . 2014. Ligand binding and activation of PPARγ by Firemaster® 550: effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect 122(11):1225–1232, PMID: , 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, Jin Y, Wu Y, Liu L, Fu Z. 2015. Exposure of male mice to two kinds of organophosphate flame retardants (OPFRs) induced oxidative stress and endocrine disruption. Environ Toxicol Pharmacol 40(1):310–318, PMID: , 10.1016/j.etap.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Arukwe A, Carteny CC, Eggen T. 2016. Lipid peroxidation and oxidative stress responses in juvenile salmon exposed to waterborne levels of the organophosphate compounds tris(2-butoxyethyl)-and tris(2-chloroethyl) phosphates. J Toxicol Environ Health A 79(13–15):515–525, PMID: , 10.1080/15287394.2016.1171978. [DOI] [PubMed] [Google Scholar]
- 52.Lu SY, Li YX, Zhang T, Cai D, Ruan JJ, Huang MZ, et al. . 2017. Effect of e-waste recycling on urinary metabolites of organophosphate flame retardants and plasticizers and their association with oxidative stress. Environ Sci Technol 51(4):2427–2437, PMID: , 10.1021/acs.est.6b05462. [DOI] [PubMed] [Google Scholar]
- 53.Ingle ME, Watkins D, Rosario Z, VélezVega CM, Calafat AM, Ospina M, et al. . 2020. An exploratory analysis of urinary organophosphate ester metabolites and oxidative stress among pregnant women in Puerto Rico. Sci Total Environ 703:134798, PMID: , 10.1016/j.scitotenv.2019.134798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forhead AJ, Fowden AL. 2014. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol 221(3):R87–R103, PMID: , 10.1530/JOE-14-0025. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF. 2018. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am J Reprod Immunol 80(4):e13017, PMID: , 10.1111/aji.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore TA, Ahmad IM, Zimmerman MC. 2018. Oxidative stress and preterm birth: an integrative review. Biol Res Nurs 20(5):497–512, PMID: , 10.1177/1099800418791028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamai EM, McElrath TF, Ferguson KK. 2019. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health 18(1):43, PMID: , 10.1186/s12940-019-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du Z, Zhang Y, Wang G, Peng J, Wang Z, Gao S. 2016. TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver. Sci Rep 6:21827, PMID: , 10.1038/srep21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu W, Gao F, Zhang H, Hiromori Y, Arakawa S, Nagase H, et al. . 2017. Activation of peroxisome proliferator-activated receptor gamma and disruption of progesterone synthesis of 2-ethylhexyl diphenyl phosphate in human placental choriocarcinoma cells: comparison with triphenyl phosphate. Environ Sci Technol 51(7):4061–4068, PMID: , 10.1021/acs.est.7b00872. [DOI] [PubMed] [Google Scholar]
- 60.Green AJ, Graham JL, Gonzalez EA, La Frano MR, Petropoulou SSE, Park JS, et al. . 2017. Perinatal triphenyl phosphate exposure accelerates type 2 diabetes onset and increases adipose accumulation in UCD-type 2 diabetes mellitus rats. Reprod Toxicol 68:119–129, PMID: , 10.1016/j.reprotox.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valero De Bernabé J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martínez D, et al. . 2004. Risk factors for low birth weight: a review. Eur J Obstet Gynecol Reprod Biol 116(1):3–15, PMID: , 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. Lancet 371(9606):75–84, PMID: , 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barker DJP. 2007. The origins of the developmental origins theory. J Intern Med 261(5):412–417, PMID: , 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 64.Ely DM, Driscoll AK. 2021. Infant mortality in the United States, 2019: data from the period linked birth/infant death file. Natl Vital Stat Rep 70(14):1–18, PMID: , 10.15620/cdc:111053. [DOI] [PubMed] [Google Scholar]
- 65.Murray SR, Shenkin SD, McIntosh K, Lim J, Grove B, Pell JP, et al. . 2017. Long term cognitive outcomes of early term (37–38 weeks) and late preterm (34–36 weeks) births: a systematic review. Wellcome Open Res 2:101, PMID: , 10.12688/wellcomeopenres.12783.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart DL, Barfield WD, Committee on Fetus and Newborn, Cummings JJ, Adams-Chapman IS, Aucott SW, et al. . 2019. Updates on an at-risk population: late-preterm and early-term infants. Pediatrics 144(5):e20192760, PMID: , 10.1542/peds.2019-2760. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman K, Stapleton HM, Lorenzo A, Butt CM, Adair L, Herring AH, et al. . 2018. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ Int 116:248–254, PMID: , 10.1016/j.envint.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuiper JR, Stapleton HM, Wills-Karp M, Wang X, Burd I, Buckley JP. 2020. Predictors and reproducibility of urinary organophosphate ester metabolite concentrations during pregnancy and associations with birth outcomes in an urban population. Environ Health 19(1):55, PMID: , 10.1186/s12940-020-00610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo D, Liu W, Tao Y, Wang L, Yu M, Hu L, et al. . 2020. Prenatal exposure to organophosphate flame retardants and the risk of low birth weight: a nested case-control study in China. Environ Sci Technol 54(6):3375–3385, PMID: , 10.1021/acs.est.9b06026. [DOI] [PubMed] [Google Scholar]
- 70.Luo D, Liu W, Wu W, Tao Y, Hu L, Wang L, et al. . 2021. Trimester-specific effects of maternal exposure to organophosphate flame retardants on offspring size at birth: a prospective cohort study in China. J Hazard Mater 406:124754, PMID: , 10.1016/j.jhazmat.2020.124754. [DOI] [PubMed] [Google Scholar]
- 71.Bommarito PA, Welch BM, Keil AP, Baker GP, Cantonwine DE, McElrath TF, et al. . 2021. Prenatal exposure to consumer product chemical mixtures and size for gestational age at delivery. Environ Health 20(1):68, PMID: , 10.1186/s12940-021-00724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crawford KA, Hawley N, Calafat AM, Jayatilaka NK, Froehlich RJ, Has P, et al. . 2020. Maternal urinary concentrations of organophosphate ester metabolites: associations with gestational weight gain, early life anthropometry, and infant eating behaviors among mothers-infant pairs in Rhode Island. Environ Health 19(1):97, PMID: , 10.1186/s12940-020-00648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng L, Ouyang F, Liu L, Wang X, Wang X, Li YJ, et al. . 2016. Levels of urinary metabolites of organophosphate flame retardants, TDCIPP, and TPHP, in pregnant women in Shanghai. J Environ Public Health 2016:9416054, PMID: , 10.1155/2016/9416054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hernandez-Castro I, Eckel SP, Howe CG, Niu Z, Kannan K, Robinson M, et al. . 2023. Sex-specific effects of prenatal organophosphate ester (OPE) metabolite mixtures and adverse infant birth outcomes in the Maternal And Developmental Risks from Environmental and Social stressors (MADRES) pregnancy cohort. Environ Res 226:115703, PMID: , 10.1016/j.envres.2023.115703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiao Y, Ma J, Wang Y, Li W, Katzmarzyk PT, Chaput JP, et al. . 2015. Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl 5(suppl 2):S74–S79, PMID: , 10.1038/ijosup.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schellong K, Schulz S, Harder T, Plagemann A. 2012. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One 7(10):e47776, PMID: , 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong YH, Lee JE. 2021. Large for gestational age and obesity-related comorbidities. J Obes Metab Syndr 30(2):124–131, PMID: , 10.7570/jomes20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. . 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J Biochem Mol Toxicol 27(2):124–136, PMID: , 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heindel JJ, Newbold R, Schug TT. 2015. Endocrine disruptors and obesity. Nat Rev Endocrinol 11(11):653–661, PMID: , 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 80.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. 2015. Childhood obesity: causes and consequences. J Family Med Prim Care 4(2):187–192, PMID: , 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karnik S, Kanekar A. 2012. Childhood obesity: a global public health crisis. Int J Prev Med 3(1):1–7, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 82.Gillman MW, Blaisdell CJ. 2018. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr Opin Pediatr 30(2):260–262, PMID: , 10.1097/MOP.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, et al. . 2020. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) program. J Expo Sci Environ Epidemiol 30(3):397–419, PMID: , 10.1038/s41370-020-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aris IM, Kleinman KP, Belfort MB, Kaimal A, Oken E. 2019. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics 144(1):e20190076, PMID: , 10.1542/peds.2019-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, et al. . 2021. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int J Hyg Environ Health 234:113741, PMID: , 10.1016/j.ijheh.2021.113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894, PMID: , 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 87.Kuiper JR, O’Brien KM, Ferguson KK, Buckley JP. 2021. Urinary specific gravity measures in the US population: implications for the adjustment of non-persistent chemical urinary biomarker data. Environ Int 156:106656, PMID: , 10.1016/j.envint.2021.106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuiper JR, O’Brien KM, Welch BM, Barrett ES, Nguyen RHN, Sathyanarayana S, et al. . 2022. Combining urinary biomarker data from studies with different measures of urinary dilution. Epidemiology 33(4):533–540, PMID: , 10.1097/EDE.0000000000001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54(10):615–627, PMID: , 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 90.Choi G, Buckley JP, Kuiper JR, Keil AP. 2022. Log-transformation of independent variables: must we? Epidemiology 33(6):843–853, PMID: , 10.1097/EDE.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunlop AL, Essalmi AG, Alvalos L, Breton C, Camargo CA, Cowell WJ, et al. . 2021. Racial and geographic variation in effects of maternal education and neighborhood-level measures of socioeconomic status on gestational age at birth: findings from the ECHO cohorts. PLoS One 16(1):e0245064, PMID: , 10.1371/journal.pone.0245064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothman KJ. 1990. No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46, PMID: , 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Ingle ME, Watkins D, Rosario Z, Vélez Vega CM, Huerta-Montanez G, Calafat AM, et al. . 2019. The association of urinary organophosphate ester metabolites and self-reported personal care and household product use among pregnant women in Puerto Rico. Environ Res 179(pt A):108756, PMID: , 10.1016/j.envres.2019.108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, et al. . 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health 16(1):40, PMID: , 10.1186/s12940-017-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rovet JF. 2014. The role of thyroid hormones for brain development and cognitive function. Endocr Dev 26:26–43, PMID: , 10.1159/000363153. [DOI] [PubMed] [Google Scholar]
- 96.Williams GR. 2008. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20(6):784–794, PMID: , 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 97.Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth, Korevaar TIM, Derakhshan A, Taylor PN, Meima M, Chen L, et al. . 2019. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA 322(7):632–641, PMID: , 10.1001/jama.2019.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nazarpour S, Ramezani Tehrani F, Simbar M, Azizi F. 2015. Thyroid dysfunction and pregnancy outcomes. Iran J Reprod Med 13(7):387–396, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 99.Varshavsky JR, Robinson JF, Zhou Y, Puckett KA, Kwan E, Buarpung S, et al. . 2021. Organophosphate flame retardants, highly fluorinated chemicals, and biomarkers of placental development and disease during mid-gestation. Toxicol Sci 181(2):215–228, PMID: , 10.1093/toxsci/kfab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenfeld CS. 2015. Sex-specific placental responses in fetal development. Endocrinology 156(10):3422–3434, PMID: , 10.1210/en.2015-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desvergne B, Feige JN, Casals-Casas C. 2009. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol Cell Endocrinol 304(1–2):43–48, PMID: , 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 102.Szilagyi JT, Avula V, Fry RC. 2020. Perfluoroalkyl substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for placental peroxisome proliferator–activated receptors (PPARs). Curr Environ Health Rep 7(3):222–230, PMID: , 10.1007/s40572-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adams S, Wiersielis K, Yasrebi A, Conde K, Armstrong L, Guo GL, et al. . 2020. Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression. Reprod Toxicol 94:65–74, PMID: , 10.1016/j.reprotox.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.US EPA (US Environmental Protection Agency). 2023. America’s Children and the Environment (ACE). Health - Adverse Birth Outcomes. https://www.epa.gov/americaschildrenenvironment/health-adverse-birth-outcomes [accessed 10 January 2024].
- 105.Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. 2023. Births: final data for 2021. Natl Vital Stat Rep 72(1):1–53, PMID: , 10.15620/cdc:122047. [DOI] [PubMed] [Google Scholar]
- 106.Bastiaensen M, Gys C, Malarvannan G, Fotache M, Bombeke J, Ait Bamai Y, et al. . 2021. Short-term temporal variability of urinary biomarkers of organophosphate flame retardants and plasticizers. Environ Int 146:106147, PMID: , 10.1016/j.envint.2020.106147. [DOI] [PubMed] [Google Scholar]
- 107.Hoffman K, Daniels JL, Stapleton HM. 2014. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int 63:169–172, PMID: , 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ballesteros-Gómez A, Erratico CA, Van den Eede N, Ionas AC, Leonards PE, Covaci A. 2015. In vitro metabolism of 2-ethylhexyldiphenyl phosphate (EHDPHP) by human liver microsomes. Toxicol Lett 232(1):203–212, PMID: , 10.1016/j.toxlet.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Ballesteros-Gómez A, Van den Eede N, Covaci A. 2015. In vitro human metabolism of the flame retardant resorcinol bis(diphenylphosphate) (RDP). Environ Sci Technol 49(6):3897–3904, PMID: , 10.1021/es505857e. [DOI] [PubMed] [Google Scholar]
- 110.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. . 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384(9946):857–868, PMID: , 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.