Abstract
Background
The 2022 mpox outbreak has affected disproportionately people living with HIV (PLWH) and pre-exposure prophylaxis (PrEP) users.
Methods
We conducted a cross-sectional study to evaluate factors associated with laboratory diagnosis of mpox among suspected cases, and access differences between PrEP users and PLWH with confirmed diagnostic.
Results
394 mpox suspected cases were analyzed, 309 (78.4%) confirmed. Most patients with mpox were PLWH (54.4%) and 99 (32%) PrEP users. Mpox cases were likely to be between 25 and 39 years old (aOR=2.8; p=0.042), men who have sex with men/bisexual or transgender women (aOR=17.2; p< 0.001) and to have fever (aOR=4.7; p< 0.001), adenomegaly (aOR=7.2; p< 0.001) and multiple vesicular lesions (aOR=4.2; p< 0.001). Comparing PrEP users to PLWH with confirmed mpox, PrEP users had lesions predominantly with exclusive genital involvement (p=0.016); while PLWH had higher extragenital involvement (p=0.018).
Conclusions
PrEP users and PLWHA were the main epidemiological groups in our cohort. Recognizing the differences between vulnerable populations can contribute to the development public policies to control mpox in settings with reduced access to vaccines
Background
Mpox, formerly known as monkeypox (MPX), is a zoonotic viral disease caused by the Orthopoxvirus Monkeypox (MPXV), first described dates back over 50-year [1]. The first case of mpox in humans was published in the 1970s and until 2022 cases were concentrated in an endemic cycle associated with exposure to forest animals in Central and West African countries. (mainly Nigeria and the Democratic Republic of Congo), with a few imported cases occurring in travelers from the US, England, Israel, and Singapore [2], [3], [4], [5], [6], [7], [8]. Phylogenetically, MPXV is divided into 2 clades, one related to cases primarily reported in Central Africa (Clade I) and the other belonging to West Africa (Clade II) [9].
Since May 2022, the largest outbreak in recent mpox history has occurred outside of the African continent, with the first cases reported in the United Kingdom and Spain [10,11]. Outbreaks in several other countries were quickly identified, so that by January 6, 2023, 110 countries reported more than 84,000 cases and 74 deaths since 2022 [12]. Phylogenetic study revealed the circulation of a genomic variant related to the West African clade in the current outbreak, called Clade II strain B1 [13].
The first mpox case described in Brazil occurred in a man who has sex with men (MSM) who had returned from Spain and was diagnosed in May/2022. As of September/2022 Brazil ranked second in absolute number of cases and as of December/2022 held the top position for the number of deaths related to the current outbreak [14]. The city of São Paulo was the epicenter of mpox in Brazil, having reported approximately 3,000 cases by December/2022 [15].
The clinical and epidemiological characteristics of this outbreak are distinct from the endemic disease, highlighting the concentration of cases in MSM population, especially those belonging to the group of people living with HIV and AIDS (PLWH) and users of pre-exposure prophylaxis (PrEP) [16], [17], [18]. Intimate contact facilitated by sexual practices is considered the main route of transmission of the current MPXV outbreak [16], [17].
There are few publications evaluating the impact of HIV infection on the course and clinical outcomes related to mpox [18], [19]. Previous reports from African countries have included a small number of PLWH, and have not been able to identify differences in clinical presentation of mpox between PLWH and non-infected by HIV [19]. The current intersection of the mpox outbreak with the HIV/AIDS epidemic has enabled further investigation of this co-infection and should provide a better understanding of related risks.
This study describes the clinical and epidemiological characteristics of suspected cases of MPXV infection and aimed to investigate factors associated with the diagnosis confirmation, as well as to evaluate differences between the groups of PLWH and PrEP users diagnosed with mpox in a public specialized institution in the city of São Paulo.
Methods
Study design and research site
This is a cross-sectional study conducted at the Centro de Referência e Treinamento DST/Aids de São Paulo (CRT-DST/Aids), a reference public institution in prevention and treatment of HIV infection and other sexually transmitted infections (STI).The processing and analysis of samples collected for the diagnosis of mpox in the state of São Paulo is carried out at the Instituto Adolfo Lutz, also a public institution belonging to the state government, which performs real-time polymerase chain reaction (rt-PCR) according to the methodology established by the protocol of the Centers for Disease Control and Prevention (CDC) using swabs of skin lesions.
Recruitment of participants
Patients aged 18 years or older with one or more skin or mucosal lesion suspected of mpox who had material collected from the lesions for laboratory diagnostic investigation between 6/18/2022 and 9/22/2022 were included in the study. Suspected case was defined a person with sudden onset of an acute skin rash suggestive of mpox (deep and well-circumscribed lesions, often with central umbilication; and progression of the lesion through specific sequential stages – macules, papules, vesicles, pustules and crusts) single or multiple on any part of the body (including genital region), associated or not with adenomegaly or report of fever AND having one of the epidemiological links: report of intimate contact with casual partner(s), in the last 21 days prior to the onset of signs and symptoms OR contact with a suspected, probable or confirmed case of mpox prior to the onset of symptoms OR travel to an endemic country or country with confirmed cases of mpox in the 21 days prior to the onset of signs and symptoms.
Cases whose diagnosis of mpox could not be ruled out or confirmed by laboratory examination were excluded from the sample.
Research data
We used data from the Central de Vigilância de Emergência em Saúde Pública do Estado de São Paulo (CEVESP) database of mpox suspected cases evaluated at the CRT outpatient clinics. Missing or inconsistent data were checked in the patient's electronic medical record and corrected in the study database. The variables of interest for the study were sociodemographic variables (sex at birth, age, education, and race/ethnicity); signs and symptoms referred and observed in the first clinical evaluation; morphologic, distribution and topographic characterization of lesions; presence of HIV infection, gender and sexual orientation, number of sexual partners in the last 21 days prior to the onset of symptoms and likely route of transmission.
The description of the lesion's topography was also categorized in to “number of segments affected” and “place of lesions” for purpose of analysis. Buttocks, perianal region, and genitals were grouped in “genitals/buttocks”; upper limbs, lower limbs, palm of hands, and sole of feet were grouped in “extremities”; neck, face, and oral cavity were grouped in “cephalic/neck”. Signs and symptoms in addition to the lesions were assessed individually and grouped into number of other signs and symptoms.
We cross-checked all suspected cases regarding PrEP use and HIV infection status, as well as the last viral load and CD4 count results among PLWH, using the Logistical Medication Control System. In addition, data regarding the concomitant and in the last 12 months occurrence of STIs (serology for syphilis, PCR testing for gonococcus and chlamydia in urine and genital secretions) were extracted from the institutional laboratory system database.
The detection rates of mpox in the studied populations (PLWH, PrEP users, and STI/testing clinic users who were HIV-negative and did not take PrEP – here called as “non-HIV/non-PrEP”) were estimated using as numerator the new cases of mpox in each population group and as denominator the total number of people admitted to the respective outpatient clinics in the same period, multiplied by one hundred.
Statistical analysis
Mpox disease, defined as detectable rt-PCR (cycle threshold value less than or equal to 37), was considered the dependent variable for this analysis. The risks for mpox disease were studied for all the independent variables of interest cited above such as sociodemographic variables, variables related to clinical presentation, the presence of HIV infection, PrEP use, gender, and sexual orientation, previous and concurrent STI, and number of sexual partnerships.
Clinical-epidemiological data, behavioral information, and laboratory test results were coded and stored in a database named CeVeSP (Central/CIEVS SP) as the basis of the mpox case notification forms. The statistical program STATA 16.1 was used for data storage and analysis.
Then, a bivariate analysis was performed to verify the presence of associations among them. Chi-square (x2) tests were used for proportion differences and Student's t test and analysis of variance for differences between means. The odds ratio (OR) was used to estimate associations with a 95% confidence interval. The variables were selected to compose the model when they presented a p value equal to or less than 0.25 in the likelihood ratio test. Multivariate analysis was performed to estimate joint effects of the independent variables using logistic regression models. This model was adjusted through the progressive stepwise procedure and the inclusion of variables followed an increasing order of OR values. The importance of the variables for the final model was evaluated using the likelihood ratio test considering p < 0.05.
Ethical Aspects
This study was submitted to and approved by the CRT-DST/Aids Research Ethics Committee (opinion No. 5.638.484).
Results
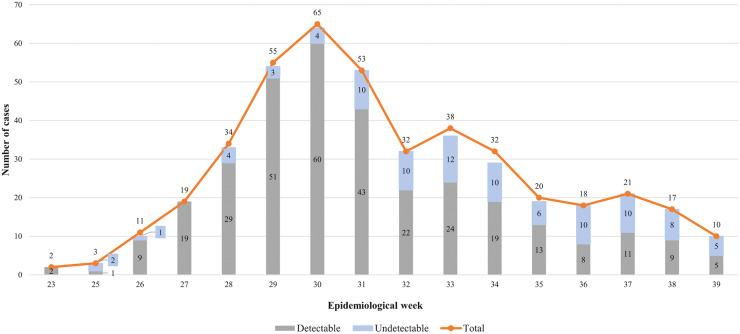
Between 6/18/2022 and 9/20/2022, 394 suspected cases of mpox aged over 18 years had medical evaluation and a confirmed or discharged diagnosis of mpox by performing rt-PCR for MPXV. There was a particular concentration of suspected and confirmed mpox cases occurring between epidemiological weeks 29 and 31, followed by a steady decline of cases among PrEP users until the final date when participants were included (Figure 1).
Figure 1.
Suspected cases of mpox according to the results of the real-time polymerase chain reaction and epidemiological week, Centro de Referência e Treinamento DST/Aids, June to September, 2022.
Among the suspected cases, 309 (78.4%) had laboratory-confirmed diagnosis of mpox. Sociodemographic and clinical characteristics distribution referring to the 394 mpox suspected patients, according to diagnostic confirmation, are shown in Table 1.
Table 1.
Distribution of sociodemographic, clinical, and behavioral characteristics of suspected cases according to the results of the mpox real-time polymerase chain reaction, Centro de Referência e Treinamento DST/Aids, June 18 to September 20, 2022.
| Characteristics | Mpox real-time polymerase chain reaction |
||||||
|---|---|---|---|---|---|---|---|
| Undetectable |
Detectable |
Total |
P-value | ||||
| (n = 85; 21.6%) |
(n = 309; 78.4%) |
(n = 394; 100.0%) |
|||||
| n | % | n | % | n | % | ||
| Sex assigned at birth | <0.001 | ||||||
| Male | 76 | 89.4 | 309 | 100.0 | 385 | 97.7 | |
| Female | 9 | 10.6 | 0 | 0.0 | 9 | 2.3 | |
| Age group (years old) | 0.01 | ||||||
| ≤ 24 | 12 | 14.1 | 22 | 7.1 | 34 | 8.6 | |
| 25 - 39 | 44 | 51.8 | 212 | 68.6 | 256 | 65.0 | |
| ≥ 40 | 29 | 34.1 | 75 | 24.3 | 104 | 26.4 | |
| Race/Ethnicitya | 0.823 | ||||||
| White | 49 | 57.6 | 164 | 53.1 | 213 | 54.1 | |
| Black | 15 | 17.6 | 52 | 16.8 | 67 | 17.0 | |
| Asian | 1 | 1.2 | 2 | 0.6 | 3 | 0.8 | |
| Pardo (mixed) | 20 | 23.5 | 86 | 27.8 | 106 | 26.9 | |
| Indigenous | 0 | 0.0 | 2 | 0.6 | 2 | 0.5 | |
| Gender and sexual orientation | <0.001 | ||||||
| Heterosexual | 16 | 18.8 | 4 | 1.3 | 20 | 5.1 | |
| Man who have sex with men/ bisexual, or transgender woman | 69 | 81.2 | 305 | 98.7 | 374 | 94.9 | |
| Years of studyb | 0.113 | ||||||
| Up to 11 | 40 | 48.8 | 114 | 39.0 | 154 | 41.2 | |
| 12 or more | 42 | 51.2 | 178 | 61.0 | 220 | 58.8 | |
| Patient category | 0.02 | ||||||
| People living with HIV/AIDS | 52 | 61.2 | 168 | 54.4 | 220 | 58.8 | |
| PrEP user | 15 | 17.6 | 99 | 32.0 | 114 | 28.9 | |
| Non-HIV / Non-PrEP | 18 | 21.2 | 42 | 13.6 | 60 | 15.2 | |
| Status according HIV infectionc | 0.371 | ||||||
| VL < 50 copies/ml / CD4 < 350 cells/mm3 | 4 | 7.7 | 7 | 4.2 | 11 | 5.0 | |
| VL < 50 copies/ml / CD4 ≥ 350 cells/mm3 | 36 | 69.2 | 136 | 81.0 | 172 | 78.2 | |
| VL ≥ 50 copies/ml / CD4 < 350 cells/mm3 | 4 | 7.7 | 7 | 4.2 | 11 | 5.0 | |
| VL ≥ 50 copies/ml / CD4 ≥ 350 cells/mm3 | 1 | 1.9 | 5 | 3.0 | 6 | 2.7 | |
| Not available | 7 | 13.5 | 13 | 7.7 | 20 | 9.1 | |
| Antiretroviral therapy usec | 0.457 | ||||||
| No | 5 | 9.6 | 11 | 6.5 | 16 | 7.3 | |
| Yes | 47 | 90.4 | 157 | 93.5 | 204 | 92.7 | |
| Topography of lesions | 0.313 | ||||||
| Cephalic/neck | 42 | 16.3 | 215 | 83.7 | 257 | 100.0 | |
| Torso | 33 | 21.6 | 120 | 78.4 | 153 | 100.0 | |
| Genitals/buttocks | 53 | 17.7 | 247 | 82.3 | 300 | 100.0 | |
| Extremities | 36 | 22.6 | 123 | 77.4 | 159 | 100.0 | |
| Number of segments affected | 0.238 | ||||||
| One | 41 | 48.2 | 152 | 49.2 | 193 | 49.0 | |
| Two | 26 | 30.6 | 73 | 23.6 | 99 | 25.1 | |
| Three | 11 | 12.9 | 46 | 14.9 | 57 | 14.5 | |
| Four | 5 | 5.9 | 36 | 11.7 | 41 | 10.4 | |
| Ignored | 2 | 2.4 | 2 | 0.6 | 4 | 1.0 | |
| Distribution of lesionsd | 0.006 | ||||||
| Only genitals/buttocks | 27 | 32.5 | 128 | 41.7 | 155 | 39.7 | |
| Genitals/buttocks and extra-genitals | 26 | 31.3 | 119 | 38.8 | 145 | 37.2 | |
| Only extra-genitals | 30 | 36.1 | 60 | 19.5 | 90 | 23.1 | |
| Morphology of lesions | 0.361 | ||||||
| Macula | 27 | 21.8 | 97 | 78.2 | 124 | 100.0 | |
| Papule | 40 | 19.4 | 166 | 80.6 | 206 | 100.0 | |
| Vesicle | 11 | 22.0 | 39 | 78.0 | 50 | 100.0 | |
| Pustule | 29 | 14.1 | 177 | 85.9 | 206 | 100.0 | |
| Scab | 41 | 20.1 | 163 | 79.9 | 204 | 100.0 | |
| Number of lesions | 0.596 | ||||||
| Single | 12 | 14.1 | 37 | 12.0 | 49 | 12.4 | |
| Multiple | 73 | 85.9 | 272 | 88.0 | 345 | 87.6 | |
| Stage of lesions | 0.055 | ||||||
| Single-phase | 31 | 36.5 | 80 | 25.9 | 111 | 28.2 | |
| Polymorphic | 54 | 63.5 | 229 | 74.1 | 283 | 71.8 | |
| Other signs and symptoms | 0.664 | ||||||
| Adenomegaly | 28 | 10.7 | 233 | 89.3 | 261 | 100.0 | |
| Fever | 16 | 8.5 | 172 | 91.5 | 188 | 100.0 | |
| Headache | 20 | 10.9 | 163 | 89.1 | 183 | 100.0 | |
| Myalgia | 22 | 12.8 | 150 | 87.2 | 172 | 100.0 | |
| Asthenia | 22 | 13.2 | 145 | 86.8 | 167 | 100.0 | |
| Back pain | 14 | 14.0 | 86 | 86.0 | 100 | 100.0 | |
| Number of other signs and symptoms | <0.001 | ||||||
| None | 32 | 37.6 | 24 | 7.8 | 56 | 14.2 | |
| 1 to 3 | 41 | 48.2 | 155 | 50.2 | 196 | 49.7 | |
| 4 or more | 12 | 14.1 | 130 | 42.1 | 142 | 36.0 | |
| STI last 12 months | 0.144 | ||||||
| No | 78 | 91,8 | 265 | 85.8 | 343 | 87.1 | |
| Yes | 7 | 8.2 | 44 | 14.2 | 51 | 12.9 | |
| Concurrent STI | 0.037 | ||||||
| No | 74 | 87.1 | 290 | 93.9 | 364 | 92.4 | |
| Yes | 11 | 12.9 | 19 | 6.1 | 30 | 7.6 | |
| Number of sexual partnerse | 0.158 | ||||||
| None | 7 | 10.1 | 16 | 6.5 | 23 | 7.3 | |
| 1 | 31 | 44.9 | 81 | 32.8 | 112 | 35.4 | |
| 2 to 5 | 20 | 29.0 | 107 | 43.3 | 127 | 40.2 | |
| 6 to 10 | 5 | 7.2 | 25 | 10.1 | 30 | 9.5 | |
| 11 or more | 6 | 8.7 | 18 | 7.3 | 24 | 7.6 | |
| Presumed transmission route | 0.086 | ||||||
| Sexual | 81 | 95.3 | 304 | 98.4 | 385 | 97.7 | |
| Healthcare associated | 1 | 1.2 | 0 | 0.0 | 1 | 0.3 | |
| Non-sexual contact | 3 | 3.5 | 5 | 1.6 | 8 | 2.0 | |
PrEP: HIV pre-exposure prophylaxis; STI: sexually transmitted infection; VL: viral load.
Comparisons performed using chi-square tests. Bold indicates statistically significant difference (P <0.05).
Ignored for three patients
Ignored for 20 patients
Not applicable for 174 patients
Ignored for four patients
Ignored for 78 patients.
Mostly frequently the suspected cases were assigned as male at birth (n= 385; 97.7%), were MSM/bisexual or transgender women (n= 374; 94.9%, being 8 transgender women), had White ethnicity (n= 213; 54.1%) and 12 or more years of schooling (n= 220; 58.8%). The median age was 33 years. A total of 220 (55.8%) people were living with HIV/AIDS and, among these, 204 (92.7%) were on antiretroviral therapy, 183 (83.2%) had viral load < 50 copies/mL, and 178 (80.9%) had CD4 count ≥ 350 cells/mm³; 114 (28.9%) were PrEP users. All patients had skin-mucosal lesions, being mostly multiple (n= 345; 87.6%), polymorphic (n= 283; 71.8%), and affecting a single body segment (n= 193; 49%). Lesions were more prevalent in genitals/buttocks (n= 300; 76.1%) and cephalic/neck (n= 257; 65.2%) segments. The risk of mpox acquisition was mostly sexual (n= 385; 97.7%) and 181 (57.3%) patients reported intercourse with more than one partner in the last 21 days prior to the onset of the first symptoms, 54 with more than 5 partners. Most patients had one to three symptoms in addition to skin-mucosal lesions (n= 196; 49.7%), with adenomegaly, fever, and headache being the most common ones (66.2%, 47.4%, and 46.4%, respectively).
There were differences in the distribution of PCR-detectable and PCR-undetectable cases in terms of sex at birth, sexual orientation, age group, categorization regarding HIV infection and PrEP use, number of symptoms associated with the cutaneous-mucosal rash, and area of distribution of lesions. Patients diagnosed with mpox had a higher proportion of persons designated as male at birth (n= 309; 100%), MSM/bisexual or transgender women (n= 305; 98.7%), aged 25 to 39 (n= 212; 68.6%), were PLWH (n= 168; 54,4%); had at least one sign or symptom associated with skin-mucosal rash (n= 285; 92,3%) and lesions frequently compromising genitals/ buttocks (n= 247; 80.5%), but fewer STI diagnosed concomitant to mpox (n= 19; 6.1%). (Table 1) The median rt-PCR cycle threshold of confirmed cases was 18, with no difference between PrEP users and PLWH.
The factors shown to be associated with a higher likelihood of laboratory confirmation of mpox among suspected cases are displayed in Table 2, as follows: be in the age group of 25 to 39 years old (aOR= 2.8; 95%CI 1.1-7.5; p= 0.042), being MSM/bisexual or transgender woman (aOR= 17.2; 95%CI 4.5-65.9; p< 0.001), having presented fever (aOR= 4.7; 95%CI 2.3-9.7; p< 0.001) or adenomegaly (aOR= 7.2; 95%CI 3.8-13.7; p< 0.001), having multiple vesicular lesions (aOR= 4.2; 95%CI 2.1-8.5; p< 0.001), and absence of another STI diagnosed during the suspected mpox clinical event (aOR= 3.2; 95%CI 1.2-8.6; p= 0.017).
Table 2.
Bivariate and multiple analysis of factors associated with mpox diagnosis, Centro de Referência e Treinamento DST/Aids, June 18 to September 20, 2022.
| Characteristics | Total | Mpox |
cOR | 95% CI (cOR) | P-value | aOR | 95% CI (aOR) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| n | % | ||||||||
| Age group (years old) | |||||||||
| ≤24 | 34 | 22 | 64.7 | 1 | - | - | 1 | - | - |
| 25-39 | 256 | 212 | 82.8 | 2.6 | 1.2 - 5.7 | 0.015 | 2.8 | 1.1 - 7.5 | 0.042 |
| ≥40 | 104 | 75 | 72.1 | 1.4 | 0.6 - 3.2 | 0.413 | 2.1 | 0.7 - 6.0 | 0.171 |
| Gender and sexual orientation | |||||||||
| Heterosexual | 20 | 4 | 20.0 | 1 | - | - | 1 | - | - |
| Man who have sex with men/bisexual, or transgender woman | 374 | 305 | 81.6 | 17.7 | 5.7 - 54.5 | < 0.001 | 17.2 | 4.5 - 65.9 | < 0.001 |
| Fever | |||||||||
| No | 201 | 132 | 65.7 | 1 | - | - | 1 | - | - |
| Yes | 188 | 172 | 91.5 | 5.6 | 3.1 - 10.1 | < 0.001 | 4.7 | 2.3 - 9.7 | < 0.001 |
| Adenomegaly | |||||||||
| No | 126 | 70 | 55.6 | 1 | - | - | 1 | - | - |
| Yes | 261 | 233 | 89.3 | 6.7 | 3.9 - 11.3 | < 0.001 | 7.2 | 3.8 - 13.7 | < 0.001 |
| Vesicle | |||||||||
| Absent | 174 | 119 | 68.4 | 1 | - | - | 1 | - | - |
| Single | 50 | 39 | 78.0 | 1.6 | 0.8 - 3.4 | 0.192 | 1.5 | 0.6 - 3.7 | 0.372 |
| Multiple | 170 | 151 | 88.8 | 3.7 | 2.1 - 6.5 | < 0.001 | 4.2 | 2.1 - 8.5 | < 0.001 |
| Concurrent sexually transmitted infection | |||||||||
| No | 364 | 290 | 79.7 | 2.3 | 1.1 - 5.0 | 0.041 | 3.2 | 1.2 - 8.6 | 0.017 |
| Yes | 30 | 19 | 63.3 | 1 | - | - | 1 | - | - |
CI: confidence interval; aOR: adjusted odds ratio; cOR: crude odds ratio.
When comparing the group of PrEP users to PLWH among the cases with laboratory-confirmed mpox diagnosis, some differences were noticed (Table 3). PrEP users had a higher proportion of 12 or more years of schooling (71.4% vs 57.4%; p= 0.027), reporting multiple sexual partnerships in the last 21 days (73.4% vs 56.2%; p= 0.035) and had lesions predominantly with exclusive genital involvement (48% vs 33,5%; p=0,016). On the other hand, PLWH had a higher proportion of involvement of any of the body segments, specially extragenital (cephalic/neck 73,5% vs 26,5%; torso 74,5% vs 25,5%; extremities 67,6% vs 32,4%; genitals/buttocks 59,1% vs 40,9%; p= 0.018), and a higher proportion of exclusive extragenital involvement (26.3% vs 13.3%; p= 0.016).
Table 3.
Distribution of sociodemographic, clinical, and behavioral characteristics of patients with mpox according to category (HIV pre-exposure prophylaxis or people living with HIV/Aids), Centro de Referência e Treinamento DST/Aids, June 18 to September 20, 2022.
| Characteristics | Category |
||||||
|---|---|---|---|---|---|---|---|
| PrEP user |
PLWH |
Total |
P-value | ||||
| (n = 99; 37.1%) |
(n = 168; 62.9%) |
(n = 267; 100.0%) |
|||||
| n | % | n | % | n | % | ||
| Sex assigned at birth | - | ||||||
| Male | 99 | 100.0 | 168 | 100.0 | 267 | 100.0 | |
| Female | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Age group (years old) | 0.582 | ||||||
| ≤ 24 | 4 | 4.0 | 6 | 3.6 | 10 | 3.7 | |
| 25 - 39 | 73 | 73.7 | 115 | 68.5 | 188 | 70.4 | |
| ≥ 40 | 22 | 22.2 | 47 | 28.0 | 69 | 25.8 | |
| Race/Ethnicitya | 0.783 | ||||||
| White | 48 | 49.0 | 91 | 54.8 | 139 | 52.7 | |
| Black | 19 | 19.4 | 26 | 15.7 | 45 | 17.0 | |
| Asian | 1 | 1.0 | 1 | 0.6 | 2 | 0.8 | |
| Pardo (Mixed) | 30 | 30.6 | 47 | 28.3 | 77 | 29.2 | |
| Indigenous | 0 | 0.0 | 1 | 0.6 | 1 | 0.4 | |
| Gender and sexual orientation | 0.784 | ||||||
| Heterosexual | 1 | 1.0 | 1 | 0.6 | 2 | 0.7 | |
| Man who have sex with men/ bisexual, or transgender woman | 98 | 99.0 | 167 | 99.4 | 265 | 99.3 | |
| Years of studyb | 0.027 | ||||||
| Up to 11 | 26 | 28.6 | 69 | 42.6 | 95 | 37.5 | |
| 12 or more | 65 | 71.4 | 93 | 57.4 | 158 | 62.5 | |
| Topography of lesions | 0.018 | ||||||
| Cephalic/neck | 22 | 26.5 | 61 | 73.5 | 83 | 100.0 | |
| Torso | 27 | 25.5 | 79 | 74.5 | 106 | 100.0 | |
| Genitals/buttocks | 85 | 40.9 | 123 | 59.1 | 208 | 100.0 | |
| Extremities | 36 | 32.4 | 75 | 67.6 | 111 | 100.0 | |
| Number of segments affected | 0.151 | ||||||
| One | 54 | 54.5 | 73 | 43.5 | 127 | 47.6 | |
| Two | 22 | 22.2 | 43 | 25.6 | 65 | 24.3 | |
| Three | 16 | 16.2 | 25 | 14.9 | 41 | 15.4 | |
| Four | 6 | 6.1 | 26 | 15.5 | 32 | 12.0 | |
| Ignored | 1 | 1.0 | 1 | 0.6 | 2 | 0.7 | |
| Distribution of lesions | 0.016 | ||||||
| Only genitals/buttocks | 47 | 48.0 | 56 | 33.5 | 103 | 38.9 | |
| Genitals/buttocks and extra-genitals | 38 | 38.8 | 67 | 40.1 | 105 | 39.6 | |
| Only extra-genitals | 13 | 13.3 | 44 | 26.3 | 57 | 21.5 | |
| Morphology of lesions | 0.52 | ||||||
| Macula | 31 | 34.8 | 58 | 65.2 | 89 | 100.0 | |
| Papule | 62 | 42.5 | 84 | 57.5 | 146 | 100.0 | |
| Vesicle | 55 | 33.1 | 111 | 66.9 | 166 | 100.0 | |
| Pustule | 56 | 36.6 | 97 | 63.4 | 153 | 100.0 | |
| Scab | 51 | 35.4 | 93 | 64.6 | 144 | 100.0 | |
| Number of lesions | 0.166 | ||||||
| Single | 15 | 15.2 | 16 | 9.5 | 31 | 11.6 | |
| Multiple | 84 | 84.8 | 152 | 90.5 | 236 | 88.4 | |
| Stage of lesions | 0.501 | ||||||
| Single-phase | 26 | 26.3 | 38 | 22.6 | 64 | 24.0 | |
| Polymorphic | 73 | 73.7 | 130 | 77.4 | 203 | 76.0 | |
| Other signs and symptoms | 0.894 | ||||||
| Adenomegaly | 78 | 39.4 | 120 | 60.6 | 198 | 100.0 | |
| Fever | 55 | 36.2 | 97 | 63.8 | 152 | 100.0 | |
| Headache | 49 | 34.8 | 92 | 65.2 | 141 | 100.0 | |
| Myalgia | 45 | 34.9 | 84 | 65.1 | 129 | 100.0 | |
| Asthenia | 49 | 38.9 | 77 | 61.1 | 126 | 100.0 | |
| Back pain | 24 | 33.3 | 48 | 66.7 | 72 | 100.0 | |
| Number of other signs and symptoms | 0.513 | ||||||
| None | 6 | 6.1 | 17 | 10.1 | 23 | 8,6 | |
| 1 to 3 | 51 | 51.5 | 81 | 48.2 | 132 | 49.4 | |
| 4 or more | 42 | 42.4 | 70 | 41.7 | 112 | 41.9 | |
| STI last 12 months | 0.441 | ||||||
| No | 82 | 82.8 | 145 | 86.3 | 227 | 85.0 | |
| Yes | 17 | 17.2 | 23 | 13.7 | 40 | 15.0 | |
| Concurrent STI | 0.757 | ||||||
| No | 94 | 94.9 | 158 | 94.0 | 252 | 94.4 | |
| Yes | 5 | 5.1 | 10 | 6.0 | 15 | 5.6 | |
| Number of sexual partnersc | 0.035 | ||||||
| None | 5 | 6.7 | 9 | 6.6 | 14 | 6.6 | |
| 1 | 15 | 20.0 | 51 | 37.2 | 66 | 31.1 | |
| 2 to 5 | 36 | 48.0 | 57 | 41.6 | 93 | 43.9 | |
| 6 to 10 | 8 | 10.7 | 13 | 9.5 | 21 | 9.9 | |
| 11 or more | 11 | 14.7 | 7 | 5.1 | 18 | 8.5 | |
| Presumed transmission route | 0.59 | ||||||
| Sexual | 97 | 98.0 | 166 | 98.8 | 263 | 98.5 | |
| Non-sexual contact | 2 | 2.0 | 2 | 1.2 | 4 | 1.5 | |
PrEP: HIV pre-exposure prophylaxis; PLWH: people living with HIV/Aids; STI: sexually transmitted infection.
Comparisons performed using chi-square tests. Bold indicates statistically significant difference (P <0.05).
Ignored for three patients
Ignored for 14 patients
Ignored for 55 patients.
There were no deaths in this case series and most patients had mild to moderate course of illness. Only seven patients diagnosed with mpox were hospitalized, five of them coinfected with HIV. The criteria used for hospitalization among PLWH were the need for additional measures to control pain (2/5), treatment of secondary infection (2/5) and large number of lesions (2/5). Others hospitalizations occurred among PrEP users (2/7) - one due to extensive proctitis and the other due to keratitis, the latter leading to the use of antiviral medication (tecovirimat).
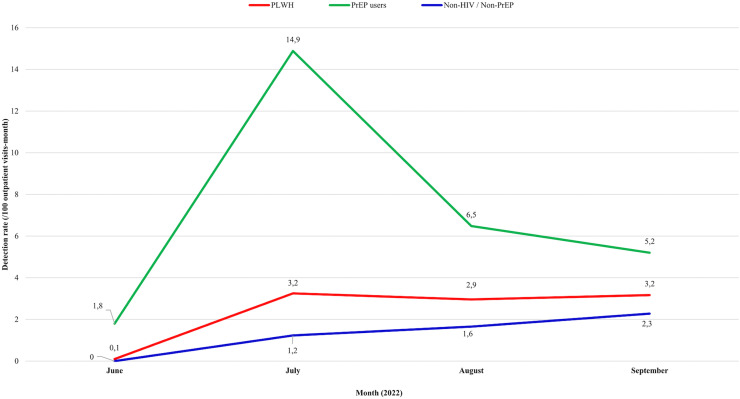
The detection rates in CRT-DST/Aids at the peak of the São Paulo outbreak, which occurred in July/2022, were 14.9/100 outpatient visits-month among PrEP users, 3.2/100 outpatient visits-month among PLWH, and 1.2/100 outpatient visits-month among non-HIV/non-PrEP patients (Figure 2).
Figure 2.
Mpox detection rate per 100 outpatient visits-month in the discriminated groups, Centro de Referência e Treinamento DST/Aids, June 18 to September 20, 2022. PrEP: HIV pre-exposure prophylaxis; PLWH: people living with HIV/AIDS.
Discussion
In this cohort from a single center in Brazil, we had 309 confirmed cases of mpox, with no fatal outcomes. A notable proportion were PLWH (56,4%) and PrEP users, who together accounted for 86.4% of mpox cases. In a global series of mpox (N= 528) cases published compiling data from 16 countries, 41% of cases were patients with immunodeficiency virus infection [20].
Most of mpox patients were young adult, with an absolute predominance of cases among MSM/bisexuals and transgender women. These epidemiologic findings are very similar to others case series and observational studies published since the beginning of the outbreak [20], [21], [22], [23], [24].
In our cohort, a wide variety of systemic symptoms (adenomegaly, fever and headache) were associated with the occurrence of cutaneous lesions, which were mostly multiple (87.6%) and polymorphic (71.8%), commonly affecting the genitals (76.1%). This polymorphism of lesions found in different anatomical sites, predominantly in the genitals associated with the presence of systemic signs has also been previously described in almost all series published on the 2022 outbreak [20], [21], [22], [23].
In addition to some sociodemographic and epidemiological characteristics (specially being MSM), the clinical findings found in our analysis that were most likely to confirm the diagnosis were the presence of multiple vesicles, enlarged lymph nodes and fever. Fever, adenomegaly and and systemic signs were also associated with a diagnosis of mpox in another observational cohort in Brazil [22].
The report of sexual exposure (97.7%) associated with more than 1 sexual partner (57.3%) in confirmed cases highlights the role of sexual contact in the transmission of the 2022 mpox outbreak [21], [22].
PrEP users represented an important population vulnerable to mpox with a massive concentration of cases. [20], [21], [22], [23], [24] We found higher detection rates of mpox among PrEP users when compared to other groups, including PLWH, but no association between the use of prophylaxis and greater likelihood of confirmation of diagnosis of mpox.
When comparing PrEP users to PLWH with mpox, PrEP users had higher level of education and more partners. The search for PrEP reveals sexual behavior of increased risk for HIV acquisition, and in the CRT-DST/Aids PrEP has been mainly accessed by MSM with university education level or complete higher education, that should have contributed to the pattern of detection rates evolution in this group. It is not clear why the mpox outbreak has cooled down within a few months of its start, even in the absence of a vaccine. The strong mobilization of the LGBTQIA+ community aimed in establishing behaviors changing to curb the advance of the outbreak and seems to have impacted differently the groups. It may have contributed to the fastest reduction observed in the detection rate among PrEP users, a population that is sexually more vulnerable and that revealed a higher number of partners, but which may nevertheless have greater greater ability to access and understand the general recommendations for controlling the disease and to assimilate the need to change their risk behavior for acquiring the disease. The impact was smaller among PLHW possibly because transmission was related to exposures involving other contexts, with fewer sexual partnerships and lower level of education. The absence of impact on the detection rate of mpox among the population not infected with HIV or using PrEP, in turn, may reveal deficiencies in communication about the disease for less sexually vulnerable populations, whose perception of risk may be intrinsically lower, favoring the maintenance of risky practices for the acquisition of mpox and increase of these rates during the study period.
There are publications in literature that highlight the possibility of greater extent, severity, and organ involvement of mpox in people with severe immunosuppression, including PLWH on irregular use of antiretroviral therapy [25], [26]. In a CDC report of mpox hospitalized cases in the US, 57 patients had severe manifestations of the disease from August to October 2022, 47 (82%) of whom were HIV-infected[27] Most studies, however, failed to find any difference between the clinical presentation in PLWH and non-HIV-infected patients, possibly because these studies concentrated on PLWH with good immune status and virological control [25], [26]. A surveillance compilation that included PLWH described cases of mpox had higher rash burden especially in those with CD4 < 500 [23].
Severe immunosuppression (CD4 < 200) was associated with and serious cutaneous manifestations and deaths in a global series of people living with HIV [26].
In our study, we also had a high proportion of PLWH virologically suppressed and with good immune status. Comparing PrEP users and PLWH with mpox, the only statistically significant clinical difference found was greater extragenital involvement among PLWH. Considering that both have similar sexual risk that involves the same likely route of inoculation of MPXV, we believe that this difference may be related to some fragility of the immune response innate and/or adaptive of PLWH, which failed to contain viral spread to areas distant from the virus entrance site.
Unlike other publications which identified a high prevalence of STI concomitant with mpox [22], [23], [24] in our study the occurrence of other STIs in the diagnosis of mpox was low (6.1%) and inversely associated with mpox confirmation. We hypothesized that it could mean noncompliance with the institutional protocol, which proposes the investigation of other STIs during the management of all mpox suspected cases. The existence of very suggestive clinical presentations of this diagnosis must have contributed for clinicians to ignore the possibility of coinfection with other STIs, focusing on ruling out the emerging disease in our country and prioritizing other tests in less typical cases.
Our article has some limitations. We included cases self-identified as suspect for mpox and that sought the CRT-DST/Aids for evaluation, so the findings relate exclusively to this sample. Another issue is that we used the suspected cases notification form of the state government, which has some limitations in risk and clinical characterization. In addition, we had fairly representation of PLWH with severe immunosuppression and without virological control, which may have contributed for not identifying substantial differences in the clinical presentation in this group.
In conclusion, we found higher rates of mpox detection among PrEP users followed by PLWH and an outbreak concentrated in young MSM/bisexual and transgender women. The restricted availability and indication of antivirals for the treatment of mpox, as well as the absence of vaccines in Brazil until the conclusion of our study, make educational strategies directed to professionals and community, and those related to the reduction of risk behavior, essential in combating the circulation of the virus. Despite the importance of spreading the education campaigns, it is important to warn against triggering stigma and discrimination
The knowledge of the behavioral component related to mpox transmission and the possible lower ability of PLWH to contain the spread of MPXV, especially in situations of advanced immunosuppression, can contribute to the development of more effective public policies in contexts of antiviral and vaccine shortage, which should prioritize access to PLWH.
Authors' contributions
AFC, SQR, MF, RSN, AOK, JVRM, MCG, RAS, RR, AT, RJCF, AMCS, WDAP, AA, and MVT conceived and designed the study. AFC, SQR, RSN, AOK, JVRM RJCF, and MVT defined the study methods. AFC, SQR, MVT, RSN, VM, AT, LRR, and Mpox-CRT working group worked in data and sample collection. AFC, SQR, MVT, and AOK worked in data curation and formal analysis. AA was responsible for laboratory procedures. AFC, RAS, MCG, and MVT were responsible for the study management. AFC, SQR, RSN, JVRM, WDAP, RR, AA, and MVT supervised the project. AFC, SQR, and MVT had full access and validated the data; AFC, SQR, MF, RSN, AOK, and MVT analyzed an interpreted the data. SQR and MVT defined the data visualization. AFC, SQR, MF, RSN, AOK, RAS, AT, and MVT drafted the manuscript. AFC, SQR, MF, RSN, RAS, and MVT edited the final version of the manuscript. All authors revised and approved the final version of the manuscript, and were responsible for the final decision to submit for publication.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Ethical considerations
This study was approved by the Ethics Review Board at CRT-DST/Aids.
Funding
The authors declare no financial support.
Data sharing
Deidentified participants data collected will be made available from the corresponding author on reasonable request and after authors approval.
Footnotes
Adresses:
References
- 1.Fine PEM, Jezek Z, Grab B, Dixon H. The Transmission Potential of Monkeypox Virus in Human Populations. Int J Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. https://academic.oup.com/ije/article-lookup/doi/10.1093/ije/17.3.643 [cited 2023 Jan 26]Available from: [DOI] [PubMed] [Google Scholar]
- 2.Von Magnus P, Andersen EA, Petersen KB. Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Path Microbiol Scand. 1959;46:159. [Google Scholar]
- 3.Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58(2):165–182. PMID: 6249508. [PMC free article] [PubMed] [Google Scholar]
- 4.Public Health England. Monkeypox case confirmed in England 2019. Available from: https://www.gov.uk/government/news/monkeypox-case-confirmed-in-england.
- 5.Yong SEF, Ng OT, Ho ZJM, Mak TM, Marimuthu K, Vasoo S, et al. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. http://wwwnc.cdc.gov/eid/article/26/8/19-1387_article.htm [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance. 2018 Sep 20;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2018.23.38.1800509 [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. http://wwwnc.cdc.gov/eid/article/26/4/19-1164_article.htm [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erez N, Achdout H, Milrot E, Schwartz Y, Wiener-Well Y, Paran N, et al. Diagnosis of Imported Monkeypox, Israel, 2018 - Volume 25, Number 5—May 2019 - Emerging Infectious Diseases journal - CDC. [cited 2023 Jan 26]; Available from: https://wwwnc.cdc.gov/eid/article/25/5/19-0076_article. [DOI] [PMC free article] [PubMed]
- 9.Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. https://linkinghub.elsevier.com/retrieve/pii/S0042682205003302 [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UK Health Security Agency. Investigation into monkeypox outbreak in England: technical briefing 1. 2022. https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-1 (accessed July 12, 2022).
- 11.Disease Outbreak News. [cited 2023 Jan 26]. Available from: https://www.who.int/emergencies/disease-outbreak-news.
- 12.CDC. Mpox in the U.S. Centers for Disease Control and Prevention. 2023 [cited 2023 Jan 26]. Available from: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html.
- 13.Scarpa F, Sanna D, Azzena I, Cossu P, Locci C, Angeletti S, et al. Genetic Variability of the Monkeypox Virus Clade IIb B.1. JCM. 2022 Oct 28;11(21):6388. doi: 10.3390/jcm11216388. https://www.mdpi.com/2077-0383/11/21/6388 [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO-Health Emergency Dashboard. [cited 2023 Jan 29]. Available from: https://extranet.who.int/publicemergency/
- 15.Prefeitura Municipal de São Paulo. Boletim Mpox da cidade de São Paulo, 2022 Dec 21. [cited in 20/12/2022]. Available from: https://www.prefeitura.sp.gov.br/cidade/secretarias/upload/saude/vigilancia_em_saude/boletim_mpox_21_12_2022.pdf.
- 16.Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance. 2022 Jun 2;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2022.27.22.2200421 [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022 Jul 28 doi: 10.1136/bmj-2022-072410. https://www.bmj.com/lookup/doi/10.1136/bmj-2022-072410 [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minhaj FS, Ogale YP, Whitehill F, Schultz J, Foote M, Davidson W, et al. Monkeypox Outbreak — Nine States, May 2022. MMWR Morb Mortal Wkly Rep. 2022 Jun 10;71(23):764–769. doi: 10.15585/mmwr.mm7123e1. http://www.cdc.gov/mmwr/volumes/71/wr/mm7123e1.htm?s_cid=mm7123e1_w [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogoina D, Izibewule JH, Ogunleye A, Ederiane E, Anebonam U, Neni A, et al. The 2017 human monkeypox outbreak in Nigeria—Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE. 2019 Apr 17;14(4) doi: 10.1371/journal.pone.0214229. https://dx.plos.org/10.1371/journal.pone.0214229 Ukwaja KN, editor. [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox Virus Infection in Humans across 16 Countries — April–June 2022. N Engl J Med. 2022 Aug 25;387(8):679–691. doi: 10.1056/NEJMoa2207323. http://www.nejm.org/doi/10.1056/NEJMoa2207323 [cited 2023 Jan 28]Available from: [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann C, Jessen H, Wyen C, Grunwald S, Noe S, Teichmann J, Krauss AS, Kolarikal H, Scholten S, Schuler C, Bickel M, Roll C, Kreckel P, Köppe S, Straub M, Klausen G, Lenz J, Esser S, Jensen B, Rausch M, Unger S, Pauli R, Härter G, Müller M, Masuhr A, Schäfer G, Seybold U, Schellberg S, Schneider J, Monin MB, Wolf E, Spinner CD, Boesecke C. Clinical characteristics of monkeypox virus infections among men with and without HIV: A large outbreak cohort in Germany. HIV Med. 2023;24(4):389–397. doi: 10.1111/hiv.13378. Epub 2022 Sep 4. PMID: 36059149. [DOI] [PubMed] [Google Scholar]
- 22.Silva MST, Coutinho C, Torres TS, Peixoto E, Ismério R, Lessa F, et al. Ambulatory and hospitalized patients with suspected and confirmed mpox: an observational cohort study from Brazil. The Lancet Regional Health - Americas. 2023;17 doi: 10.1016/j.lana.2022.100406. https://linkinghub.elsevier.com/retrieve/pii/S2667193X2200223X [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelo Kristina M, et al. Epidemiological and clinical characteristics of patients with monkeypox in the GeoSentinel Network: a cross-sectional study. The Lancet. Infectious diseases. 2023;23(2):196–206. doi: 10.1016/S1473-3099(22)00651-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarín-Vicente E.J., Alemany A., Agud-Dios M., Ubals M., Suñer C., Antón A., Arando M., Arroyo-Andrés J., Calderón-Lozano L., Casañ C., Cabrera J.M., Coll P., Descalzo V., Folgueira M.D., García-Pérez J.N., Gil-Cruz E., González-Rodríguez B., Gutiérrez-Collar C., Hernández-Rodríguez Á., López-Roa P., Mitjà O. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet (London, England) 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MJ, Cash-Goldwasser S, Marx GE, Schrodt CA, Kimball A, Padgett K, et al. Severe Monkeypox in Hospitalized Patients — United States, August 10–October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022 Nov 4;71(44):1412–1417. doi: 10.15585/mmwr.mm7144e1. http://www.cdc.gov/mmwr/volumes/71/wr/mm7144e1.htm?s_cid=mm7144e1_w [cited 2023 Jan 26]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitjà O, Alemany A, Marks M, et al. Mpox in people with advanced HIV infection: a global case series [published correction appears in Lancet. 2023 Apr 8;401(10383):1158] Lancet. 2023;401(10380):939–949. doi: 10.1016/S0140-6736(23)00273-8. [DOI] [PubMed] [Google Scholar]