Abstract
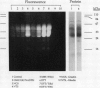
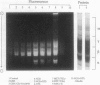
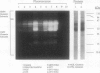
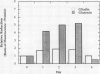
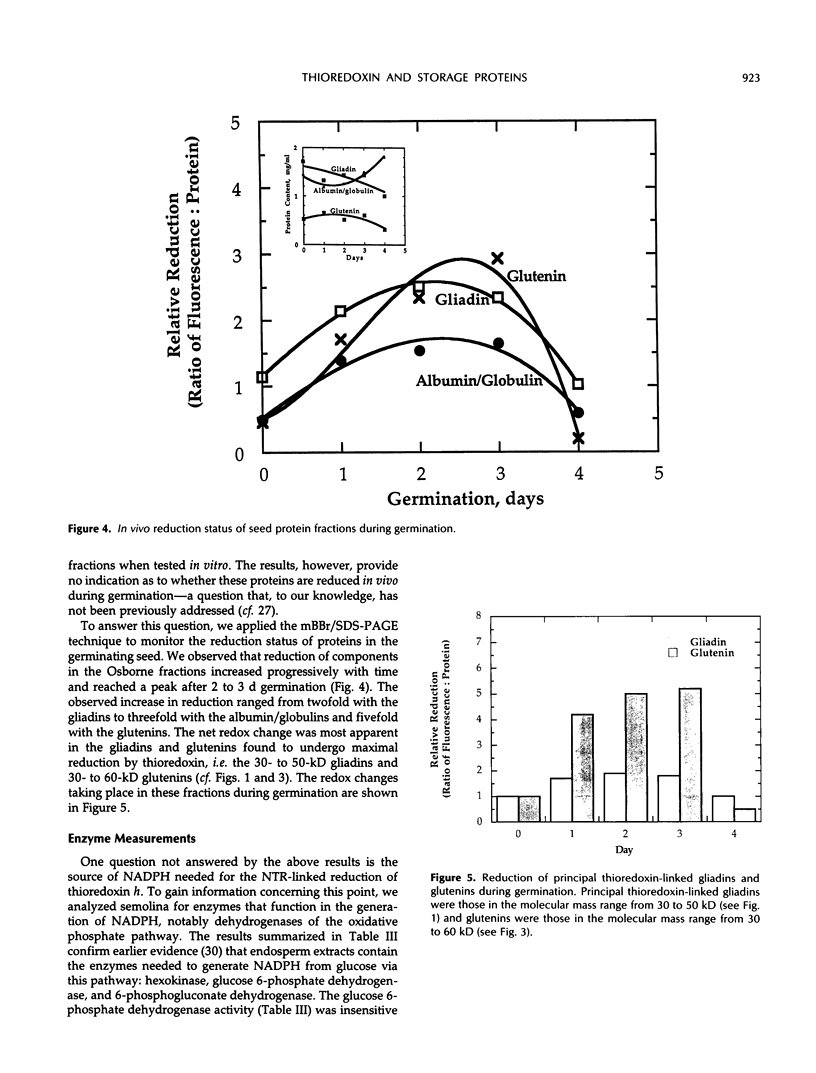
Gliadins and glutenins, the major storage proteins of wheat endosperm (Triticum durum, Desf. cv Monroe), were reduced in vitro by the NADP/thioredoxin system (NADPH, NADP-thioredoxin reductase and thioredoxin; in plants, the h type) from either the same source or the bacterium Escherichia coli. A more limited reduction of certain members of these protein groups was achieved with the reduced form of glutathione or glutaredoxin, a protein known to replace thioredoxin in certain bacterial and mammalian enzyme systems but not known to occur in higher plants. Endosperm extracts contained the enzymes necessary to reduce NADP by the oxidative pentose phosphate pathway (hexokinase, glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase). The gliadins and glutenins were also reduced in vivo during germination--an event that accompanied their proteolytic breakdown. The results suggest that thioredoxin, reduced by NADPH generated via the oxidative pentose phosphate pathway, functions as a signal in germination to enhance metabolic processes such as the mobilization of storage proteins and, as found earlier, the activation of enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodenstein-Lang J., Buch A., Follmann H. Animal and plant mitochondria contain specific thioredoxins. FEBS Lett. 1989 Nov 20;258(1):22–26. doi: 10.1016/0014-5793(89)81606-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991 Jul;288(1):1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Crawford N. A., Droux M., Kosower N. S., Buchanan B. B. Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys. 1989 May 15;271(1):223–239. doi: 10.1016/0003-9861(89)90273-7. [DOI] [PubMed] [Google Scholar]
- Fickenscher K., Scheibe R. Purification and properties of the cytoplasmic glucose-6-phosphate dehydrogenase from pea leaves. Arch Biochem Biophys. 1986 Jun;247(2):393–402. doi: 10.1016/0003-9861(86)90598-9. [DOI] [PubMed] [Google Scholar]
- Florencio F. J., Yee B. C., Johnson T. C., Buchanan B. B. An NADP/thioredoxin system in leaves: purification and characterization of NADP-thioredoxin reductase and thioredoxin h from spinach. Arch Biochem Biophys. 1988 Nov 1;266(2):496–507. doi: 10.1016/0003-9861(88)90282-2. [DOI] [PubMed] [Google Scholar]
- Johnson T. C., Cao R. Q., Kung J. E., Buchanan B. B. Thioredoxin and NADP-thioredoxin reductase from cultured carrot cells. Planta. 1987;171:321–331. [PubMed] [Google Scholar]
- Johnson T. C., Wada K., Buchanan B. B., Holmgren A. Reduction of purothionin by the wheat seed thioredoxin system. Plant Physiol. 1987 Oct;85(2):446–451. doi: 10.1104/pp.85.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss F., Wu M. X., Wong J. H., Balogh A., Buchanan B. B. Redox active sulfhydryls are required for fructose 2,6-bisphosphate activation of plant pyrophosphate fructose-6-phosphate 1-phosphotransferase. Arch Biochem Biophys. 1991 Jun;287(2):337–340. doi: 10.1016/0003-9861(91)90487-4. [DOI] [PubMed] [Google Scholar]
- Kobrehel K., Yee B. C., Buchanan B. B. Role of the NADP/thioredoxin system in the reduction of alpha-amylase and trypsin inhibitor proteins. J Biol Chem. 1991 Aug 25;266(24):16135–16140. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Marcus F., Chamberlain S. H., Chu C., Masiarz F. R., Shin S., Yee B. C., Buchanan B. B. Plant thioredoxin h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys. 1991 May 15;287(1):195–198. doi: 10.1016/0003-9861(91)90406-9. [DOI] [PubMed] [Google Scholar]
- Scheibe R., Geissler A., Fickenscher K. Chloroplast glucose-6-phosphate dehydrogenase: Km shift upon light modulation and reduction. Arch Biochem Biophys. 1989 Oct;274(1):290–297. doi: 10.1016/0003-9861(89)90441-4. [DOI] [PubMed] [Google Scholar]
- Scheibe R. Redox-modulation of chloroplast enzymes : a common principle for individual control. Plant Physiol. 1991 May;96(1):1–3. doi: 10.1104/pp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]