Abstract
Shigella spp. are the major cause of bacillary dysentery worldwide. To identify immune effectors associated with protection of the naive host during infection, the susceptibility to pulmonary Shigella infection of each of various mouse strains that have a targeted deletion in a specific aspect of the immune system was evaluated. Our results demonstrate that mice deficient in gamma interferon are 5 orders of magnitude more susceptible to Shigella than are wild-type mice, whereas mice deficient in B and T lymphocytes or in T lymphocytes alone exhibit no difference in susceptibility. Significantly lower numbers of shigellae were recovered from immunocompetent compared with gamma-interferon-deficient mice after infection. While immunocompetent mice were able to clear a sublethal Shigella inoculum by day 5 postinfection, progressively increasing numbers of shigellae were cultured from the lungs of gamma interferon-deficient mice over the same period. Histopathology of the lungs from immunocompetent mice infected with a sublethal Shigella inoculum showed mild inflammatory changes, whereas the lungs from gamma interferon-deficient mice demonstrated progressively worsening acute bronchiolitis with ulceration. Further, the time to death in gamma interferon-deficient mice correlates inversely with the size of the Shigella inoculum. To identify the cellular source of gamma interferon, we infected SCID mice, T-cell-receptor-deficient mice, beige mice (a mouse strain deficient in natural killer [NK] cell activity), and mice depleted of NK cells using anti-asialo-GM1. Each NK cell-deficient mouse strain exhibited a 10-fold-greater susceptibility to Shigella infection than immunocompetent mice. To test the protective effects of gamma interferon in vitro, survival of intracellular Shigella was examined in primary macrophages from wild-type mice, primary macrophages from gamma interferon-deficient mice, a macrophage cell line, and a fibroblast cell line. Following activation with gamma interferon, each cell type eradicated intracellular Shigella, while nonactivated macrophages fostered Shigella replication and nonactivated fibroblast cells fostered both Shigella replication and intercellular spread. Taken together, these data establish that NK cell-mediated gamma interferon is essential to resistance following primary Shigella infection.
Shigellosis is an invasive disease of the human intestinal tract that is responsible for 200 million cases of bacillary dysentery and 650,000 fatalities annually (15). The disease process is thought to involve bacterial translocation across M cells within the gastrointestinal tract, phagocytosis by macrophages with subsequent lysis of the phagosomes, and induction of apoptosis in infected macrophages with release of proinflammatory mediators (12, 26, 30, 31). Inflammation further promotes Shigella invasion and intercellular spread between gut epithelial cells, which leads to necrosis (20, 21). These processes are thought to account for the diarrhea and dysentery that occur in the course of shigellosis.
Immune mechanisms necessary for protection against shigellosis are poorly defined due to the lack of a convenient infection model. While both macrophage and epithelial cell lines can be infected with shigellae, the relative contributions of macrophage and epithelial-cell infection to pathogenesis are unclear. It is becoming increasingly evident that the host inflammatory response plays an important role in Shigella pathogenesis. Therefore, manipulable models which provide intact immune systems are necessary in order to elucidate the relationship between host cell infection, the inflammatory response following infection, and pathogenesis. Recently, a murine model of intranasal Shigella infection has been reported (16, 28). Following intranasal infection with a lethal dose of Shigella, mice die of an acute pneumonitis (28). Virulent shigellae are able to invade bronchial and alveolar epithelium and elicit both acute suppurative infiltrates and epithelial necrosis, which resembles the lesions seen in intestinal shigellosis (28, 29). In lung lavage fluid of intranasally infected mice, tumor necrosis factor alpha and gamma interferon levels peak at 24 h postinfection, suggesting activation of the Th1 type of T-helper-cell response (28). This is consistent with the intracytoplasmic residence of shigellae within infected cells. Mice inoculated intranasally with sublethal doses of wild-type Shigella or candidate Shigella vaccine strains show partial protective immunity to future intranasal challenges with a lethal Shigella inoculum (16, 28).
The immune mechanisms which mediate resistance in both primary Shigella infection and subsequent exposures remain undefined. To determine which components of the immune system confer protection against primary Shigella infection in vivo, the susceptibility of each of various strains of mice with a targeted deletion in a specific aspect of the immune system to intranasal Shigella infection was examined. Our results show that gamma interferon is required for protection from Shigella infection in vivo and that gamma interferon-mediated activation of host cells leads to killing of shigellae in primary macrophages from wild-type and gamma interferon-deficient mice as well as in macrophage- and fibroblast-derived cell lines in vitro.
MATERIALS AND METHODS
Mouse strains.
The immunocompetent mouse strains BALB/c and C57BL/6, T-cell-receptor-deficient mice (C57BL/6 tcrβ × tcrδ/tcrβ × tcrδ), and the natural killer (NK) cell-deficient mouse strain beige (C57BL/6 bgJ/bgJ) were obtained from Jackson Laboratory (Bar Harbor, Maine). Immunocompetent 129SvEv mice were obtained from Taconic (Germantown, N.Y.). Mice with severe combined immunodeficiency (BALB/c scid/scid) were obtained from Harris Goldstein. Major histocompatibility complex class II-deficient (Aβ0−/−), major histocompatibility complex class I-deficient (homozygously negative for β2-microglobulin [β2M−/−]) and gamma interferon knockout (GKO−/−) mice, which were constructed in the C57BL/6 × 129SvEv background and had been backcrossed several times onto the C57BL/6 background, were obtained from Barry R. Bloom. To confirm that the mixed-strain background of these mice would not confound our results, we examined the susceptibilities of the stem cell parental 129SvEv mouse strain (Taconic), homozygous GKO+/+ littermates of GKO−/− mice that had been maintained by sibling matings, and gamma interferon-deficient mice on the C57BL/6 genetic background (Jackson Laboratory) as described in Results.
Mice were between 5 and 7 weeks of age at the time of infection and were sex matched between groups. Mice were housed in a pathogen-free environment at the Albert Einstein College of Medicine. Care of animals was in accordance with guidelines set by the American Association for Accreditation of Laboratory Animal Care and the Albert Einstein College of Medicine.
Bacteria.
The wild-type Shigella flexneri serotype 2a strain 2457T and its virulence plasmid-cured derivative, BS103, have been described (17). Strains were routinely grown in tryptic soy broth.
Cytokines.
Recombinant murine, rat, and human gamma interferons were obtained from Gibco BRL (Grand Island, N.Y.).
Mouse infections.
For mouse infections, S. flexneri was subcultured from an overnight culture to mid-exponential phase (optical density at 600 nm, 0.5 to 1.0), washed with phosphate-buffered saline (PBS), and adjusted to the appropriate concentration prior to inoculation. Numbers of bacteria per inoculum were confirmed by plating serial dilutions of the inoculum. To determine the lethal dose of Shigella, groups of 4 to 10 mice of each strain were inoculated intranasally with serial 10-fold dilutions of the wild-type S. flexneri serotype 2a strain 2457T as described previously (28). Briefly, mice were anesthetized intramuscularly with a mixture of 0.6 mg of ketamine hydrochloride (Ketaset; Aveco Co., Fort Dodge, Iowa) and 0.18 mg of xylazine hydrochloride (Rompun; Mobay Corp., Shawnee, Kans.) in 100 μl of saline. An inoculum of Shigella resuspended in 30 μl of saline was then introduced dropwise into the nares. Mice were examined 2 to 4 h after infection to confirm recovery from the anesthetic and were monitored daily thereafter for survival. Saline controls were included in all experiments to control for mortality and morbidity that might occur as a result of the experimental technique. To quantitate the effects of gamma interferon on Shigella eradication in vivo, C57BL/6 and GKO−/− mice were infected with 105 CFU of 2457T. At days 1, 3, and 5 postinfection, lungs from these mice were surgically removed and homogenized. Dilutions of lung lysates were plated onto trypic soy agar containing Congo red (0.01%).
NK cell depletion.
In vivo depletion of NK cells was achieved by intravenous injection of 0.2 ml of PBS containing 40 μl (39 μg/ml) of rabbit anti-asialo-GM1 antiserum (Wako BioProducts) at 2 days prior to infection and at 1 and 4 days postinfection as described by the manufacturer. To verify that differences in susceptibilities observed following NK cell depletion were not due to nonspecific effects of rabbit antibody, intravenous injection of 0.2 ml of PBS containing 40 μl (40 μg/ml) of rabbit immunoglobulin G (Sigma Chemical Co.) was used as a control.
Cell lines.
Primary bone marrow-derived macrophages were harvested from the femurs of GKO−/− and C57BL/6 mice and cultured in Dulbecco’s modified Eagle medium supplemented with 20% fetal calf serum (FCS) and 30% L929 cell supernatant for at least 7 days prior to infection. Mouse macrophage J774 cells, rat lung fibroblast L2 cells, human cervical epithelioid carcinoma HeLa cells, and mouse areolar connective tissue L929 cells (L cells) were obtained from the American Type Culture Collection. J774 cells were maintained in Dulbecco’s modified Eagle medium supplemented with 10% FCS and 5% NCTC-109. L2, HeLa, and L cells were maintained in minimal essential medium (MEM) supplemented with 10% FCS and 1× nonessential amino acids.
In vitro infections.
For infections of all cell lines, shigellae, diluted in MEM, were centrifuged (700 × g) onto monolayers of adherent cells within tissue culture dishes for 10 min at 30°C. For infection of primary bone marrow macrophages, 2.4 × 105 CFU of 2457T were used to infect 2.2 × 105 to 2.4 × 105 cells seeded in 48-well tissue culture plates 12 h prior to infection. For infection of J774 cells, 1.25 × 106 to 1.50 × 106 CFU of 2457T were used to infect a confluent 35-mm-diameter petri dish of cells. At 15 min postcentrifugation, the bacterial suspension was replaced with DMEM supplemented with FCS (10%), NCTC 109 (5%), and gentamicin (50 μg/ml). Gentamicin kills extracellular but not intracellular shigellae. To quantitate the number of viable intracellular shigellae, adherent cells were washed three times with MEM at the indicated time points and lysed with 0.1% sodium deoxycholate in saline. Serial dilutions of the cell lysate were then plated onto tryptic soy agar containing Congo red (0.01%).
For infection of fibroblast cells, 5.0 × 105 to 7.0 × 105 CFU of 2457T were used to infect a confluent monolayer within a 35-mm petri dish. At 80 min postcentrifugation, the bacterial suspension was replaced with MEM supplemented with FCS (10%) and gentamicin (25 μg/ml). Quantitation of viable shigellae in fibroblast cell lines was carried out for 15 h postinfection, whereas quantitation in macrophage cells was carried out for only 6 h, since Shigella induces apoptosis of macrophages beginning at approximately 3 h postinfection (31); Shigella infection is not known to induce apoptosis of fibroblast cells. Assays of Shigella plaque formation on fibroblast cell monolayers were performed as previously described (19). Cells were examined for the formation and size of bacterial plaques at 24 and 48 h postinfection.
For experiments involving gamma interferon activation of primary macrophages, as well as macrophage and fibroblast cell lines, species-specific gamma interferon was added to a final concentration of 100 U/ml to the media in which cells were maintained both overnight prior to infection and during all steps of the infection.
Statistics.
The statistical significance of data was determined by the independent Student t test, with a P value of <0.05 being taken as significant.
RESULTS
Susceptibility of different immune knockout mouse strains to primary Shigella infection.
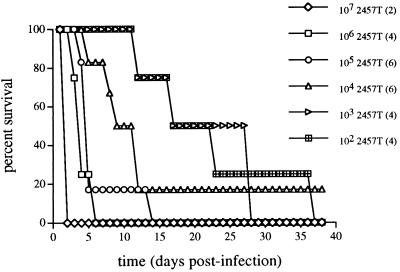
Mice with targeted deletions in specific aspects of the immune system are valuable tools to study the immune effectors necessary for protection against various infectious agents (4, 10, 13, 14). To assess which immune cells or factors are important in protection following primary Shigella infection, the susceptibility of various immune-deficient mouse strains to intranasal infection was determined (Fig. 1). The lethal dose of S. flexneri for both C57BL/6 and BALB/c immunocompetent mice is 107 CFU, a finding consistent with the previously reported 50% lethal dose of 107 CFU for strain 2457T in BALB/c mice (16, 28). Further, Shigella organisms killed prior to inoculation by exposure to UV light or the virulence-plasmid-cured derivative of 2457T, strain BS103 (17), which is unable to invade nonphagocytic cells, do not cause morbidity in C57BL/6 mice (data not shown).
FIG. 1.
Susceptibilities of different mouse strains to intranasal Shigella infection. Age- and sex-matched groups of 4 to 10 mice were infected with serial 10-fold dilutions of wild-type S. flexneri 2457T in saline or with saline alone as described in Materials and Methods. C57BL/6 anti-asialo-GM1 are mice depleted of NK cells as described in the text. Reported lethal doses represent the Shigella inoculum that caused ≥90% mortality.
Gamma interferon-deficient mice (GKO−/−) exhibited susceptibility to Shigella infection that was increased by 5 orders of magnitude, whereas no differences in susceptibility were observed in mice that were β2M−/−, Aβ0−/−, homozygously negative for β and δ T-cell-receptor subunits (TCRβ−/−δ−/−), or SCID compared with C57BL/6 or BALB/c mice (Fig. 1). To confirm that the observed difference in susceptibility of the GKO−/− mice was not due to mouse strain background differences, we examined the susceptibilities of the stem cell parental 129SvEv mouse strain and of the homozygous GKO+/+ littermates of GKO−/− mice having the background of the animals used in subsequent studies presented here that had been maintained by sibling matings. Each of these mouse strains has the same susceptibility as C57BL/6 mice (data not shown). Further, gamma interferon-deficient mice crossed extensively into the C57BL/6 genetic background have the same increased susceptibility as GKO−/− mice used in this study.
To determine the contribution of NK cell-produced gamma interferon to protection, we examined the susceptibilities of the NK cell-deficient mouse strain beige and C57BL/6 mice depleted of NK cells by using rabbit anti-asialo-GM1 antiserum. Each NK cell-deficient mouse strain had a 10-fold-increased susceptibility to Shigella infection compared with immunocompetent mice (Fig. 1), whereas no difference in susceptibility was observed in mice treated with rabbit serum immunoglobulin G.
Effect of gamma interferon on Shigella survival in vivo.
To determine whether differences in susceptibility between mouse strains correlate with the ability of the mice to clear the infection, the number of viable Shigella organisms present within the lungs of infected C57BL/6 and GKO−/− mice was quantitated at various time points following infection with 105 CFU of wild-type Shigella (Table 1). Significantly increased numbers of viable shigellae were recovered from the lungs of GKO−/− mice as compared with C57BL/6 mice at days 1, 3, and 5 postinfection (P < 0.05, GKO−/− versus C57BL/6 mice at each time point). A 3,600-fold decrease was observed in the numbers of shigellae in C57BL/6 mice from day 1 to day 5 postinfection (P < 0.05). The progressive fall in the number of shigellae recovered from C57BL/6 mice, beginning at inoculation and continuing over time, indicates that in the presence of gamma interferon, killing begins upon infection and clearance of the infection is achieved by day 5 postinfection for a Shigella inoculum 2 logs below the lethal dose. Following infection with the same dose, there was a fivefold increase in the number of shigellae recovered from the lungs of GKO−/− mice (P < 0.05) within the same period. These data indicate that the role of gamma interferon in mediating protection from Shigella infection is in the promotion of Shigella clearance. Since a significant percentage of GKO−/− mice infected with 105 CFU of 2457T die by day 5 postinfection (see Fig. 3), GKO−/− mice that survived to day 5 are more likely to be better able to survive; thus, the number of viable shigellae isolated from the lungs of these animals probably represents an underestimate of the number that theoretically would have been seen at this time point.
TABLE 1.
Survival of Shigella within the lungs of C57BL/6 immunocompetent mice or GKO−/− gamma interferon-deficient mice after infection with 105 CFU of wild-type Shigella strain 2457T
| Day postinfection | No. of viable shigellae per mouse in strain:
|
P value | |
|---|---|---|---|
| C57BL/6 | GKO−/− | ||
| 1 | 1.4 × 104 | 1.8 × 105 | 0.0004 |
| 3 | 7.8 × 101 | 2.7 × 105 | 0.013 |
| 5 | 4.0 × 100 | 9.6 × 105 | 0.0016 |
FIG. 3.
Time to death in gamma interferon-deficient mice following infection with a lethal dose of S. flexneri wild-type strain 2457T (102 CFU) and serial 10-fold increases above the lethal dose. The number of mice infected per dose is indicated in parentheses.
Histopathology of infected gamma interferon-deficient and wild-type mice.
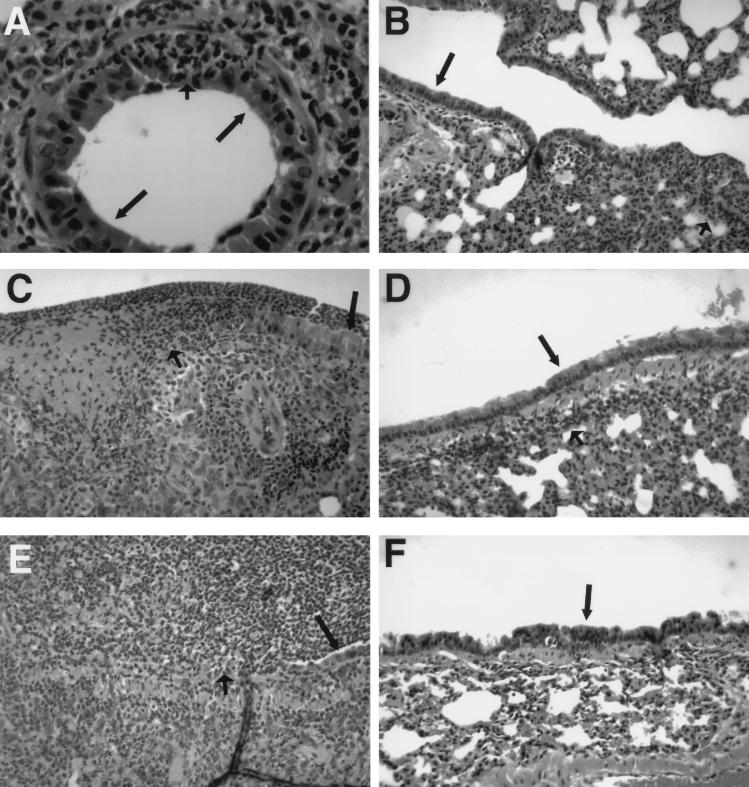
The increased susceptibility of GKO−/− versus C57BL/6 mice to intranasal Shigella infection is supported by histopathologic examination. After infection with 105 organisms, GKO−/− mice show early acute bronchiolitis at day 1 postinfection (Fig. 2A), followed by multifocal mucosal ulceration and intense mixed inflammatory infiltrate by day 3 (Fig. 2C). This progresses to nearly complete mucosal ulceration with exuberant acute neutrophilic exudate filling airway lumina by day 5 (Fig. 2E). In contrast, C57BL/6 animals infected with the same-size Shigella inoculum show a mild peribronchiolar and interstitial inflammatory infiltrate without mucosal ulceration beginning at day 1 postinfection (Fig. 2B). By day 3 postinfection, the chronic inflammation is mildly increased (Fig. 2D). This inflammation is completely resolved by day 5 (Fig. 2F). The histopathology observed in GKO−/− mice following intranasal Shigella infection with 105 organisms shows a progression that is similar to histopathologic changes previously observed in immunocompetent mice following administration of a lethal dose of Shigella (28).
FIG. 2.
Hematoxylin-and-eosin-stained sections of lung from GKO−/− (A, C, and E) and C57BL/6 (B, D, and F) mice after infection with a 105-CFU inoculum of S. flexneri. (A) On day 1, bronchioles show early acute inflammation (short arrow) with no disruption in bronchiolar epithelium (long arrows). (C) On day 3, bronchiolar epithelium (long arrow) shows ulceration (short arrow) with polymorphonuclear cell exudate, fibrin, and intense acute and chronic inflammation in the bronchiolar wall. (E) On day 5, near-complete epithelial destruction is evident with residual epithelium (long arrow). The luminal space is filled with polymorphonuclear cells, with obstruction of the airway (short arrow). (B) On day 1, a longitudinal section of terminal bronchiole shows absence of acute bronchiolitis. A mild acute and chronic lymphocytic pneumonitis is present (long arrow, bronchiolar epithelium). (D) On day 3, mild peribronchiolar chronic lymphocytic inflammation (short arrow) is seen adjacent to intact bronchiolar epithelium (long arrow). (F) On day 5, inflammatory infiltrate is resolved (long arrow, bronchiolar epithelium). Magnifications: ×80 (A) and ×30 (B to F).
Time to death in gamma interferon-deficient mice following infection with various Shigella inocula.
To determine whether lethality as determined by the time to death correlates with size of the Shigella inoculum, we examined the time to death in gamma interferon-deficient mice following infection with 10-fold increases in bacterial inoculum above the lethal dose (Fig. 3). The time to death in gamma interferon-deficient mice was observed to correlate inversely with the Shigella inoculum size. The average time to death following infection with 106 CFU of Shigella was 3.3 ± 1.2 (mean ± standard deviation) days compared with 21.3 ± 10.8 days after infection with 102 CFU (P < 0.05). These data suggest that lethality in mice caused by Shigella is dose dependent.
Effect of gamma interferon activation on Shigella intracellular survival in bone marrow-derived macrophages from GKO−/− and C57BL/6 mice.
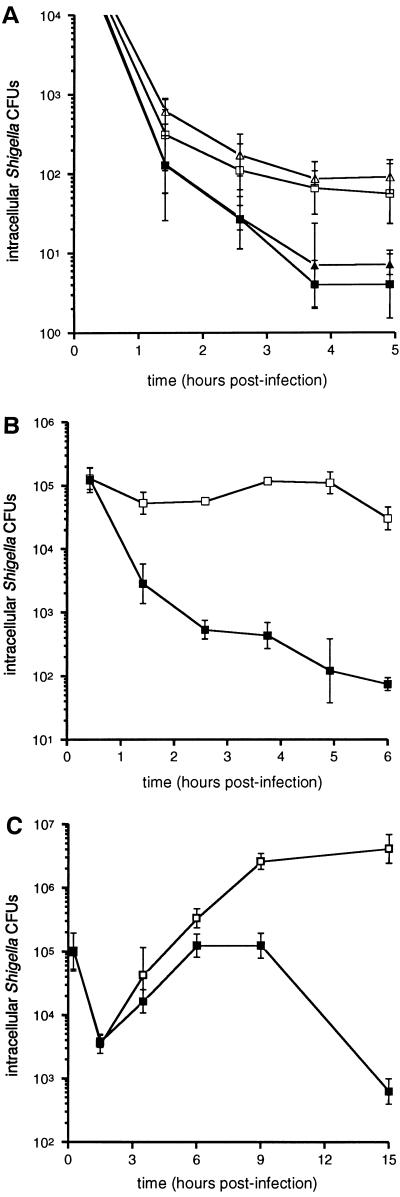
To determine whether the in vivo difference observed between GKO−/− and C57BL/6 mice correlates with the effects of gamma interferon activation of primary macrophages obtained from these mice in vitro, the effects of gamma interferon pretreatment on the intracellular viability of Shigella in bone marrow-derived macrophages was evaluated. Beginning at 2.5 h postinfection and at all time points observed thereafter, significant differences in intracellular Shigella survival were observed between gamma interferon-activated and nonactivated macrophages derived from either C57BL/6 or GKO−/− mice (Fig. 4A). No significant differences in Shigella survival were observed in macrophages from C57BL/6 and GKO−/− mice under either condition.
FIG. 4.
(A) Intracellular survival of Shigella in bone marrow-derived macrophages from C57BL/6 (squares) and GKO−/− mice (triangles). Cells were either not activated (open symbols) or activated (solid symbols) with 100 U of gamma interferon per ml. Each datum point represents the mean of six independent determinations. (B) Intracellular survival of Shigella within J774 macrophagelike cells with (solid squares) or without (open squares) activation with gamma interferon (100 U/ml). Each datum point represents the mean of five independent determinations. (C) Intracellular survival of Shigella within L2 cells with (solid squares) or without (open squares) activation with gamma interferon (100 U/ml). Each datum point represents the mean of four independent determinations. Bars indicate one standard deviation.
Effect of gamma interferon activation of J774 cells on intracellular Shigella survival.
To further evaluate the effects of gamma interferon activation of cells in vitro, the intracellular viability of Shigella in the J774 macrophage cell line was examined (Fig. 4B). Beginning at the first hour postinfection and at all time points observed thereafter, significantly lower numbers of intracellular shigellae were recovered from cells activated with gamma interferon as compared with cells not activated. In nonactivated cells, a significant (twofold) increase (P < 0.05) in the numbers of intracellular shigellae was observed between 3 and 5 h postinfection, demonstrating the ability of Shigella to replicate within these cells.
Effects of gamma interferon activation of fibroblast cells on Shigella plaque formation and intracellular survival.
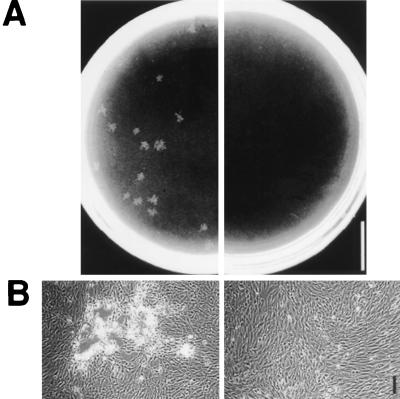
The ability to spread from cell to cell is critical to Shigella pathogenesis (3, 27). The intercellular spread of Shigella can be assessed by its ability to form plaques on confluent monolayers of cells in tissue culture. To evaluate the effects of gamma interferon activation of cells on their ability to foster Shigella intercellular spread, confluent monolayers of L2, HeLa, and L cells were pretreated with recombinant rat, human, and mouse gamma interferon, respectively, and assessed for bacterial plaque formation following Shigella infection. In all cell lines tested, no bacterial plaques were formed in cells activated with species-specific gamma interferon, whereas bacterial plaques were consistently formed in cells either not activated with gamma interferon (Fig. 5) or activated with gamma interferon from another species (data not shown).
FIG. 5.
Effects of gamma interferon activation on the ability of Shigella to spread from cell to cell. Bacterial plaque formation in L2 cell monolayers at 48 h postinfection with (right panels) or without (left panels) activation by 100 U of gamma interferon per ml. Bars: 1 cm (A) and 100 μm (B).
To determine whether the inability of Shigella to form plaques in gamma interferon-pretreated cells is due to decreased cellular invasion or to increased intracellular Shigella killing, we quantitated the numbers of viable intracellular shigellae from infected L2-cell monolayers that had been pretreated or not pretreated with gamma interferon. Beginning at 6 h postinfection and at all time points observed thereafter, significantly lower numbers of shigellae were recovered from cell monolayers activated with gamma interferon as compared with cells not activated (Fig. 4C). Notably, there were no significant differences in the numbers of recoverable shigellae at any time point prior to 6 h postinfection (Fig. 4C). Thus, gamma interferon activation of L2 fibroblast cells leads to increased killing of intracellular shigellae.
To confirm that the decreased numbers of intracellular Shigella in gamma interferon-activated cells is not due to the effect of decreased plasma membrane integrity or increased cell death, we examined the ability of gamma interferon-activated cells to exclude the dye trypan blue at 15, 24, and 48 h postinfection. At all time points, gamma interferon-activated cells demonstrated trypan blue exclusion to the same extent as uninfected monolayers or monolayers not activated with gamma interferon (data not shown).
DISCUSSION
This study establishes the essential requirement for gamma interferon in host protection against primary Shigella infections. Immune knockout mice lacking this cytokine exhibit an increase of 5 orders of magnitude in susceptibility to Shigella infection compared with mice not lacking this cytokine. The deleterious effects of gamma interferon deficiency could be counteracted in vitro by providing exogenous gamma interferon to infected primary macrophages from either wild-type or gamma interferon-deficient mice or to infected macrophage or fibroblast cell lines. Following infection, significant Shigella replication occurs in each of these cell types in the absence of gamma interferon, as well as in the lungs of gamma interferon-deficient mice. In contrast, isolated primary macrophages and macrophage and fibroblast cell lines activated with gamma interferon are able to promote killing of Shigella. In a similar fashion, Shigella is readily cleared from the lungs of immunocompetent mice and is not cleared from the lungs of gamma interferon-deficient mice. These data, taken together with the observation that the time to death following infection in gamma interferon-deficient mice decreases with increasing doses of Shigella, support the requirement for Shigella replication in pathogenesis and establish that the protective role of gamma interferon is to promote clearance of intracellular Shigella.
Consistent with these results are previously reported data that have suggested that gamma interferon plays a protective role following Shigella infection. Examination of rectal biopsy specimens has demonstrated increased levels of gamma interferon cytokine and mRNA, as well as increased expression of the gamma interferon receptor, in shigellosis patients compared with healthy controls. In shigellosis patients, the expression of gamma interferon is twofold higher during convalescence than during the acute stage (22, 23).
The two known cellular sources of gamma interferon are T cells and NK cells (1, 7). In the present study, mice with complete T-cell deficiency (TCRβ−/−δ−/− and SCID), deficiency in specific subsets of T cells (β2M−/− and Aβ0−/−), or NK cell deficiency (beige), as well as C57BL/6 mice depleted of NK cells by using anti-asialo-GM1, were examined to determine the cellular source of gamma interferon necessary for resistance. Our data suggest that NK cells are the major source of gamma interferon following primary Shigella infection, since a 10-fold increase in susceptibility was observed in both the NK cell-deficient mouse strain (beige) and the NK cell-depleted mice relative to the wild-type mice, whereas no difference in susceptibility was observed in T-cell-deficient mice. These findings are consistent with previous data demonstrating NK cells to be the major source of gamma interferon following primary infection with another intracellular pathogen, Listeria monocytogenes (1, 7). Depletion of NK cells in vivo using both monoclonal and polyclonal antibodies to NK cell antigens led to exacerbation of Listeria infection, whereas depletion of T cells had no effect (7). In these studies, as in the present study, the observation that gamma interferon-depleted mice are more susceptible to bacterial infection than wild-type mice experimentally depleted of NK cells suggests that antibody depletion of NK cells is incomplete (7). In the present study, mice with NK cell deficiency (beige) demonstrate the same increased susceptibility to Shigella infection as mice experimentally depleted of NK cells. The observation that beige mice are more resistant to Shigella infection than gamma interferon-deficient mice is likely a result of the previously observed leakiness in NK cell deficiency in the beige mouse (24, 25).
In this study, the essential role of gamma interferon in the clearance of intracellular Shigella from bone marrow-derived mouse macrophages, a mouse macrophage cell line, and human, rat, and mouse fibroblast cell lines is definitively demonstrated. The role of gamma interferon in the activation of macrophages to kill other intracellular pathogens has been described extensively (2, 8, 9); the induction of reactive oxygen and nitrogen intermediates is felt to mediate this effect (6). In contrast, while the role of gamma interferon in the activation of fibroblast killing of intracellular pathogens has been described, the mechanism of this effect has not been well characterized (5). We have demonstrated that reactive nitrogen intermediates do not mediate this effect in either macrophages or fibroblasts (unpublished data). Further studies will be performed to evaluate the mechanism of gamma interferon induction of Shigella killing by macrophage and fibroblast cells.
Two previous studies have suggested that gamma interferon may alter the course of Shigella infection in activated HeLa, FS-1, or primary rabbit kidney cells (11, 18). By measuring 3H incorporation following actinomycin D inhibition of host cell transcription and then counting the number of intracellular bacteria within infected cells, Gober et al. (11) concluded that interferons are able to suppress Shigella intracellular growth. However, this study examined the numbers of intracellular shigellae only at 7 h postinfection. Since actinomycin D inhibits host cell transcription, signaling by gamma interferon would also be expected to be inhibited, making interpretation of this result difficult. Using an agarose overlay procedure over infected cell monolayers, Niesel et al. have found evidence that treatment with gamma interferon decreases Shigella invasiveness (18). In this study, the quantitation of the numbers of focal areas of bacterial infection was performed at 60 min postinfection; thus, the observed reduction in the number of Shigella colonies from intracellular bacteria could reflect a decreased intracellular survival of Shigella within the first hour postinfection equally well as a decrease in the efficiency of invasion. Our data clearly demonstrate that the efficiency of invasion is not altered by pretreatment of cells with gamma interferon, since there were no significant differences in the numbers of recoverable intracellular Shigella at any time point prior to 6 h postinfection, but that Shigella intracellular survival in gamma interferon-activated cells is dramatically curtailed at later time points postinfection. These data, taken together with the marked increased susceptibility of gamma interferon-deficient mice compared with that of immunocompetent mice, establish that gamma interferon is essential to host resistance to primary Shigella infection.
ACKNOWLEDGMENTS
We thank B. R. Bloom, J. Chan, and L.-A. Pirofski for helpful discussions, L.-A. Pirofski for critically reviewing the manuscript, and B. R. Bloom and H. Goldstein for their generous contribution of mice.
This work was supported by NIH grants T32 GM07288 (S.S.W.) and AI35817 (M.B.G.), a Pew Scholars Award in Biomedical Sciences (M.B.G.), and Established Investigator and Grant-in-Aid awards (M.B.G.) from the American Heart Association.
REFERENCES
- 1.Bancroft G J, Sheehan K C F, Schreiber R D, Unanue E R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- 2.Beckerman K P, Rogers H W, Corbett J A, Schreiber R D, McDaniel M L, Unanue E R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. J Immunol. 1993;150:888–895. [PubMed] [Google Scholar]
- 3.Bernardini M, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti P. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalton D, Pitts-Meek S, Keshav S, Figari I, Bradley A, Stewart T. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 5.Darnell J, Jr, Kerr I, Stark G. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 6.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 7.Dunn P L, North R J. Early gamma interferon protection by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2990. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flesch I E, Kaufmann S H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flesch I E, Kaufmann S H. Role of cytokines in tuberculosis. Immunobiology. 1993;189:316–339. doi: 10.1016/S0171-2985(11)80364-5. [DOI] [PubMed] [Google Scholar]
- 10.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gober L L, Friedman-Kien A E, Havell E A, Vilcek J. Suppression of the intracellular growth of Shigella flexneri in cell cultures by interferon preparations and polyinosinic-polycytidylic acid. Infect Immun. 1972;5:370–376. doi: 10.1128/iai.5.3.370-376.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale T L. Shigella vaccines. In: Ala’Aldeen D A A, Hornaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. New York, N.Y: John Wiley & Sons, Ltd.; 1995. pp. 179–204. [Google Scholar]
- 13.Harty J, Bevan M. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 15.Katz S. New vaccine development establishing priorities, disease of importance in developing countries. Washington, D.C: National Academy Press; 1986. [PubMed] [Google Scholar]
- 16.Mallett C, Van De Verg L, Collins H, Hale T. Evaluation of Shigella vaccine safety and efficacy in an intranasal challenged mouse model. Vaccine. 1993;11:190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- 17.Maurelli A T, Baudry B, d’Hauteville H, Hale T L, Sansonetti P J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niesel D W, Hess C B, Cho Y J, Klimpel D K, Klimpel G R. Natural and recombinant interferons inhibit epithelial cell invasion by Shigella spp. Infect Immun. 1986;52:828–833. doi: 10.1128/iai.52.3.828-833.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oaks E V, Wingfield M E, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perdomo J J, Gounon P, Sansonetti P J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perdomo O J J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;1994:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raqib R, Ljungdahl A, Lindberg A A, Andersson U, Andersson J. Local entrapment of interferon-gamma in the recovery from Shigella dysenteriae type 1 infection. Gut. 1996;38:328–336. doi: 10.1136/gut.38.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raqib R, Ljungdahl A, Lindberg A A, Wretlind B, Andersson U, Andersson J. Dissociation between cytokine mRNA expression and protein production in shigellosis. Eur J Immunol. 1996;26:1130–1138. doi: 10.1002/eji.1830260526. [DOI] [PubMed] [Google Scholar]
- 24.Roder J, Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979;278:451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- 25.Roder J C. The beige mutation in the mouse. J Immunol. 1979;123:2168–2173. [PubMed] [Google Scholar]
- 26.Sansonetti, P. J. 1991. Genetic and molecular basis of epithelial cell invasion by Shigella species. Rev. Infect. Dis. 13(Suppl. 4):S285–S295. [DOI] [PubMed]
- 27.Sansonetti P J, Arondel J, Fontaine A, d’Hauteville H, Bernardini M L. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 28.Van De Verg L L, Mallett C P, Collins H H, Larsen T, Hammack C, Hale T L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63:1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voino-Yasenetsky M V, Voino-Yasenetsky M K. Experimental pneumonia caused by bacteria of the Shigella group. Acta Morphol Acad Sci Hung. 1962;11:439–454. [PubMed] [Google Scholar]
- 30.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin-1 is released by macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]