Abstract
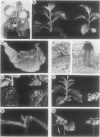
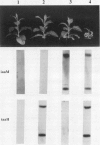
Transgenic tobacco (Nicotiana tabacum) SR1 plants expressing the Agrobacterium tumefaciens nopaline transferred DNA iaaH gene were transformed with a 35S-iaaM construct. The transformants displayed several morphological aberrations, such as adventitious root formation on stem and leaves, dwarfism, epinastic leaf growth, increased apical dominance, and an overall retardation in development. In addition, xylem lignification was higher than in wild type. Free and conjugated indoleacetic acid (IAA) levels were quantified by gas chromatography-multiple ion monitoring-mass spectrometry in leaves and internodes of wild-type plants and two transformed lines with different phenotypes. Both transformed lines contained elevated levels of free and conjugated IAA, which was associated with increased transcription of the iaaM gene. The line with the highest IAA level also had the most altered pattern of growth and development. These IAA-overproducing plants will provide a model system for studies on IAA metabolism, IAA interactions with other phytohormones, and IAA roles in regulating plant growth and development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M., Miller C. O. Hormonal control of tobacco crown gall tumor morphology. Plant Physiol. 1982 Feb;69(2):389–392. doi: 10.1104/pp.69.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feung C. S., Hamilton R. H., Mumma R. O. Metabolism of Indole-3-Acetic Acid: III. Identification of Metabolites Isolated from Crown Gall Callus Tissue. Plant Physiol. 1976 Nov;58(5):666–669. doi: 10.1104/pp.58.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn A. G., Clarke L. E., Pearson L., White J. The role of cytosine methylation in the control of nopaline synthase gene expression in a plant tumor. J Mol Appl Genet. 1983;2(3):315–329. [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Primig M., Trnovsky J., Matzke A. J. Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J. 1989 Mar;8(3):643–649. doi: 10.1002/j.1460-2075.1989.tb03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C. P., Hein M. B., Klee H. J. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 1991 Mar;5(3):438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Sitbon F., Sundberg B., Olsson O., Sandberg G. Free and Conjugated Indoleacetic Acid (IAA) Contents in Transgenic Tobacco Plants Expressing the iaaM and iaaH IAA Biosynthesis Genes from Agrobacterium tumefaciens. Plant Physiol. 1991 Feb;95(2):480–485. doi: 10.1104/pp.95.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigocki A. C., Owens L. D. Cytokinin-to-Auxin Ratios and Morphology of Shoots and Tissues Transformed by a Chimeric Isopentenyl Transferase Gene. Plant Physiol. 1989 Nov;91(3):808–811. doi: 10.1104/pp.91.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Reeves S., Thomashow M. F. Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes synthesis of indoleacetic acid. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5071–5075. doi: 10.1073/pnas.81.16.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Hugly S., Buchholz W. G., Thomashow L. S. Molecular basis for the auxin-independent phenotype of crown gall tumor tissues. Science. 1986 Feb 7;231(4738):616–618. doi: 10.1126/science.3511528. [DOI] [PubMed] [Google Scholar]