Abstract
Background and Purpose
Stress-related disorders are often intertwined with alcohol use disorder (AUD). The endocannabinoid (eCB) system, which comprises the lipid mediators anandamide (AEA) and 2-arachidonoylglycerol (2-AG), plays an important homeostatic role in the regulation of stress circuits and has emerged as a therapeutic target to treat stress disorders and AUD. Extensive research has elucidated the role of AEA, but less is known about 2-AG-mediated signaling.
Experimental Approach
We pharmacologically enhanced the eCB signaling by inhibiting the 2-AG metabolizing enzyme, monoacylglycerol lipase (MAGL), in male and female Marchigian-Sardinian alcohol preferring (msP) rats, a model of innate alcohol preference and stress hypersensitivity, and control Wistar rats. We tested the acute effect of the selective MAGL inhibitor MJN110 in alleviating symptoms of alcohol drinking, anxiety, irritability, and fear in both male and female rats.
Key Results
A single systemic administration of MJN110 increased 2-AG levels in the central amygdala, prelimbic, and infralimbic cortex, but did not acutely alter alcohol drinking. MAGL inhibition reduced aggressive behaviors in female msPs and increased defensive behaviors in male msPs, during the irritability test. Moreover, in the novelty-induced hypophagia test, MJN110 selectively enhanced palatable food consumption in females, mitigating stress-induced food suppression. Lastly, msP rats showed increased conditioned fear behavior compared to Wistar rats, and MJN110 reduced context-associated conditioned fear responses, but not cue-probed fear expression, in male msPs.
Conclusion and Implications
Acute inhibition of MAGL attenuated some stress-related responses in msP rats but not voluntary alcohol drinking. Our results provide new insights into the sex dimorphism documented in stress-induced responses and suggest that sex-specific endocannabinoid-based approaches should be considered in the clinical development of therapeutics.
Keywords: Endocannabinoid system, 2-AG, monoacylglycerol lipase, stress, alcohol use disorder, sex differences
1. INTRODUCTION
Stress-related disorders, including anxiety disorders and post-traumatic stress disorder (PTSD), affect over 30% of adults in the United States and represent one of the most prevalent mental health morbidities in the world (Kessler et al., 2012). Symptoms of these disorders include hyperarousal, sleep disorders, excessive fear response even in the absence of an immediate threat, and irritability (Ressler et al., 2022). Additionally, stress-related disorders are often intertwined with, and possibly a risk factor for, substance use disorders such as alcohol use disorder (AUD). AUD is often co-diagnosed with anxiety and stress disorders, whereby alcohol is used for self-medication purposes to alleviate anxiety, distress, and negative affect state. In AUD patients alcohol intake progressively increases, initiating a vicious feed-forward cycle with subsequent worsening of anxiety symptoms and dysphoria that results in comorbidity (Kushner et al., 2000).
The endogenous cannabinoid, or endocannabinoid (eCB), system plays an important homeostatic role in the regulation of stress circuits (Worley et al., 2018) and has emerged as a putative therapeutic target to treat stress, anxiety, fear-related behaviors, and AUD (Bedse et al., 2019; Natividad et al., 2017; Shonesy et al., 2014). The eCB system is a retrograde signaling system that regulates multiple physiological functions in the central and peripheral nervous systems. N-arachidonoylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG) are the two most studied lipid-derived endogenous ligands that exert biological effects via the activation of cannabinoid receptors type 1 and 2 (CB1 and CB2). AEA and 2-AG are mainly degraded by two serine hydrolases, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively. Preclinical evidence supports the hypothesis that serine hydrolase inhibitors augment brain eCB levels and thereby may therapeutically reduce symptoms of stress and anxiety (Pavon et al., 2021; Zhang et al., 2015; Zhong et al., 2014).
Recent development of pharmacological tools to selectively inhibit MAGL activity has made it possible to study the contribution of 2-AG signaling in mediating these responses (Blankman & Cravatt, 2013). 2-AG is produced on demand in postsynaptic neurons by the hydrolytic activity of diacylglycerol-lipase alpha (DAGLa) and canonically acts as a retrograde transmitter by activating Gi/o-coupled CB receptors located at the presynaptic level to reduce neurotransmitter release (Kano et al., 2009; Piomelli, 2003; Stella et al., 1997). Preclinical studies have demonstrated that augmenting 2-AG signaling can reduce anxiety-like behaviors and treat stress-related symptoms (Bedse et al., 2017; Bluett et al., 2017; Bosch-Bouju et al., 2016; Ivy et al., 2020; Lisboa et al., 2017; Lutz et al., 2015; McLaughlin et al., 2014; Patel & Hillard, 2009), whereas genetic or pharmacological inhibition of 2-AG synthesis increases anxiety-like behaviors, hinders extinction of conditioned fear, and increases susceptibility to stress-induced anxiety (Bluett et al., 2017; Cavener et al., 2018; Shonesy et al., 2014). In addition, studies have shown that stress induces bidirectional changes in brain eCB levels. Stress exposure reduces amygdala AEA levels, and its reduction after stress correlate with anxious-like behaviors (Bluett et al., 2014). In contrast, stress can increase amygdala 2-AG levels, which determines termination and adaptation to stress, as well as changes in emotional memory (Hill et al., 2010; Patel et al., 2009; Patel et al., 2005), suggesting that 2-AG regulates stress signaling once the stress response is activated. Similarly, effects of alcohol exposure on eCB contents have been described and the interactions between alcohol and eCB system at the molecular, synaptic, and behavioral level have been recently reviewed (Kunos, 2020; Wolfe et al., 2022). For instance, acute alcohol exposure has been associated with decreased 2-AG in prefrontal cortex (Rubio et al., 2007), while chronic intermittent alcohol with reduction of baseline 2-AG levels in the central nucleus of the amygdala (CeA) (Serrano et al., 2018).
Genetically-selected Marchigian Sardinian alcohol-preferring (msP) rats display comorbid symptoms of excessive alcohol drinking and enhanced anxiety compared to their genetic counterpart Wistar rats, and they resemble a post-dependence phenotype (Ciccocioppo et al., 2006; Ciccocioppo et al., 1999). It has been hypothesized that elevated alcohol intake in msP rats is driven, at least in part, by the attempt to attenuate anxiety-like behaviors and ‘self-medicate’. The msPs display pronounced aberrations in the brain stress signaling, including genetically determined overexpression of corticotropin-releasing factor 1 (CRF1) receptors in various brain regions (Ayanwuyi et al., 2013; Hansson et al., 2007; Hansson et al., 2006). More recently, the discovery of dysregulated eCB signaling in msPs raises the possibility that the eCB system critically underlies the comorbid expression of behavioral anxiety and excessive alcohol drinking (Natividad et al., 2017; Natividad et al., 2021). Additionally, previous work has shown that FAAH inhibitors can be successfully employed to increase AEA levels and regulate drinking behaviors (Stopponi et al., 2018), but much less is known about the role of 2-AG. Lastly, sex-dependent effects of endocannabinoid modulation of stress-related disorders have started to be elucidated (Henricks et al., 2017; Morena et al., 2021), but more studies are needed to examine potential sex-specific effects of MAGL inhibition.
Here, we used systemic administration of the brain-permeant MAGL inhibitor, MJN110, to pharmacologically block MAGL enzymatic activity and test the hypothesis that the modulation of the endocannabinoid 2-AG can decrease voluntary alcohol drinking and stress-related behaviors in male and female msP rats. First, we assessed MJN110 efficacy to significantly increase brain 2-AG levels using liquid chromatography-mass spectrometry (LC-MS/MS). Then, we assessed the effects of augmenting the 2-AG signaling on voluntary alcohol drinking and stress-induced behavioral adaptations such as novelty-induced anxiety behaviors, irritability-like behaviors, and cued fear expression in a rodent model resembling post-dependent states and affective impairments (Hansson et al., 2007; Hansson et al., 2006).
2. MATERIALS AND METHODS
2.1. Animals
We used a total of 292 rats. Adult male (n = 64, ~450 g) and female (n = 81, ~250 g) msP rats, obtained from the School of Pharmacy, University of Camerino (Camerino, Italy), were bred at The Scripps Research Institute (La Jolla, CA, USA). Adult male (n = 66, ~450 g) and female (n = 81, ~250 g) Wistar rats from which msPs originate were purchased from Charles River Laboratories (Wilmington, MA, USA). Rats were housed in a temperature- and humidity-controlled vivarium on a 12-hour reverse light/dark cycle (lights off at 8:00 a.m.), with food and water available ad libitum. Rats were pair-housed, separated by a perforated clear plexiglass divider to habituate them to the behavioral test conditions, while also reducing isolation stress (Steinman et al., 2021). The rats were randomly assigned to the different treatment groups. The in vivo experimental system in rat replicates aspects of the human alcohol use disorder and stress mechanisms (Borruto et al., 2021). We conducted all procedures in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with The Scripps Research Institute Institutional Animal Care and Use Committee policies (IACUC, Protocol number: 09–0006) and are in line with the ARRIVE 2.0 and BJP guidelines.
2.2. Drug preparation and administration
MJN110 was synthesized as described in Niphakis et al. (2013) and provided by the Cravatt laboratory at The Scripps Research Institute (La Jolla, CA, USA).
Rats were injected with a single intraperitoneal (i.p.) dose of MJN110 (5 mg kg−1 or 10 mg kg−1; 1 mL kg−1) or vehicle (Ethanol/PEG-40 Castor Oil/saline = 1/1/18) 3 hours prior to each test or brain collection. The doses and pretreatment time were chosen based on previous work demonstrating that the acute systemic administration of MJN110 at 5 mg kg−1 dose yields maximal MAGL inhibition in the rat brain, while having no effect on other measured endocannabinoids, including AEA (Burston et al., 2016; Niphakis et al., 2013).
2.3. Two-bottle choice alcohol access procedure
The two-bottle choice (2-BC) procedure (free choice between water and 10% v/v alcohol) was used to measure voluntary alcohol drinking and preference (Leeman et al., 2010). Animals were given free access to water and alcohol (10% v/v) 24 hours/day, for 15 days to establish a stable drinking baseline and preference for alcohol (80–90% preference alcohol vs water in msP rats). Once the baseline was reached, the access to alcohol was then decreased to 2 hours, starting from 2 hours into the animals’ dark active phase, for 4 days. After reaching a stable 2 hour-drinking baseline, on the 5th day rats were injected with either vehicle or MJN110 (10 mg kg−1 or 5 mg kg−1), 3 hours prior to the beginning of the 2-BC session (2 hours). Fluids were offered through graduated drinking tubes equipped with metallic spouts and intake was measured by weighing the tubes at the end of each drinking session. The drinking tubes were switched daily to avoid the development of side preference. Animals had free access to food. Alcohol intake was calculated as absolute value of consumption at each time interval and expressed as g/kg body weight to control for body weight variability (Devgun & Dunbar, 1990).
2.4. Novelty induced hypophagia (NIH)
Anxiety-like behavior was assessed using novelty-induced hypophagia (NIH) procedures as previously described (Vozella et al., 2021). Exposure to a novel environment elicits a stressful reaction in rodents that can interfere with normal behavior, including food consumption as the animals face the choice between engaging in feeding and the fear of novelty (Bechtholt et al., 2007). Here, 24 hours before undergoing testing, the animals received home cage exposure to a novel palatable food (50% sucrose, chocolate-flavored pellets, 45 mg, 3.4 kcal/g, 5TUL, Test Diets, St. Louis, MO, USA) in their housing room during their dark phase, to familiarize the rats with the food pellets. Upon exposure to the palatable food (~1 g), rats were left undisturbed and allowed to consume the whole amount while monitored by the experimenter to confirm that each rat tasted the novel food. The next day, the rats were treated with MJN110 (5 mg kg−1) or vehicle and left undisturbed for 3 hours prior to evaluation under novel testing conditions that are perceived as stressful (i.e., white lights, unfamiliar double-size cage, 60-dB background noise, different room). Rats were placed in the novel environment and let explore. Treatment-blind experimenters measured the latency to first approach the food, latency to consume the chocolate pellets, and the total intake of the novel palatable food in the unfamiliar environment over a 10 min trial. Rats that did not approach the food within the 10 min trial were assigned 600 sec latencies. Rats that did not eat any pellet were assigned intake 0 g kg−1. We also performed a separate control experiment on a separate set of animals where rats were exposed to the relevant test diet (i.e., highly palatable chocolate pellets) and tested in the familiar home cage 24 hours after the first home cage exposure to the novel food. Intake data are expressed as g pellet intake/kg body weight.
2.5. Irritability test
Irritability-like behavior was measured using the bottle-brush procedure (Kimbrough et al., 2017). The testing was performed in a behavioral room under dim red lights, 3 hours after treatment with MJN110 (5 mg kg−1) or vehicle. Each individual rat was placed in a clear plastic cage (27 × 48 × 20 cm) containing clean bedding, facing the back of the cage. For each trial, a treatment-blind experimenter inserted a bottle brush into the cage and rotated it in five phases, each one lasting 3 sec: 1) rotating towards the rat, 2) forcing interaction by touching the whiskers, 3) withdrawing from the rat, 4) rotating upright, and 5) holding upright without rotation. The testing consists of 10 trials per rat with 10 sec intervals between each trial. The following were scored as aggressive-like responses: biting, boxing, siding, following, mounting. The following were scored as defensive-like responses: avoiding, digging/burying, freezing, jumping, startling, vocalizing. Grooming, rearing, and exploring were additionally recorded during the trial. Data are expressed as the sum of all the aggressive-like behaviors and sum of all the defensive-like behaviors.
2.6. Fear conditioning
Rats were placed in 30.5 × 25 × 28 cm chambers (Med Associates, St. Albans, VT, USA), which were cleaned before and in between rats using 70% isopropanol. Chambers were equipped with an infrared camera for behavioral observation and recordings. On conditioned fear acquisition days (day 1 and 2), rats were first acclimatized to the chamber for 5 min without lights or any stimuli. This 5-min period was used to examine initial freezing behavior before the presentation of the cued stimulus. Rats were then presented with six conditioned stimulus (CS, light) – unconditioned stimulus (US, foot shock) pairings that were separated by variable (60±20 sec) intertrial intervals (ITI). Each light cue lasted 20 sec and co-terminated with a 2 sec 0.6 mA electric foot shock. At the end of the conditioning session, rats were left in the dark chamber for an additional 2 min. On the conditioned fear expression day (day 3), half of the rats of each sex and genotype were treated with vehicle and the other half with MJN110 (5 mg kg−1) 3 hours before being placed in the same chamber as per conditioned fear acquisition day 1 and day 2. After 5 min in the chamber, rats were presented with 18 CS presentations (20 sec) (no shock) separated by a 60±20 sec intertrial interval, followed by final 2 min without any stimulus presentation. Animals were videorecorded during testing, and freezing behavior was manually scored, blind to drug treatment, by measuring the absence of any movement except for respiratory-related movements during the CS + ITI duration.
2.7. Tissue collection
A separate batch of rats (n = 40) was used for brain tissue collection and liquid chromatography-mass spectrometry (LC-MS/MS) analysis. Rats were intraperitoneally injected with either vehicle or MJN110 (5 mg kg−1) and 3 hours later deeply anesthetized with isoflurane and rapidly decapitated while unconscious using a guillotine. Brains were removed and flash frozen in dry ice-cold isopentane. Prelimbic cortex (PrL, 1 mm), infralimbic cortex (IL, 1 mm) and central amygdala (CeA, 0.8 mm) punches were dissected from cryostat-sectioned slices (400 μm).
2.8. Lipid extraction and analyses
Rat brain punches were thawed on ice and homogenized using a glass douncer or a mechanical mortar and pestle gun in 500 μL ice-cold DPBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA, Catalog #14190144) in a cold room. Dounce homogenized samples were then probe sonicated briefly (8 pulses, 10% power). An aliquot (10 μL) of each homogenized sample was reserved for protein concentration quantification using DC Protein Assay (BioRad, Hercules, CA, USA, Catalog #5000112). The remaining homogenized lysate was then immediately transferred into 3 mL ice-cold chloroform:methanol (2:1) spiked with the internal standards 2-AG-d5 (500 pmol, Cayman, Ann Arbor, MI, USA, Catalog #362162) and AEA-d4 (100 pmol, Cayman, Ann Arbor, MI, USA, Catalog #10011178), and an additional 500 μL DBPS was added to the extraction mixture. Samples were vortexed for 30 sec, centrifuged at 1400 g for 3 min at 4 ºC and the lower organic phase was collected. Additional 1 mL chloroform and 100 μL 3 N HCl were added to the remaining aqueous fraction; samples were then vortexed for 30 sec, centrifuged at 1400 g for 3 min at 4 ºC, and the lower organic mixture was collected and combined with the first organic fraction. Samples were dried down using a stream of N2, resuspended in 100 μL of chloroform:methanol (2:1) and transferred into LC-MS/MS vials.
2-AG and AEA were quantified using LC-MS/MS. Samples were injected onto a 50 mm × 4.6 mm 5 μm Gemini C18 column (Phenomenex, Torrance, CA, USA) coupled to a guard column (Gemini C18: 4 × 3 mm) using HPLC (Agilent 1290 Infinity II LC System, Santa Clara, CA, USA, RRID:SCR_019375) with a flow rate between 0.1 mL min−1 and 0.5 mL min−1. The aqueous buffer (Buffer A) contained 5% methanol and 0.1% formic acid. The organic buffer (Buffer B) contained 60% isopropanol, 35% methanol, and 0.1% formic acid. Lipids were eluted using the following gradient: 0–5 min, 0% Buffer B, 0.1 mL min−1; 5–5.01 min 0–60% Buffer B, 0.4 mL min−1; 5.01–20 min, 60–100% Buffer B, 0.4 mL min−1; 20–20.01 min, 100–0% Buffer B, 0.5 mL min−1; 20.01–33 min, 100% Buffer B, 0.5 mL min−1; 33–34 min, 100–0% Buffer B, 0.5 mL min−1; 34–35 min, 0% Buffer B, 0.5 mL min−1. Eluted lipids were detected using the Agilent 6470 Triple Quadrupole Liquid Chromatography Mass Spectrometer System (Agilent, Santa Clara, CA, USA, RRID:SCR_019421) via multiple reaction monitoring (MRM) using an electrospray ionization (ESI) source in positive mode. MS analysis was performed using ESI with the following parameters: gas temperature: 350 ºC; gas flow: 9 L min−1; nebulizer: 35 psi; sheath gas temperature: 375 ºC; sheath gas flow: 11 L min−1; capillary: 4000 V; nozzle voltage/charging: 500 V. MRM transitions were specific for each lipid. 2-AG precursor ion: 379; product ion: 287; dwell: 100; fragmentation (F): 100 V; collision (C): 8 V; collision acceleration (CA): 7 V. 2-AG-d5 precursor ion: 384; product ion: 287; dwell: 100; F: 100 V; C: 8 V; CA: 7 V. AEA precursor ion: 348.3; product ion: 62.1; dwell: 100; F: 100 V; C: 40 V; CA: 7 V. AEA-d4 precursor ion: 352.3; product ion: 66.1; dwell: 100; F: 100 V; C: 40 V; CA: 7 V. Lipids were quantified by Agilent Masshunter Quantitative Analysis software, version 10 (Agilent, Santa Clara, CA, USA, RRID:SCR_015040) by integrating their peak area and normalizing relative to the peak area of the internal standard, as follows:
Quantified lipids were then normalized per the total amount of protein per sample.
2.9. Experimental design overview
This report consists of four behavioral assessments (two-bottle choice alcohol drinking, irritability, NIH, conditioned fear expression) and one lipidomic study that were conducted in five separate cohorts of rats.
Cohort 1: lipid measurement. 10 male and 10 female Wistar rats and 10 male and 10 female msP rats were subjects. Rats were euthanized 3 hours after MJN110 or vehicle administration, and brains were collected for LC-MS/MS analysis (Figure 1 and Supplementary Figure 1).
Cohort 2: two-bottle choice alcohol drinking. 20 male and 19 female Wistar rats and 20 male and 19 female msP rats were subjects (Figure 2).
Cohort 3: irritability and NIH. 16 male and 16 female Wistar rats and 14 male and 16 female msP rats were subjects (Figures 3–4).
Cohort 4: NIH control experiment. 16 female Wistar rats and 16 female msP rats were subjects (Supplementary Figure 2).
Cohort 5: conditioned fear expression. 20 male and 20 female Wistar rats and 20 male and 20 female msP rats were subjects (Figure 5).
Figure 1. MJN110 increases 2-AG in prefrontal cortex subregions and central amygdala.
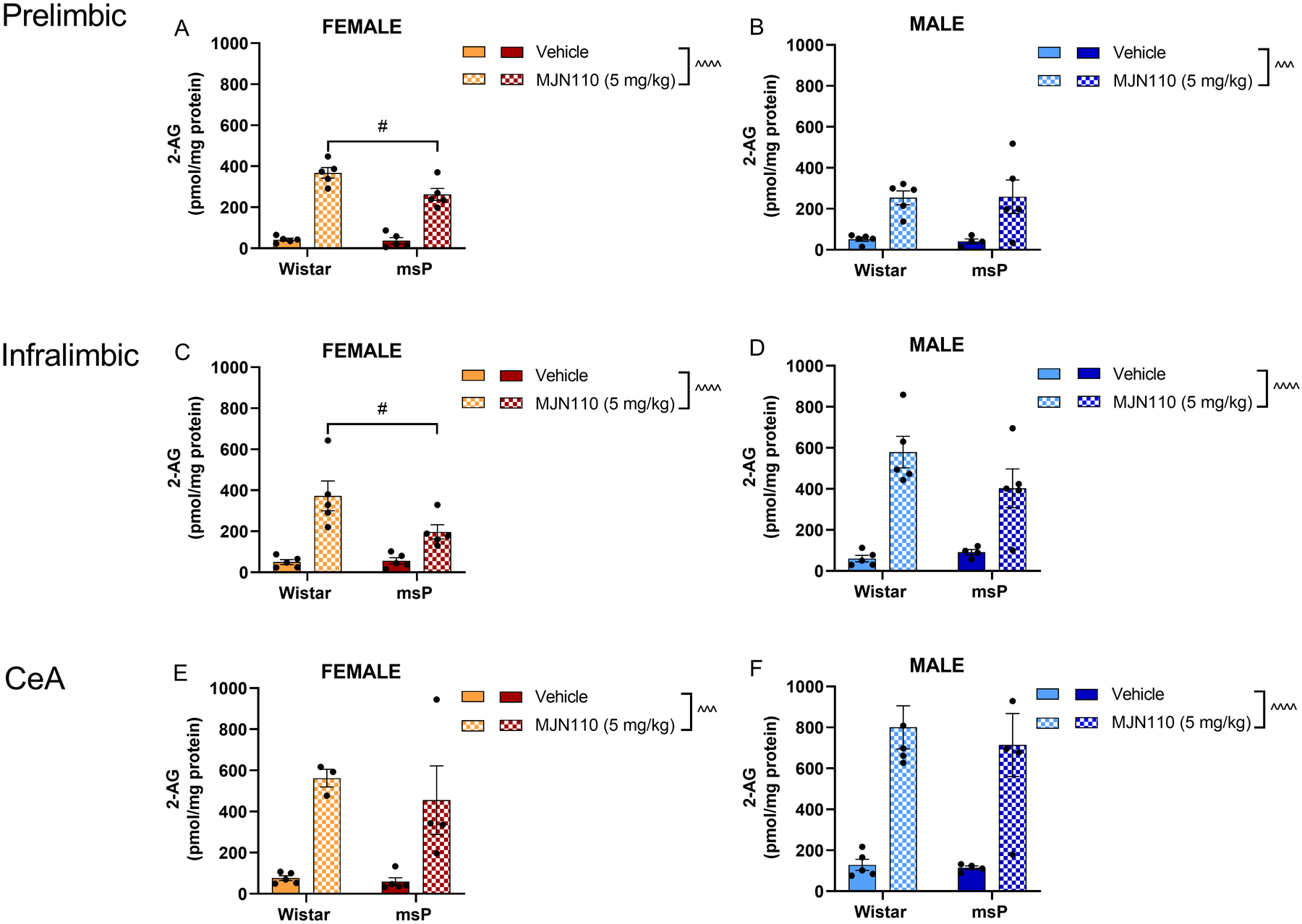
2-AG levels were measured by LC-MS/MS 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female prelimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). B) Male prelimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). C) Female infralimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). D) Male infralimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). E) Female central amygdala (CeA) (Wistar vehicle, n = 5; Wistar MJN110, n = 3; msP vehicle, n = 5; msP MJN110, n = 4). F) Male central amygdala (CeA) (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). Results are expressed as mean ± SEM. 1 rat in the male msP vehicle group was identified as an outlier and excluded across the three brain regions analyzed. ^ p < 0.05, ^^ p < 0.01, ^^^ p < 0.001, ^^^^ p < 0.0001, main effect of the treatment, two-way ANOVA. # p < 0.05, MJN110-treated Wistars vs MJN110-treated msPs, two-way ANOVA followed by Tukey’s multiple comparisons test.
Figure 2. Acute MJN110 does not affect voluntary alcohol intake.
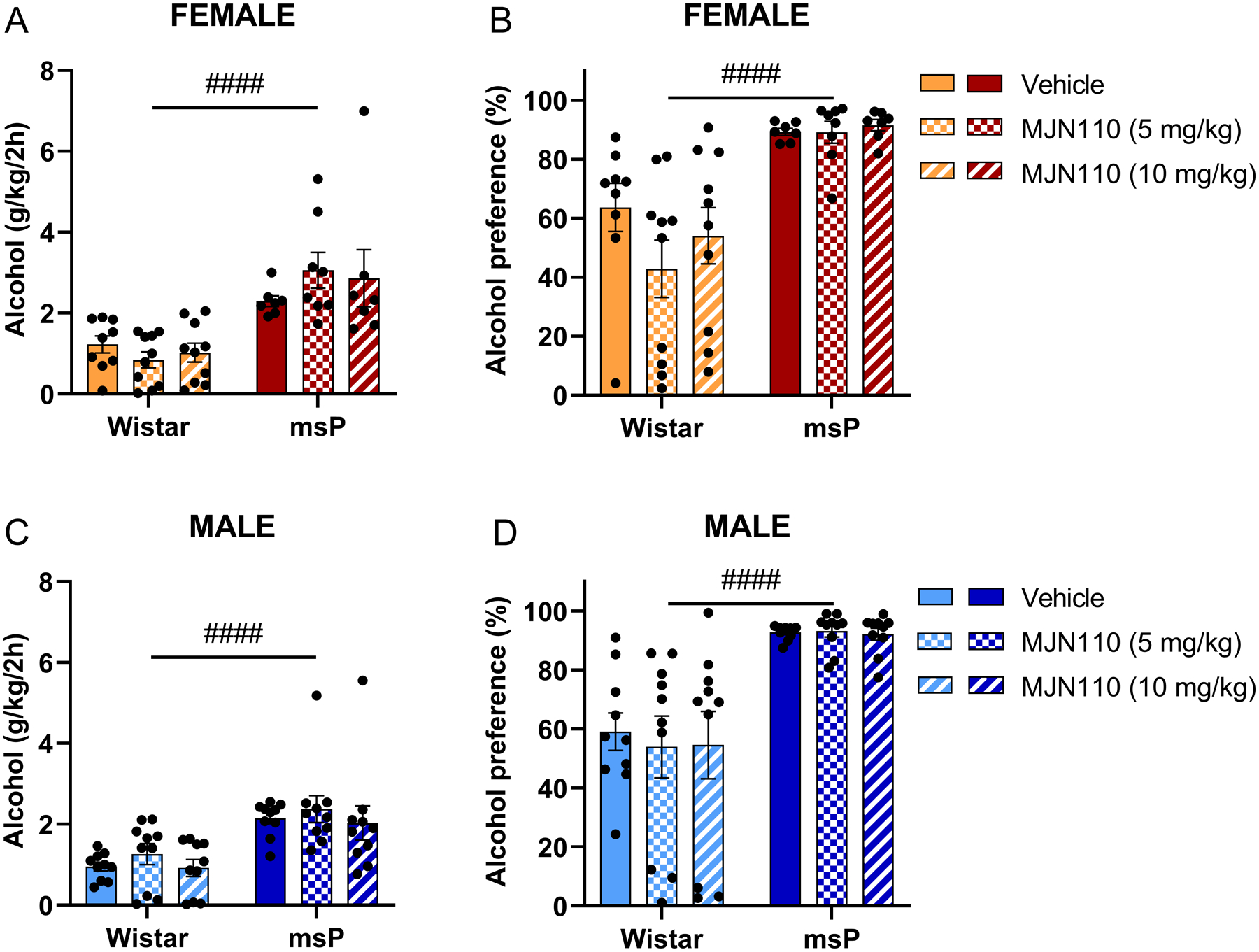
Rats were tested in the two-bottle choice drinking procedure (10% v/v alcohol) 3 hours after a single MJN110 administration (5 mg kg−1 and 10 mg kg−1, i.p.). A) Female alcohol intake (g/kg/2h). B) Female alcohol preference. C) Male alcohol intake (g/kg/2h). D) Male alcohol preference. Results are expressed as mean ± SEM. Female Wistar vehicle, n = 9; female Wistar MJN110, n = 10; female msP vehicle, n = 9; female msP MJN110, n = 10; male Wistar vehicle, n = 10; male Wistar MJN110, n = 10; male msP vehicle, n = 10; male msP MJN110, n = 10. #### p < 0.0001 main effect of the genotype, two-way ANOVA.
Figure 3. Acute MJN110 reduces anxiety-like novelty-induced hypophagia in female rats.
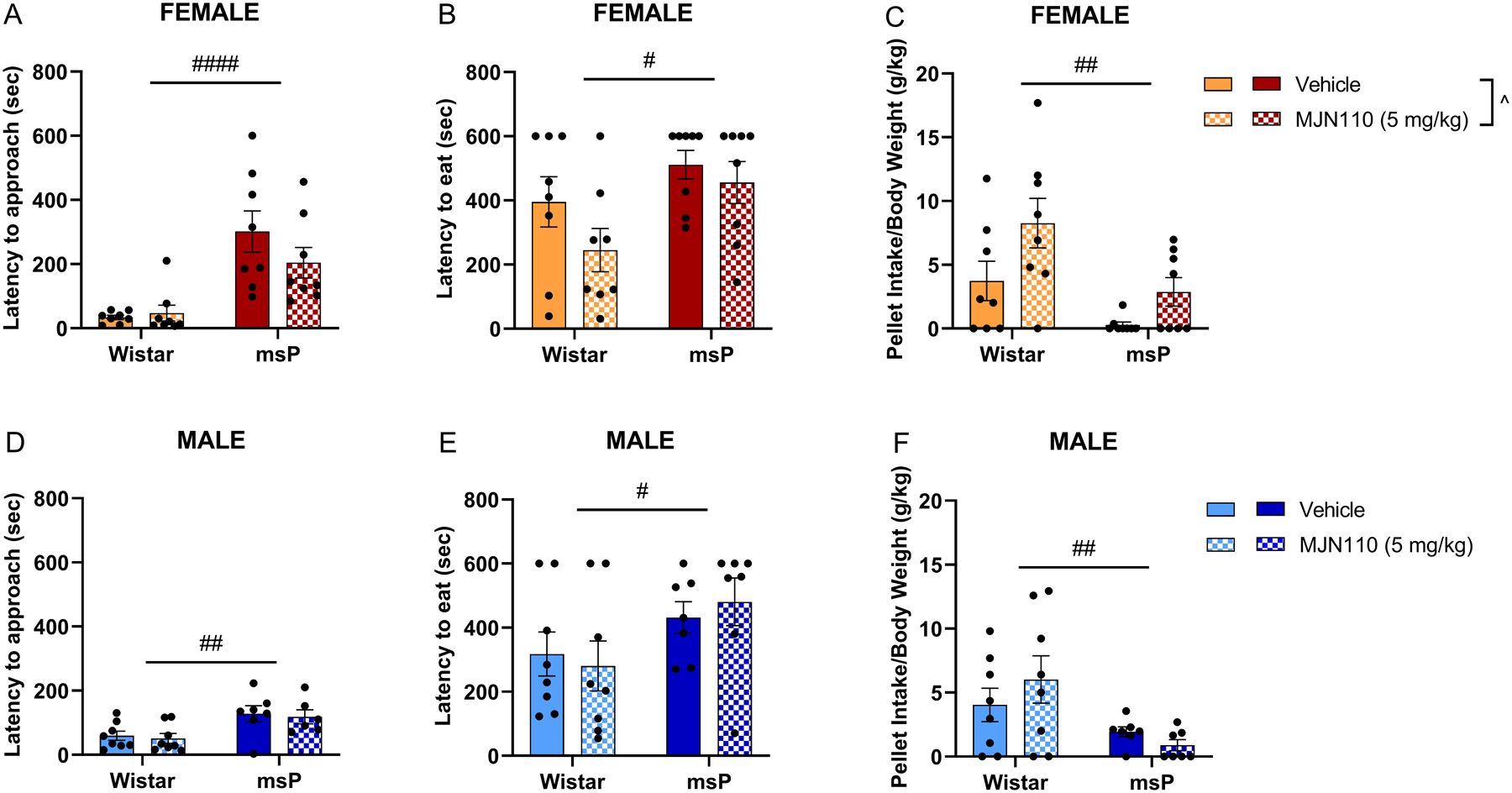
Rats were tested in a 10 min novelty induced hypophagia (NIH) test 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female latency to approach. B) Female latency to eat. C) Female pellet intake. D) Male latency to approach. E) Male latency to eat. F) Male pellet intake. Results are expressed as mean ± SEM. Female Wistar vehicle, n = 8; female Wistar MJN110, n = 8; female msP vehicle, n = 8; female msP MJN110, n = 8; male Wistar vehicle, n = 8; male Wistar MJN110, n = 8; male msP vehicle, n = 7; male msP MJN110, n = 7. ^ p < 0.05 main effect of the treatment; # p < 0.05, ## p < 0.01, #### p < 0.0001 main effect of the genotype, two-way ANOVA.
Figure 4. Acute MJN110 reduces irritability-like behaviors in msP rats.
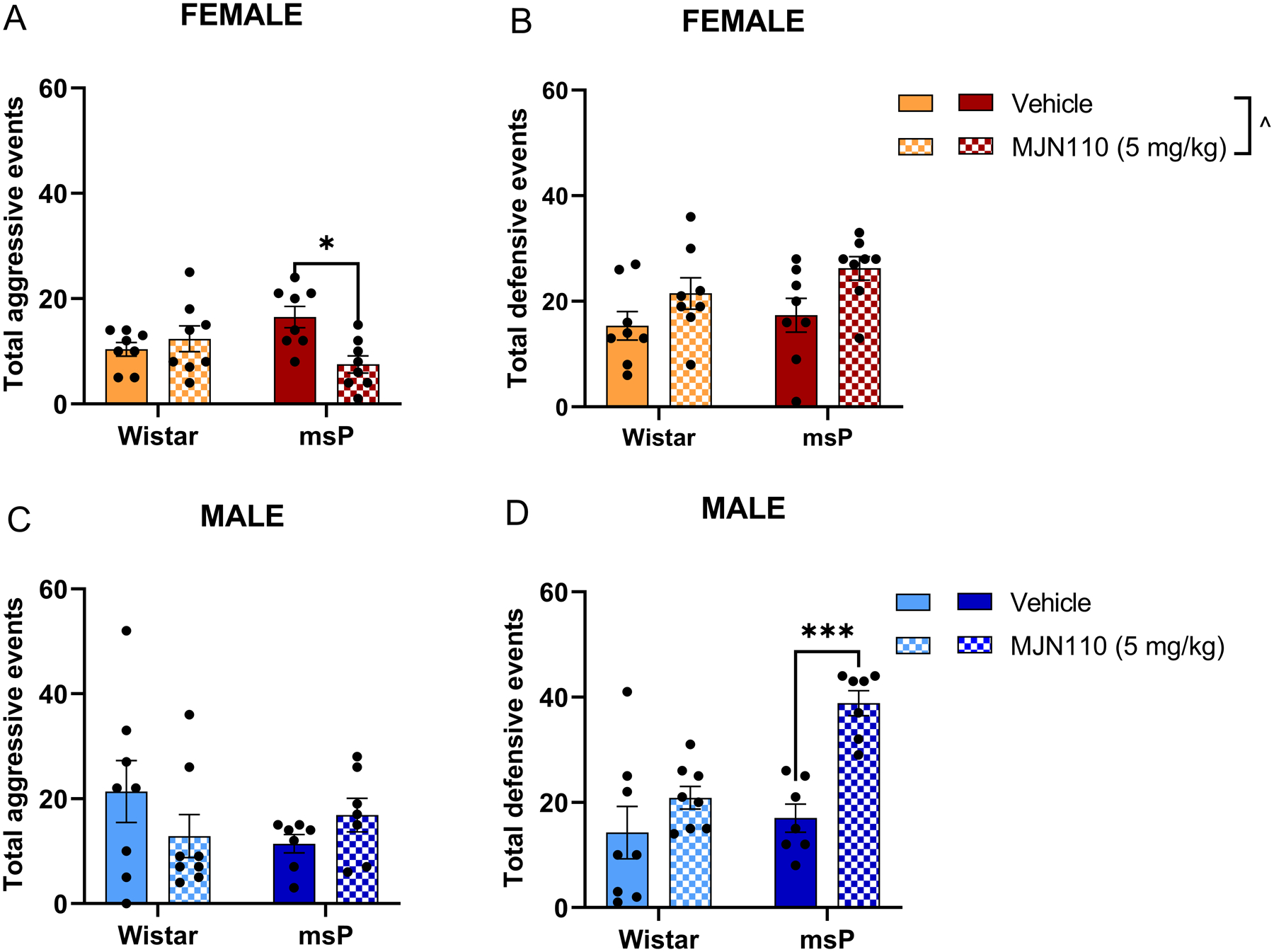
Rats were tested for irritability using the bottle-brush test 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female total aggressive events. B) Female total defensive events. C) Male total aggressive events. D) Male total defensive events. Results are expressed as mean ± SEM. Female Wistar vehicle, n = 8; female Wistar MJN110, n = 8; female msP vehicle, n = 8; female msP MJN110, n = 8; male Wistar vehicle, n = 8; male Wistar MJN110, n = 8; male msP vehicle, n = 7; male msP MJN110, n = 7. ^ p < 0.05 main effect of the treatment, two-way ANOVA; * p < 0.05, *** p < 0.001, vehicle vs MJN110, two-way ANOVA followed by Tukey’s multiple comparisons test.
Figure 5. MJN110 does not reduce cue-induced fear expression but reduces fear in the absence of cues selectively in male msP rats.
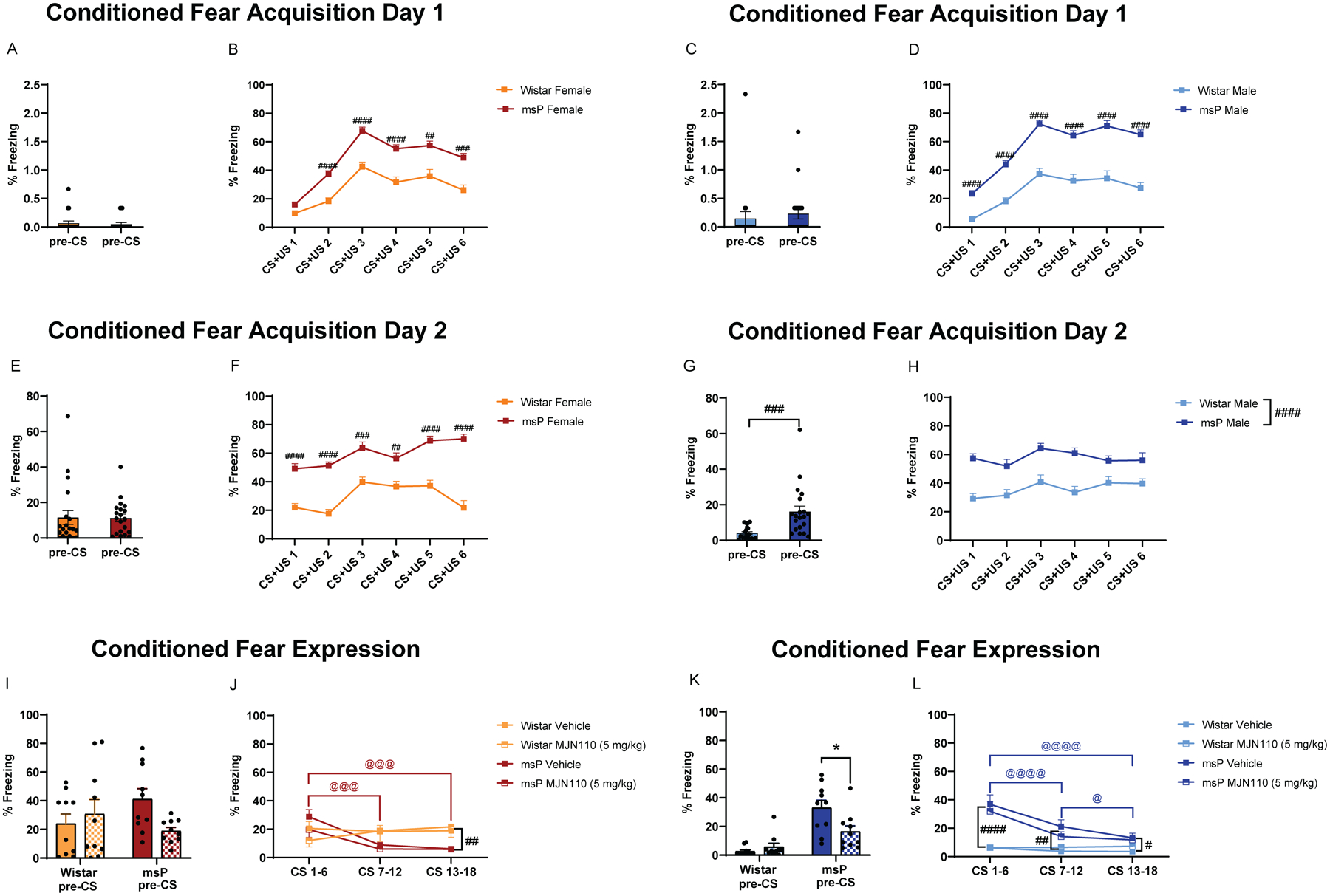
Rats were tested for conditioned fear expression 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). Female conditioned fear acquisition day 1. A) % freezing during 5 min before CS+US presentation. B) % freezing during CS+US pairing presentations. Male conditioned fear acquisition day 1. C) % freezing during 5 min before CS+US presentation. D) % freezing during CS+US pairing presentations. Female conditioned fear acquisition day 2. E) % freezing during 5 min before CS+US presentation. F) % freezing during CS+US pairing presentations. Male conditioned fear acquisition day 2. G) % freezing during 5 min before CS+US presentation. H) % freezing during CS+US pairing presentations. Female conditioned fear expression. I) % freezing during 5 min before CS presentation. J) % freezing during CS presentations. The 18 CS were analyzed as 3 bins of 6 sequential CS. Male conditioned fear expression. K) % freezing during 5 min before CS presentation. L) % freezing during CS presentations. The 18 CS were analyzed as 3 bins of 6 sequential CS. Results are expressed as mean ± SEM. Female Wistar vehicle, n = 10; female Wistar MJN110, n = 10; female msP vehicle, n = 10; female msP MJN110, n = 10; male Wistar vehicle, n = 10; male Wistar MJN110, n = 10; male msP vehicle, n = 10; male msP MJN110, n = 10. A, C, E, G ### p < 0.001 unpaired t-test. B, D, F, H # p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001, Wistar vs msP rats, RM two-way ANOVA followed by Bonferroni’s multiple comparisons test. I, K * p < 0.05, msP vehicle vs msP MJN110, two-way ANOVA followed by Tukey’s multiple comparisons test. J, L RM two-way ANOVA followed by Bonferroni’s multiple comparisons test, # p < 0.05, ## p < 0.01, #### p < 0.0001, Wistar vs msP rats; @ p < 0.05, @@@ p < 0.001, @@@@ p < 0.0001 msP CS 1–6 vs CS 7–12 or CS 13–18.
2.10. Statistical analyses
Two-bottle choice alcohol drinking, irritability, NIH and lipid measures were analyzed using two-way analyses of variance (ANOVA) with treatment (vehicle vs MJN110) and genotype (Wistar vs msP) as between subject factors. Significant interaction effects were followed by Tukey’s multiple comparisons test. Fear conditioning data containing repeated CS were analyzed using a repeated measures (RM) two-way or three-way ANOVA with genotype (two-way) or genotype and drug treatment (three-way) as between subjects’ factors, and trial (e.g., CS+US pairing or CS presentation) as repeated, within-subject factors. Significant interaction effects were followed by Bonferroni’s multiple comparisons test. Freezing during the pre-CS acclimation period (context-only, no cue exposure) was also monitored and analyzed separately using unpaired t-test or two-way ANOVA, as appropriate. To better evaluate any difference in drug effects in the early or late phases of the conditioned fear expression sessions, we divided the 18 conditioned stimuli into 3 blocks of 6 consecutive conditioned stimuli each (Block 1: CS1–6; Block 2: CS7–12; Block 3: CS13–18). We scored the time spent freezing per each block. Data are expressed as a percentage of time spent freezing over the total duration of the block. Since the main goal of our study was to investigate MJN110 effects in a genetic rat model of high alcohol preference and high anxiety, and because of the underlying sexual dimorphism of the endocannabinoid system (for review see Lopez 2010 and Craft 2013), male and female rats were herein analyzed separately. Studies were designed to generate groups of comparable size, using randomization and blinded analysis. Group size, indicated for each experiment in the figure legends, is the number of independent values (i.e., individual rats) and the statistical analysis was done using these independent values. Sample sizes are based on experience from our laboratory and the Cravatt laboratory (Niphakis et al., 2013) for similar studies. All datasets were derived from ≥7 rats (behavioral experiments) or 3–5 rats (biochemical experiment, (Niphakis et al., 2013)) from each sex and genotype. While processing the samples for lipid quantification, 2 MJN110-treated female Wistar CeA samples and 1 MJN110-treated female msP CeA sample were damaged, reducing the number of final quantifiable samples to 3 and 4 respectively. All data are presented as mean ± standard error of the mean (SEM). The significance level was determined at p < 0.05. Outliers in the lipid quantification were determined using Grubbs’ outlier test and excluded. 2 female msPs/group were excluded in the 2-BC procedure due to bottle leakage on the test day.
The data and statistical analysis comply with the recommendations and requirements on experimental design and analysis in pharmacology (Curtis et al., 2018). All statistical analyses were performed, and all graphs were generated using GraphPad Prism V9 (GraphPad, San Diego, CA, USA).
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2020/21 (Alexander Christopoulos, et al., 2021; Alexander, Cidlowski, et al., 2021; Alexander, Fabbro, et al., 2021; Alexander, Mathie, et al., 2021).
3. RESULTS
3.1. Single systemic administration of MJN110 increases 2-AG in prefrontal cortex subregions and central amygdala.
First, we wanted to determine whether the MJN110 dosage (5 mg kg−1) and timepoint (3 hours) we selected would elevate 2-AG levels in cortical regions associated with stress, negative affect, and alcohol drinking behavior, such as the medial prefrontal cortex (mPFC), which includes the prelimbic (PrL) and infralimbic (IL) subregions (Klenowski, 2018), and the central nucleus of the amygdala (CeA) (Gilpin et al., 2015). We focused on the 5 mg kg−1 MJN110 dose, which has been previously shown to decrease motivated behaviors in rats (Feja et al., 2020). The results revealed a main effect of the MJN110 treatment in increasing 2-AG in the prelimbic cortex (Figure 1A, F(1, 16) = 167.5, p < 0.0001; Figure 1B, F(1, 15) = 19.50, p = 0.0005) and infralimbic cortex (Figure 1C, F(1, 16) = 31.60, p < 0.0001; Figure 1D, F(1, 15) = 40.44, p < 0.0001) of both female and male rats, regardless of the genotype. Similarly, following a single MJN110 administration, 2-AG levels were significantly elevated in the CeA of both female (Figure 1E, F (1, 13) = 29.06, p = 0.0001) and male (Figure 1F, F(1, 15) = 39.99, p < 0.0001) rats.
No changes were observed in AEA levels across the brain regions analyzed, sex, or genotype, suggesting a selective inhibition of MAGL, and not FAAH, by MJN110 administration (Supplementary Figure 1). The 5 mg kg−1 dose was then selected and used for all the other behavioral assessments.
3.2. Single systemic administration of MJN110 does not affect voluntary alcohol intake.
To elucidate the acute role of 2-AG signaling in the regulation of voluntary alcohol drinking behavior, we tested the MAGL inhibitor MJN110 in the two-bottle choice free access procedure in non-selected Wistar and genetically selected msP rats. Consistent with previous work (Ciccocioppo et al., 2006), we found that msP displayed significantly higher alcohol intake and preference than their Wistar controls in both female rats (Figure 2A, F(1, 45) = 36.81, p < 0.0001; Figure 2B, F(1, 45) = 33.29, p < 0.0001) and male rats (Figure 2C, F(1, 54) = 26.89, p < 0.0001; Figure 2D, F(1, 54) = 42.17, p < 0.0001). Systemic administration of MJN110 (5 mg kg−1 or 10 mg kg−1, i.p.) did not alter the total alcohol intake (Figure 2A, C) or preference (Figure 2B, D) in either female or male, and Wistar or msP rats.
3.3. Single systemic administration of MJN110 reduces anxiety-like behaviors in female rats.
Consistent with our previous work (Vozella et al., 2021), female and male msP rats displayed a greater latency to approach the palatable food (Figure 3A, F(1, 28) = 25.48, p < 0.0001; Figure 3D, F(1, 25) = 12.69, p = 0.0015) and greater latency to consume the palatable food (Figure 3B, F(1, 28) = 6.285, p = 0.0183; Figure 3E, F(1, 26) = 5.086, p = 0.0328) under novelty stress conditions as compared to their Wistar counterparts. Similar genotype-dependent effects were observed with total food pellet intake when compared to Wistar rats (Figure 3C, F(1, 28) = 10.55, p = 0.0030; Figure 3F, F(1, 26) = 8.452, p = 0.0074). Higher latency and lower pellet intake indicate higher sensitivity to novelty stress (Bechtholt et al., 2007; Carr et al., 2011). Notably, a single administration of MJN110 (5 mg kg−1, i.p.) did not alter the latency to approach or latency to eat the palatable food in either sex or genotype but induced an overall increase of pellet intake in females, regardless of genotype (Figure 3C, F(1, 28) = 6.825, p = 0.0143). To rule out generalized appetite-related action of MJN110 (Feja et al., 2020), in a separate cohort of females we performed a control experiment. Rats were tested in their home cage (familiar environment) 24 hours after the first exposure to the novel palatable food. Acute administration of MJN110 did not change total home cage palatable food pellet intake in either Wistar or msP female rats (Supplementary Figure 2).
3.4. Single systemic administration of MJN110 reduces irritability-like behaviors in msP rats.
Acute treatment with MJN110 produced a robust reduction of total aggressive-like signs (i.e., biting, boxing, siding, following, mounting) in female msP (p = 0.0119) but not Wistar rats (Figure 4A). MJN110 also exerted a main effect in elevating total defensive-like behaviors (i.e., avoiding, digging/burying, freezing, jumping, startling, vocalizing) in females (Figure 4B, F(1, 28) = 7.236, p = 0.0119). When administered in male animals, MJN110 did not have any effect on aggressive signs (Figure 4C), however it significantly increased the number of total defensive events in male msP rats (p = 0.0007) but not in Wistar rats (Figure 4D). These results indicate that pharmacological inhibition of MAGL alleviates irritability-like behaviors in rats showing innate hypersensitivity to stress and anxiety but with different coping responses between female and male rats. For a summary of individual behaviors, refer to Supplementary Table 1.
3.5. Single systemic administration of MJN110 does not suppress cue-induced fear expression but reduces fear in the absence of cues in male msP rats.
On conditioned fear acquisition day 1, we did not observe any genotype (Wistar vs msP) difference in the freezing response during the chamber pre-conditioning (pre-CS) 5-min period in both females (Figure 5A) and males (Figure 5C). However, during the CS-US trial presentations, we found a significant genotype × CS-US trial interaction in both females (F(5, 190) = 4.361, p = 0.0009) and males (F(5, 190) = 5.096, p = 0.0002). Post-hoc comparisons indicated significantly higher freezing behavior in female msP rats as compared to female Wistars in response to CS-US 2, 3, 4, 5 and 6 (Figure 5B). Male msP rats showed significantly higher freezing behavior as compared to Wistars in response to CS-US 1, 2, 3, 4, 5 and 6 (Figure 5D).
On conditioned fear acquisition day 2, we did observe a significant genotype difference in the freezing response during the initial, pre-CS 5-min period in males (Figure 5G, t = 3.728, df = 38, p = 0.0006), but not in females (Figure 5E). During the CS-US trial presentations, we found a significant genotype × CS-US trial interaction in females (Figure 5F, F(5, 190) = 4.932, p = 0.0003) and a significant main effect of genotype in males (Figure 5H, F(1, 38) = 26.05, p < 0.0001). Post-hoc comparisons indicated that female msP rats showed significantly higher freezing behavior as compared to Wistars during presentations of CS-US 1, 2, 3, 4, 5 and 6 (Figure 5F).
On the next day, we tested whether a single injection of MJN110 (5 mg kg−1) suppressed conditioned fear expression. Rats were treated with MJN110 3 hours prior to testing for conditioned fear expression where, after the pre-CS 5 min period, 18 CS were presented but in absence of the US (shock). MJN110 did not alter the freezing response during the 5 min pre-CS period in female Wistars, but it exerted a trending, nonsignificant effect in female msPs (Figure 5I). Notably, MJN110 significantly decreased the freezing response during the pre-CS period in msP males (p = 0.0128), but not in Wistar males (p = 0.9267) (Figure 5K). MJN110 did not significantly reduce the mean duration of freezing in the ITI following CS presentations in either sex, suggesting that in the present model an acute MAGL inhibition does not interfere with a cue-conditioned fear memory recall, but might ameliorate non-cued fear responses (i.e., contextual conditioned fear and non-associative freezing).
Interestingly, we found a significant genotype × CS-US trial interaction in both females (F(2, 72) = 16.29, p < 0.0001) and males (F(2, 72) = 22.76, p < 0.0001) during the CS presentations. Post-hoc comparisons indicated that female msP rats showed a greater fear extinction than female Wistars as indicated by a lower freezing response by the last trial block, CS13–18 (p = 0.0079) (Figure 5J). Similar behavior was observed in male msPs, which showed progressively reduced freezing response across the 3 trial blocks (Figure 5L), even though their freezing response remained significantly higher than Wistars across all CS trials (CS 1–6, p < 0.0001; CS 7–17, p = 0.0015; CS 13–18, p = 0.0314).
4. DISCUSSION
In the present study we sought to determine whether acute MAGL inhibition using MJN110 could suppress the innate vulnerabilities to stress and alcohol drinking in genetically-selected msP rats compared to their counterpart Wistar rats. We found that MJN110 ameliorates the stress-related responses in msP rats, but not voluntary alcohol drinking, in a sex-dependent manner. Recent evidence has shown that the eCB signaling regulates synaptic plasticity within the amygdala-PFC circuit under stressful experiences (Marcus et al., 2020) and fundamental sex differences have been identified within the amygdala-PFC fear circuit (Gruene et al., 2015). Here we used a single systemic administration of the selective MAGL inhibitor MJN110 to first assess whether the dose (5 mg kg−1) and time point (3 hours) we selected were sufficient to achieve significant elevation of 2-AG in brain regions involved in the regulation of drinking behaviors and stress response: the mPFC (prelimbic and infralimbic subregions) and the CeA. The i.p. route of administration was chosen here to reduce stress confounds of oral gavage administration, relevant with the stress-sensitive msP rats, and to facilitate comparison with previous behavioral work, most of which used i.p. administration. Because MJN110 is reportedly orally active, future translationally-oriented work will compare effects of oral administration. mPFC regulates emotions and plays an important role in the processing of and behavioral responses to stressful or threatening stimuli. The amygdala regulates physiological and behavioral responses to stress (Gilpin et al., 2015; Zhang et al., 2021), and a growing body of work has demonstrated that eCB signaling in the amygdala is involved in the regulation of emotional states (Lim et al., 2016; Marsicano et al., 2002; Morena et al., 2019). After we treated male and female Wistar and msP rats with a single dose of MJN110 (5 mg kg−1, 3 hours), we measured 2-AG and AEA levels in prelimbic cortex, infralimbic cortex, and CeA. We were able to quantify 2-AG and AEA in each dissected brain region and found a general and consistent elevation of 2-AG levels in each brain region analyzed, but no changes in AEA levels, for both Wistars and msPs. MJN110 exhibits good potency for rat MAGL compared to the JZL184 inhibitor (Niphakis et al., 2013) and, consistent with previous reports, it produced a 5 to 10-fold increase of 2-AG at the 5 mg kg–1 dose. Additionally, since MJN110 achieves partial inhibition of MAGL, it does not lead to drug tolerance through CB1 receptor desensitization. We then selected this 5 mg kg−1 dose to test MJN110 efficacy in ameliorating symptoms that are often associated with alcohol drinking or stress-related behaviors.
Previous studies have characterized the role of the eCB AEA/FAAH signaling system on alcohol-related behaviors, showing for instance that local injection of FAAH inhibitor URB597 into the CeA or the basolateral amygdala reduced alcohol self-administration in msP rats (Stopponi et al., 2018). Additionally, a single systemic administration of MAGL inhibitor MJN110 reduced self-administration in alcohol-dependent rats and the MAGL inhibitor JZL184 reduced voluntary alcohol drinking in dependent mice (Serrano et al., 2018). Although we predicted MJN110 would attenuate voluntary alcohol drinking and preference in the 2-BC procedure in the high alcohol preferring msP rat model, we observed no effects of MJN110 on reducing 2-BC drinking in either msP or Wistar rats, even when we tested a higher dose (10 mg kg−1). This discrepant finding may be due to differences in drinking procedures (voluntary 2-BC drinking vs operant self-administration), to the higher MJN110 dose (20 mg kg−1), and, most importantly, previous alcohol history (dependent vs non-dependent). Consistent with Serrano and co-authors, none of the doses tested reduced alcohol intake in control, non-dependent rats.
Stress disrupts 2-AG signaling, with acute and repeated stress exposure causing a delayed increase of 2-AG levels in the prefrontal cortex and amygdala, which contributes to stress response termination (Morena et al., 2016; Sumislawski et al., 2011). Moreover, chronic stress is generally associated with a down-regulation of CB1 receptors in most brain regions except for the mPFC (McLaughlin et al., 2014). We assessed anxiety-like behaviors using the NIH procedure under stressful-perceived novel environmental conditions in male and female Wistar and msP rats treated with a single dose of MJN110 (Burston et al., 2016). We found no significant effect of the treatment on the latency to approach or to start eating the familiar palatable food. However, the increased pellet intake elicited by MJN110 in female rats exposed to a novel, stressful environment is consistent with the hypothesis that inhibition of MAGL might alleviate anxiogenic-like behavior selectively in females, possibly by acting on the maintenance of the feeding behavior under anxiogenic-like conditions rather than on its initiation. We also confirmed that appetite or feeding per se were not playing a role (Feja et al., 2020) by testing a separate group of female rats in their own familiar home cage (Supplementary Figure 2).
The mPFC is involved in executive functions such as the ability to attend to or ignore stimuli and cognitive and behavioral flexibility. Structural and functional abnormalities of the mPFC have been linked to aggressive disorders (Miczek et al., 2022), thus we examined the effects of MJN110 on irritable-like behaviors. We treated male and female Wistar and msP rats with a single dose of MJN110 and assessed the efficacy of pharmacological MAGL inhibition on modulating the number of aggressive and defensive behaviors in the bottle-brush test. We have previously used the bottle brush test (Cruz et al., 2022; Kimbrough et al., 2017; Kirson et al., 2021; Somkuwar et al., 2017; Spierling et al., 2020; Steinman et al., 2021) to assess withdrawal or PTSD irritability-like behaviors. As expected, the compound did not have any effect in Wistar controls, but it did exert a significant effect in the msPs. Interestingly, we identified a clear sex difference in the endocannabinoid regulation of stress coping strategies in msP rats. In female msPs, MJN110 reduced aggressive-like behaviors, which can be classified as a more active-coping behavior. In male msPs, however, MJN110 increased defensive behaviors, which is indicative of increased passive-coping behaviors. Importantly, we observed that 5 mg kg−1 MJN110 did not reduce general locomotor activity, which serves as an indication that general active behaviors were not negatively impacted (Supplementary Table 1). Our findings in male rats are in line with recent work (Pavon et al., 2021) showing that MAGL inhibition induces different changes in stress-coping behaviors compared to FAAH inhibition and increases passive-coping behaviors in male mice (Pavon et al., 2021). Therefore, our irritability assessments with the bottle-brush test build up upon previous findings and expand our understanding of sex-dependent mechanisms associated with endocannabinoid-mediated stress responses.
Lastly, we examined the effect of MAGL inhibition on cue-induced fear expression. A separate cohort of male and female Wistar and msP rats was fear conditioned in response to six light cue stimuli (CS) paired with 0.6 mA electric shocks (US) across 2 consecutive days. On the third day, rats were tested for conditioned fear expression after a single systemic administration of MJN110. Our experimental design allowed us to observe a significant fear conditioning in msP vs Wistar rats of both sexes. Importantly, the freezing behavior and the absence of any movements should not be attributed to locomotor activity differences between msP and Wistar rats (Cannella et al., 2016). We choose relatively mild conditions for our protocol (0.6 mA shocks) because msP rats are particularly vulnerable to stress. On the conditioned fear acquisition day 2, msP females showed a significantly higher freezing % versus Wistars across the 6 CS+US, reaching 70% freezing by CS-US6. Similar behavior was observed in males, with the conditioned freezing reaching 60%. Notably, on conditioned fear expression test day (day 3), we found a significant effect of MJN110 in reducing freezing response during the pre-conditioning period, selectively in male msPs. We did observe a similar trend in females, although not statistically significant. Interestingly, acute 2-AG elevation seemed to affect only the pre-CS expression of fear but not the contingent, cue-induced expression of fear (i.e., light CS presentation), indicating a selective role in the regulation of contextual conditioning or possibly non-associative fear versus cue-induced expression of fear memory. Notably, while the MAGL inhibitor JZL184 induces hypomotility and decreases mobility time in male mice, MJN110 does not affect the overall time spent mobile (Ignatowska-Jankowska et al., 2015). Our results partially align with recent studies (Morena et al., 2021) showing that MJN110 administered prior to fear extinction tests did not reduce cued fear memory expression and extinction in male rats but acutely reduced freezing at the last CS presentations. Interestingly, in the same paradigm, MJN110 affected darting behavior in females, a sign of increased active coping behavior. Overall, this evidence points to the hypothesis that increased 2-AG signaling promoted active over passive fear and stress-coping responses in female rats. This shift from passive to active forms of acute coping might be explained by the established role of CB1 receptors on modulating glutamatergic neurons (Metna-Laurent et al., 2012), suggesting that in females elevated 2-AG signaling may preferentially engage this signaling to promote this behavioral transition. Our results, however, are opposed to another study in a male mice line of high anxiety where inhibition of 2-AG hydrolysis increased active, but not passive responses (Heinz et al., 2017).
We acknowledge some limitations of our studies that will be addressed in future work. First, female rats were left to cycle freely, and the estrous cycle was not monitored at the time of testing. Given that stress coping responses and freezing behavior during fear expression vary with estrous phases (Gruene et al., 2015; Wiersielis et al., 2016) and estrous cycle modulates CB1 receptor density and affinity (Rodriguez de Fonseca et al., 1994), as well as AEA and 2-AG levels across different brain regions (Bradshaw et al., 2006; Gonzalez et al., 2000), future studies will address this point. Additionally, only acute effects of MAGL inhibition are reported herein, but the overall effects of the elevation of eCBs is determined by cannabinoid receptor expression and post-receptor signaling, which were not assessed in the present study. Future studies that involve repeated or chronic administration will be translationally informative because the long-term effects of sustained endocannabinoid elevation also will depend on potential downstream changes in CB1 expression and signaling cascades. Second, we used only one dose of MJN110 in most of our behavioral studies (anxiety, irritability, and conditioned fear expression). Future studies will evaluate MJN110 across a wider range of doses, which might uncover dose-dependent effects and reveal more genotype- and sex-dependent differences. We will also perform brain region site-specific administrations of MJN110 to examine potential interactions between local 2-AG elevations and other co-factors (e.g., corticosterone/glucocorticoid receptor signaling) that would help tease apart and identify target regions or circuits involved in our behavioral assessments. Lastly, our conditioned fear experiment design didn’t allow us to discriminate whether the nature of increased pre-CS freezing we observed in the msP rats (and its reversal by MJN110) is contextual or non-associative learning.
Conclusions:
Our results showed that pharmacological enhancement of 2-AG levels did not reduce voluntary alcohol drinking in the 2-BC procedure. Importantly, MAGL inhibition by MJN110 reduced irritability-like behaviors, with potential opposing coping strategies between males and females, and ameliorated contextual or non-associative fear expression but without affecting cue-conditioned fear expression. These effects were selectively observed in msP rats, a genetic rat model of innate and inheritable vulnerability to stress. Altogether our findings suggest that MAGL inhibition is efficacious in attenuating some stress-related maladaptive behaviors, pointing to a selective role of 2-AG in specific aspect of stress symptoms, but not voluntary drinking behaviors. These data open new avenues of investigation of MAGL inhibitors as potential therapies for a subset of patients suffering from AUD and stress-related disorders. Results from this study may inform future research aiming at investigating endocannabinoid regulation of stress-dependent disorders within specific brain regions and neuronal circuits, and with a focus on sex-specific therapeutic approaches. Table 1
Table 1.
Effect of MJN110 on the individual aggressive, defensive, and general active behaviors.
| Wistar | msP | Wistar | msP | |||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | MJN110 | Vehicle | MJN110 | Vehicle | MJN110 | Vehicle | MJN110 | |
| Bite | 0.0±0.0 | 0.0±0.0 | 0.1±0.1 | 0.0±0.0 | 1.5±0.8 | 0.0±0.0 | 0.1±0.1 | 1.3±0.8 |
| Box | 1.0±0.5 | 0.9±0.6 | 1.2±0.7 | 1.5±0.6 | 4.6±2.3 | 1.9±1.3 | 0.4±0.3 | 2.1±0.9 |
| Side | 0.0±0.0 | 0.9±0.6 | 3.9±1.8 | 0.3±0.2 | 0.1±0.1 | 2.6±1.7 | 0.6±0.4 | 3.3±1.1 |
| Follow | 9.4±1.2 | 10.6±1.7 | 11.3±1.1 | 5.8±1.2 | 13.1±3.2 | 7.4±2.0 | 10.1±1.7 | 10.0±1.9 |
| Mount | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 2.0±1.0 | 1.0±0.7 | 0.1±0.1 | 0.1±0.1 |
| Rattle | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| Avoid | 10.8±1.9 | 15.6±2.0 | 10.8±1.7 | 14.9±1.9 | 6.9±1.6 | 11.4±2.2 | 10.0±1.3 | 24.9±2.1 |
| Dig | 3.4±1.4 | 0.6±0.5 | 0.0±0.0 | 0.4±0.4 | 0.3±0.3 | 0.3±3.2 | 0.1±0.1 | 2.1±1.1 |
| Freeze | 0.6±0.6 | 4.8±1.3 | 6.2±2.5 | 9.5±2.8 | 6.8±3.8 | 8.6±3.2 | 4.6±1.9 | 5.4±2.1 |
| Jump | 0.1±0.1 | 0.2±0.3 | 0.0±0.0 | 0.4±0.2 | 0.0±0.0 | 0.1±0.1 | 0.0±0.0 | 0.4±0.2 |
| Startle | 0.5±0.3 | 0.2±0.2 | 0.5±0.3 | 1.1±0.4 | 0.4±0.2 | 0.5±0.3 | 2.3±1.2 | 6.0±1.2 |
| Vocalization | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| Groom | 3.4±1.6 | 0.1±0.1 | 2.1±0.8 | 2.0±0.7 | 0.4±0.3 | 0.0±0.0 | 1.9±0.5 | 0.7±0.4 |
| Rear | 12.4±1.7 | 13.0±1.2 | 10.8±2.2 | 16.1±1.4 | 11.8±1.9 | 10.3±1.3 | 12.1±1.5 | 8.4±2.1 |
| Explore | 7.5±1.7 | 3.4±1.4 | 2.2±0.9 | 1.5±0.8 | 1.5±0.7 | 1.3±1.3 | 1.6±0.5 | 1.4±0.7 |
Data are presented as number of events’ mean ± SEM.
Supplementary Material
Supplementary Figure 2. Acute MJN110 does not change home cage palatable food intake in female rats. Rats were tested in their familiar home cage 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female latency to approach. B) Female pellet intake. Results are expressed as mean ± SEM. Wistar vehicle, n = 8; Wistar MJN110, n = 8; msP vehicle, n = 8; female msP MJN110, n = 8. ## p < 0.01, #### p < 0.0001 main effect of the genotype, two-way ANOVA.
Supplementary Figure 1. MJN110 does not change AEA in prefrontal cortex subregions and central amygdala. AEA levels were measured by LC/MS-MS 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female prelimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). B) Male prelimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). C) Female infralimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). D) Male infralimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). E) Female central amygdala (CeA) (Wistar vehicle, n = 5; Wistar MJN110, n = 3; msP vehicle, n = 5; msP MJN110, n = 4). F) Male central amygdala (CeA) (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). Results are expressed as mean ± SEM.
What is already known.
The endocannabinoid system has emerged as a molecular target for the treatment of stress-related and alcohol use disorders (AUD).
2-AG signaling is a key modulator of the stress response.
What this study adds.
MJN110 significantly inhibits the 2-AG degrading enzyme MAGL, and elevates 2-AG in brain regions involved in the processing of stress and AUD.
MJN110 alleviates stress-related symptoms in a sex-specific manner.
What is the clinical significance.
This study highlights a distinct role for 2-AG in the modulation of stress-related disorders.
Sex-specific endocannabinoid-based approaches should be considered in the clinical development of therapeutics.
ACKNOWLEDGEMENTS
This is manuscript number 30227 from The Scripps Research Institute. This study was supported by National Institutes of Health grants: AA030609 (VV), AA017447 (MR, RC), AA027700 (MR, EZ), AA021491 (MR), AA006420 (MR, EZ), and AA029841 (MR), AA013498 (MR), AA025393 (LAN). The Schimmel Family Chair (MR). The Pearson Center for Alcoholism and Addiction Research. FAPESP postdoctoral fellow 2021/12978-1 (PCB). We thank Dr. Daisuke Ogasawara for synthesizing MJN110 and for technical assistance with the compound.
The conducted research was not preregistered with an analysis plan in an independent, institutional registry.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigor of preclinical research as stated in the BJP guidelines for Design and Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
ABBREVIATIONS
- AUD
alcohol use disorder
- eCB
endocannabinoid
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- MAGL
monoacylglycerol lipase
- CB
cannabinoid receptor
- FAAH
fatty acid amide hydrolase
- DAGLa
diacylglycerol-lipase alpha
- msP
Marchigian-Sardinian alcohol preferring
- PTSD
post-traumatic stress disorder
- CeA
central amygdala
- PrL
prelimbic
- IL
infralimbic
- PFC
prefrontal cortex
- CRF1
corticotropin-releasing factor 1
- LC-MS/MS
liquid chromatography-mass spectrometry
- I.P.
intraperitoneal
- 2-BC
two-bottle choice
- NIH
novelty-induced hypophagia
- CS
conditioned stimulus
- US
unconditioned stimulus
- CS-US
conditioned stimulus-unconditioned stimulus
- ITI
intertrial interval
- DPBS
Dulbecco’s phosphate-buffered saline
- DC
detergent compatible
- HPLC
high-performance liquid chromatography
- MRM
multiple reaction monitoring
- ESI
electrospray ionization
- F
fragmentation
- C
collision
- CA
collision acceleration
- ANOVA
analyses of variance
- RM
repeated measure
- SEM
standard error of the mean
Footnotes
MJN110 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10028
JZL184 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5207
AEA, anandamide https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2364
2-AG, 2-arachidonoylglycerol https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=729
MAGL, monoacylglycerol lipase https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1399
CB1, cannabinoid receptor 1 https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56
CB2, cannabinoid receptor 2 https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57
FAAH, fatty acid amide hydrolase https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1400
DAGLa, diacylglycerol-lipase alpha https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1396
CRF1, corticotropin-releasing factor 1 https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=212
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
AVAILABILITY OF DATA
Data available on request from the authors.
REFERENCES
- Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Davies JA, Abbracchio MP, Alexander W, Al-Hosaini K, Back M, Barnes NM, Bathgate R, … Ye RD (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein-coupled receptors. Br J Pharmacol, 178 Suppl 1, S27–S156. 10.1111/bph.15538 [DOI] [PubMed] [Google Scholar]
- Alexander SP, Cidlowski JA, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Davies JA, Coons L, Fuller PJ, Korach KS, & Young MJ (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Nuclear hormone receptors. Br J Pharmacol, 178 Suppl 1(Suppl 1), S246–S263. 10.1111/bph.15540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Davies JA, Boison D, Burns KE, Dessauer C, Gertsch J, Helsby NA, Izzo AA, Koesling D, … Wong SS (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. Br J Pharmacol, 178 Suppl 1, S313–S411. 10.1111/bph.15542 [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Davies JA, Aldrich RW, Attali B, Baggetta AM, Becirovic E, Biel M, Bill RM, Catterall WA, … Zhu M (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Ion channels. Br J Pharmacol, 178 Suppl 1, S157–S245. 10.1111/bph.15539 [DOI] [PubMed] [Google Scholar]
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, & Cippitelli A (2013). Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front Psychiatry, 4, 23. 10.3389/fpsyt.2013.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtholt AJ, Hill TE, & Lucki I (2007). Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology (Berl), 190(4), 531–540. 10.1007/s00213-006-0615-9 [DOI] [PubMed] [Google Scholar]
- Bedse G, Centanni SW, Winder DG, & Patel S (2019). Endocannabinoid Signaling in the Central Amygdala and Bed Nucleus of the Stria Terminalis: Implications for the Pathophysiology and Treatment of Alcohol Use Disorder. Alcohol Clin Exp Res, 43(10), 2014–2027. 10.1111/acer.14159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G, Hartley ND, Neale E, Gaulden AD, Patrick TA, Kingsley PJ, Uddin MJ, Plath N, Marnett LJ, & Patel S (2017). Functional Redundancy Between Canonical Endocannabinoid Signaling Systems in the Modulation of Anxiety. Biol Psychiatry, 82(7), 488–499. 10.1016/j.biopsych.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, & Cravatt BF (2013). Chemical probes of endocannabinoid metabolism. Pharmacol Rev, 65(2), 849–871. 10.1124/pr.112.006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett RJ, Baldi R, Haymer A, Gaulden AD, Hartley ND, Parrish WP, Baechle J, Marcus DJ, Mardam-Bey R, Shonesy BC, Uddin MJ, Marnett LJ, Mackie K, Colbran RJ, Winder DG, & Patel S (2017). Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun, 8, 14782. 10.1038/ncomms14782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, & Patel S (2014). Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry, 4(7), e408. 10.1038/tp.2014.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto AM, Stopponi S, Li H, Weiss F, Roberto M, & Ciccocioppo R (2021). Genetically selected alcohol-preferring msP rats to study alcohol use disorder: Anything lost in translation? Neuropharmacology, 186, 108446. 10.1016/j.neuropharm.2020.108446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bouju C, Larrieu T, Linders L, Manzoni OJ, & Laye S (2016). Endocannabinoid-Mediated Plasticity in Nucleus Accumbens Controls Vulnerability to Anxiety after Social Defeat Stress. Cell Rep, 16(5), 1237–1242. 10.1016/j.celrep.2016.06.082 [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, & Walker JM (2006). Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol, 291(2), R349–358. 10.1152/ajpregu.00933.2005 [DOI] [PubMed] [Google Scholar]
- Burston JJ, Mapp PI, Sarmad S, Barrett DA, Niphakis MJ, Cravatt BF, Walsh DA, & Chapman V (2016). Robust anti-nociceptive effects of monoacylglycerol lipase inhibition in a model of osteoarthritis pain. Br J Pharmacol, 173(21), 3134–3144. 10.1111/bph.13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Kallupi M, Li HW, Stopponi S, Cifani C, Ciccocioppo R, & Ubaldi M (2016). Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology (Berl), 233(15–16), 2915–2924. 10.1007/s00213-016-4333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Schechter LE, & Lucki I (2011). Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology (Berl), 213(2–3), 499–507. 10.1007/s00213-010-1798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener VS, Gaulden A, Pennipede D, Jagasia P, Uddin J, Marnett LJ, & Patel S (2018). Inhibition of Diacylglycerol Lipase Impairs Fear Extinction in Mice. Front Neurosci, 12, 479. 10.3389/fnins.2018.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, & Massi M (2006). Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol, 11(3–4), 339–355. 10.1111/j.1369-1600.2006.00032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, & Massi M (1999). Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology (Berl), 144(2), 151–157. 10.1007/s002130050988 [DOI] [PubMed] [Google Scholar]
- Cruz B, Vozella V, Carper BA, Xu JC, Kirson D, Hirsch S, Nolen T, Bradley L, Fain K, Crawford M, Kosten TR, Zorrilla EP, & Roberto M (2022). FKBP5 inhibitors modulate alcohol drinking and trauma-related behaviors in a model of comorbid post-traumatic stress and alcohol use disorder. Neuropsychopharmacology. 10.1038/s41386-022-01497-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Sobey CG, Stanford SC, Teixeira MM, Wonnacott S, & Ahluwalia A (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devgun MS, & Dunbar JA (1990). Alcohol consumption, blood alcohol level and the relevance of body weight in experimental design and analysis. J Stud Alcohol, 51(1), 24–28. 10.15288/jsa.1990.51.24 [DOI] [PubMed] [Google Scholar]
- Feja M, Leigh MPK, Baindur AN, McGraw JJ, Wakabayashi KT, Cravatt BF, & Bass CE (2020). The novel MAGL inhibitor MJN110 enhances responding to reward-predictive incentive cues by activation of CB1 receptors. Neuropharmacology, 162, 107814. 10.1016/j.neuropharm.2019.107814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, & Roberto M (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry, 77(10), 859–869. 10.1016/j.biopsych.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Di Marzo V, Ramos JA, & Fernandez-Ruiz JJ (2000). Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun, 270(1), 260–266. 10.1006/bbrc.2000.2406 [DOI] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, & Shansky RM (2015). Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry, 78(3), 186–193. 10.1016/j.biopsych.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, & Heilig M (2007). Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol, 12(1), 30–34. 10.1111/j.1369-1600.2007.00050.x [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, & Ciccocioppo R (2006). Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A, 103(41), 15236–15241. 10.1073/pnas.0604419103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz DE, Genewsky A, & Wotjak CT (2017). Enhanced anandamide signaling reduces flight behavior elicited by an approaching robo-beetle. Neuropharmacology, 126, 233–241. 10.1016/j.neuropharm.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Henricks AM, Berger AL, Lugo JM, Baxter-Potter LN, Bieniasz KV, Petrie G, Sticht MA, Hill MN, & McLaughlin RJ (2017). Sex- and hormone-dependent alterations in alcohol withdrawal-induced anxiety and corticolimbic endocannabinoid signaling. Neuropharmacology, 124, 121–133. 10.1016/j.neuropharm.2017.05.023 [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, & Viau V (2010). Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A, 107(20), 9406–9411. 10.1073/pnas.0914661107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, & Lichtman AH (2015). Selective monoacylglycerol lipase inhibitors: antinociceptive versus cannabimimetic effects in mice. J Pharmacol Exp Ther, 353(2), 424–432. 10.1124/jpet.114.222315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy D, Palese F, Vozella V, Fotio Y, Yalcin A, Ramirez G, Mears D, Wynn G, & Piomelli D (2020). Cannabinoid CB(2) receptors mediate the anxiolytic-like effects of monoacylglycerol lipase inhibition in a rat model of predator-induced fear. Neuropsychopharmacology, 45(8), 1330–1338. 10.1038/s41386-020-0696-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, & Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiol Rev, 89(1), 309–380. 10.1152/physrev.00019.2008 [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Koenen KC, Petukhova M, Hill ED, & Consortium, W. H. O. W. M. H. S. (2012). The importance of secondary trauma exposure for post-disaster mental disorder. Epidemiol Psychiatr Sci, 21(1), 35–45. 10.1017/s2045796011000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, de Guglielmo G, Kononoff J, Kallupi M, Zorrilla EP, & George O (2017). CRF(1) Receptor-Dependent Increases in Irritability-Like Behavior During Abstinence from Chronic Intermittent Ethanol Vapor Exposure. Alcohol Clin Exp Res, 41(11), 1886–1895. 10.1111/acer.13484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Steinman MQ, Wolfe SA, Spierling Bagsic SR, Bajo M, Sureshchandra S, Oleata CS, Messaoudi I, Zorrilla EP, & Roberto M (2021). Sex and context differences in the effects of trauma on comorbid alcohol use and post-traumatic stress phenotypes in actively drinking rats. J Neurosci Res, 99(12), 3354–3372. 10.1002/jnr.24972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM (2018). Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addict Behav, 77, 102–106. 10.1016/j.addbeh.2017.09.024 [DOI] [PubMed] [Google Scholar]
- Kunos G (2020). Interactions Between Alcohol and the Endocannabinoid System. Alcohol Clin Exp Res, 44(4), 790–805. 10.1111/acer.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, & Borchardt C (2000). The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev, 20(2), 149–171. 10.1016/s0272-7358(99)00027-6 [DOI] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, & O’Malley SS (2010). Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol, 15(2), 109–124. 10.1111/j.1369-1600.2009.00192.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Igarashi M, Jung KM, Butini S, Campiani G, & Piomelli D (2016). Endocannabinoid Modulation of Predator Stress-Induced Long-Term Anxiety in Rats. Neuropsychopharmacology, 41(5), 1329–1339. 10.1038/npp.2015.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa SF, Gomes FV, Terzian AL, Aguiar DC, Moreira FA, Resstel LB, & Guimaraes FS (2017). The Endocannabinoid System and Anxiety. Vitam Horm, 103, 193–279. 10.1016/bs.vh.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, & Hillard CJ (2015). The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci, 16(12), 705–718. 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DJ, Bedse G, Gaulden AD, Ryan JD, Kondev V, Winters ND, Rosas-Vidal LE, Altemus M, Mackie K, Lee FS, Delpire E, & Patel S (2020). Endocannabinoid Signaling Collapse Mediates Stress-Induced Amygdalo-Cortical Strengthening. Neuron, 105(6), 1062–1076 e1066. 10.1016/j.neuron.2019.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, & Lutz B (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418(6897), 530–534. 10.1038/nature00839 [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, & Gorzalka BB (2014). A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev, 42, 116–131. 10.1016/j.neubiorev.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Soria-Gomez E, Verrier D, Conforzi M, Jego P, Lafenetre P, & Marsicano G (2012). Bimodal control of fear-coping strategies by CB(1) cannabinoid receptors. J Neurosci, 32(21), 7109–7118. 10.1523/JNEUROSCI.1054-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Akdilek N, Ferreira VMM, Leonard MZ, Marinelli LR, & Covington HE 3rd. (2022). To fight or not to fight: activation of the mPFC during decision to engage in aggressive behavior after ethanol consumption in a novel murine model. Psychopharmacology (Berl), 239(10), 3249–3261. 10.1007/s00213-022-06208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Aukema RJ, Leitl KD, Rashid AJ, Vecchiarelli HA, Josselyn SA, & Hill MN (2019). Upregulation of Anandamide Hydrolysis in the Basolateral Complex of Amygdala Reduces Fear Memory Expression and Indices of Stress and Anxiety. J Neurosci, 39(7), 1275–1292. 10.1523/JNEUROSCI.2251-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Nastase AS, Santori A, Cravatt BF, Shansky RM, & Hill MN (2021). Sex-dependent effects of endocannabinoid modulation of conditioned fear extinction in rats. Br J Pharmacol, 178(4), 983–996. 10.1111/bph.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, & Hill MN (2016). Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology, 41(1), 80–102. 10.1038/npp.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C, Polis I, Ciccocioppo R, Roberto M, & Parsons LH (2017). Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biol Psychiatry, 82(7), 500–510. 10.1016/j.biopsych.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Steinman MQ, McGinn MA, Sureshchandra S, Kerr TM, Ciccocioppo R, Messaoudi I, Edwards S, & Roberto M (2021). Impaired hypothalamic feedback dysregulates brain glucocorticoid signaling in genetically-selected Marchigian Sardinian alcohol-preferring rats. Addict Biol, 26(3), e12978. 10.1111/adb.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB 3rd, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, & Cravatt BF (2013). Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci, 4(9), 1322–1332. 10.1021/cn400116z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, & Hillard CJ (2009). Role of endocannabinoid signaling in anxiety and depression. Curr Top Behav Neurosci, 1, 347–371. 10.1007/978-3-540-88955-7_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, & Winder DG (2009). Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology, 34(13), 2699–2709. 10.1038/npp.2009.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, & Hillard CJ (2005). Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci, 21(4), 1057–1069. 10.1111/j.1460-9568.2005.03916.x [DOI] [PubMed] [Google Scholar]
- Pavon FJ, Polis IY, Stouffer DG, Cravatt BF, Roberto M, Martin-Fardon R, Rodriguez de Fonseca F, Parsons LH, & Serrano A (2021). Selective inhibition of monoacylglycerol lipase is associated with passive coping behavior and attenuation of stress-induced dopamine release in the medial prefrontal cortex. Neurobiol Stress, 14, 100293. 10.1016/j.ynstr.2021.100293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D (2003). The molecular logic of endocannabinoid signalling. Nat Rev Neurosci, 4(11), 873–884. 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Berretta S, Bolshakov VY, Rosso IM, Meloni EG, Rauch SL, & Carlezon WA Jr. (2022). Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol, 18(5), 273–288. 10.1038/s41582-022-00635-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, & Fernandez-Ruiz JJ (1994). Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci, 54(3), 159–170. 10.1016/0024-3205(94)00585-0 [DOI] [PubMed] [Google Scholar]
- Rubio M, McHugh D, Fernandez-Ruiz J, Bradshaw H, & Walker JM (2007). Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci Lett, 421(3), 270–274. 10.1016/j.neulet.2007.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Pavon FJ, Buczynski MW, Schlosburg J, Natividad LA, Polis IY, Stouffer DG, Zorrilla EP, Roberto M, Cravatt BF, Martin-Fardon R, Rodriguez de Fonseca F, & Parsons LH (2018). Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology, 43(9), 1840–1850. 10.1038/s41386-018-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Bluett RJ, Ramikie TS, Baldi R, Hermanson DJ, Kingsley PJ, Marnett LJ, Winder DG, Colbran RJ, & Patel S (2014). Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep, 9(5), 1644–1653. 10.1016/j.celrep.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, & Mandyam CD (2017). Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology, 84, 17–31. 10.1016/j.psyneuen.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling S, de Guglielmo G, Kirson D, Kreisler A, Roberto M, George O, & Zorrilla EP (2020). Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food. Neuropsychopharmacology, 45(4), 579–588. 10.1038/s41386-019-0538-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Kirson D, Wolfe SA, Khom S, D’Ambrosio SR, Spierling Bagsic SR, Bajo M, Vlkolinsky R, Hoang NK, Singhal A, Sureshchandra S, Oleata CS, Messaoudi I, Zorrilla EP, & Roberto M (2021). Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol Psychiatry, 26(7), 3093–3107. 10.1038/s41380-020-00920-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, & Piomelli D (1997). A second endogenous cannabinoid that modulates long-term potentiation. Nature, 388(6644), 773–778. 10.1038/42015 [DOI] [PubMed] [Google Scholar]
- Stopponi S, Fotio Y, Domi A, Borruto AM, Natividad L, Roberto M, Ciccocioppo R, & Cannella N (2018). Inhibition of fatty acid amide hydrolase in the central amygdala alleviates co-morbid expression of innate anxiety and excessive alcohol intake. Addict Biol, 23(6), 1223–1232. 10.1111/adb.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumislawski JJ, Ramikie TS, & Patel S (2011). Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology, 36(13), 2750–2761. 10.1038/npp.2011.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozella V, Cruz B, Natividad LA, Benvenuti F, Cannella N, Edwards S, Zorrilla EP, Ciccocioppo R, & Roberto M (2021). Glucocorticoid Receptor Antagonist Mifepristone Does Not Alter Innate Anxiety-Like Behavior in Genetically-Selected Marchigian Sardinian (msP) Rats. Int J Mol Sci, 22(6). 10.3390/ijms22063095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersielis KR, Wicks B, Simko H, Cohen SR, Khantsis S, Baksh N, Waxler DE, & Bangasser DA (2016). Sex differences in corticotropin releasing factor-evoked behavior and activated networks. Psychoneuroendocrinology, 73, 204–216. 10.1016/j.psyneuen.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe SA, Vozella V, & Roberto M (2022). The Synaptic Interactions of Alcohol and the Endogenous Cannabinoid System. Alcohol Res, 42(1), 03. 10.35946/arcr.v42.1.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley NB, Hill MN, & Christianson JP (2018). Prefrontal endocannabinoids, stress controllability and resilience: A hypothesis. Prog Neuropsychopharmacol Biol Psychiatry, 85, 180–188. 10.1016/j.pnpbp.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Zhang JY, Holmes A, & Pan BX (2021). Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol Psychiatry, 89(9), 847–856. 10.1016/j.biopsych.2020.12.026 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang W, Zhong P, Liu SJ, Long JZ, Zhao L, Gao HQ, Cravatt BF, & Liu QS (2015). Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus, 25(1), 16–26. 10.1002/hipo.22344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, Zhang HT, Cravatt BF, & Liu QS (2014). Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology, 39(7), 1763–1776. 10.1038/npp.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2. Acute MJN110 does not change home cage palatable food intake in female rats. Rats were tested in their familiar home cage 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female latency to approach. B) Female pellet intake. Results are expressed as mean ± SEM. Wistar vehicle, n = 8; Wistar MJN110, n = 8; msP vehicle, n = 8; female msP MJN110, n = 8. ## p < 0.01, #### p < 0.0001 main effect of the genotype, two-way ANOVA.
Supplementary Figure 1. MJN110 does not change AEA in prefrontal cortex subregions and central amygdala. AEA levels were measured by LC/MS-MS 3 hours after a single MJN110 administration (5 mg kg−1, i.p.). A) Female prelimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). B) Male prelimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). C) Female infralimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). D) Male infralimbic cortex (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). E) Female central amygdala (CeA) (Wistar vehicle, n = 5; Wistar MJN110, n = 3; msP vehicle, n = 5; msP MJN110, n = 4). F) Male central amygdala (CeA) (Wistar vehicle, n = 5; Wistar MJN110, n = 5; msP vehicle, n = 5; msP MJN110, n = 5). Results are expressed as mean ± SEM.
Data Availability Statement
Data available on request from the authors.


