An 83-year-old man with a history of melanoma resected from the chest 3 years prior presented with 2 weeks of confusion. Brain MRI identified a 4.4 cm enhancing mass (Figure 1A–B). He underwent subtotal resection. Pathology demonstrated glioblastoma (GBM), IDH-wild type, MGMT promoter unmethylated. Next-generation sequencing revealed microsatellite stability with low tumor mutation burden (3 mutations/Mb), CDK4 amplification, TERT promoter mutation, TP53 mutation, and no EGFR or PDGFRA alterations.
Figure 1.
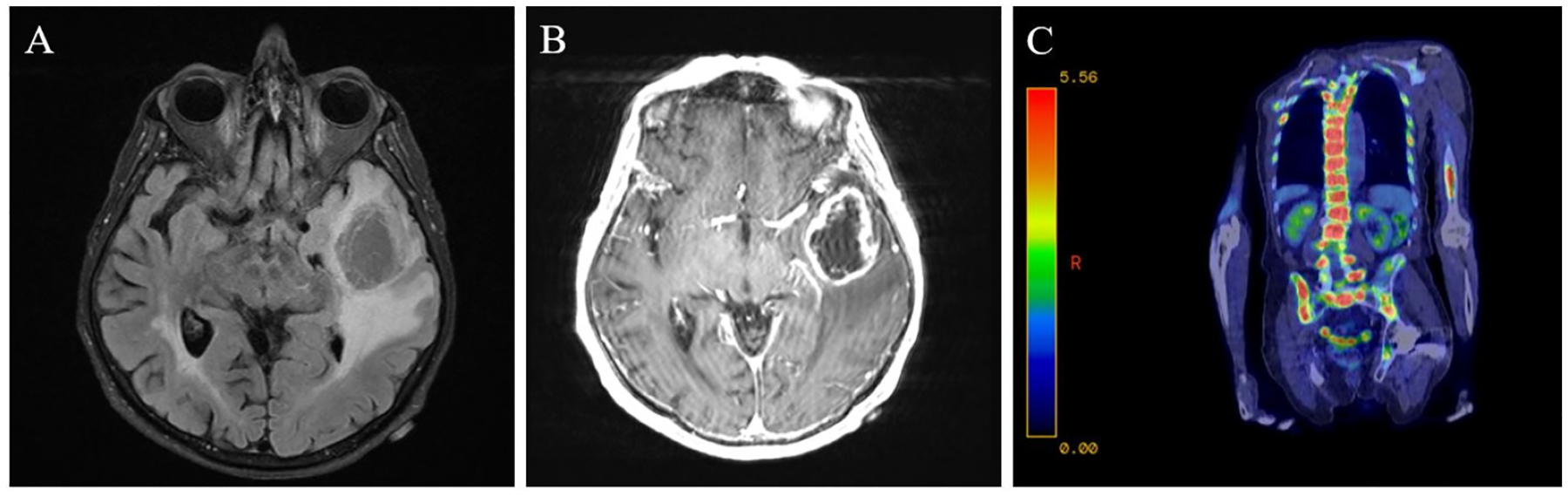
Axial T2 FLAIR MRI of the brain demonstrating a left temporal lobe mass with surrounding vasogenic edema (A) and ring-enhancement on MRI of the brain with contrast (B). PET-CT scan showing extensive metastases to the vertebrae and pelvis (C).
He received proton beam radiation therapy (40 Gy) with concurrent and adjuvant temozolomide as part of a clinical trial. Twenty-two months after initial diagnosis, he had radiographic progression. He was treated with bevacizumab with pembrolizumab, added on a compassionate-use basis due to his strong interest in receiving immunotherapy.
Thirteen months later, a lumbar spine CT scan obtained for fall evaluation revealed lytic lesions. A subsequent PET scan showed extensive additional metastases (Figure 1C). CT-guided bone biopsy confirmed the diagnosis of metastatic GBM. The neoplastic cells stained positive for SOX10, synaptophysin, synaptophysin B, GFAP and OLIG2. The patient experienced rapid clinical deterioration and died four days later.
Extracranial metastasis from GBM is rare, estimated to occur in <2% of cases.1 This rarity has been attributed to the blood brain barrier, the lack of traditional lymphatics within the brain, immunologic suppression of extracranial GBM growth, and difficulty invading extracranial extracellular matrices.2 GBM’s poor prognosis also temporally limits its opportunity to metastasize.3
GBM may spread hematogenously, as circulating tumor cells were detected in 21% of patients’ peripheral blood.4 Some GBM cells can evade the immune response, especially in immunocompromised patients.2 Dissemination through cerebrospinal fluid has also been suggested, especially in those with a ventriculoperitoneal shunt.1 Direct invasion of the skull, the glymphatic system, and transneural spread along peripheral nerves may be other routes for GBM travel.2,3
Risk factors for GBM metastasis include male gender and age <60 at diagnosis.1 Gliosarcomas, accounting for 2% of all glioblastomas, more frequently metastasize extracranially.6 EGFR amplification has been discovered in cases of GBM metastasis with circulating tumor cells.4 BRAF-targeted therapy has achieved a clinical reponse in both intracranial and extracranial metastatic GBM.7 While our patient did not have a diagnosis of gliosarcoma and no EGFR or BRAF mutations were identified in his tumor, bevacizumab has also been associated with early GBM metastasis, which he received at the time of his first progression.5
Metastatic GBM most commonly involves bone (38%), lymph nodes (37%), lungs (32%), and liver (18%),1 all active on our patient’s PET scan. Osseous metastases, which may be lytic or sclerotic, are mostly to thoracic vertebrae and may spread hematogenously via the surrounding extensive venous plexus.8
Due to the limited number of cases, optimal management and prognostic implications of extracranial GBM metastases remain unclear.1 Incidence of extracranial metastasis will likely increase with development of improved therapeutics for GBM. Despite its rarity, clinicians should be aware of GBM’s potential to metastasize, as this pattern of spread can significantly impact patient’s quality of life.
Figure 2.

Low-power (A) and high-power (B) images of tumor pathology from pubic bone biopsy with a spicule of trabecular bone visible in the lower left aspect of Figure 2A. The tumor was highly cellular, composed of compact small round cells with scant cytoplasm and scattered mitotic features. Tumor immunostains were positive for OLIG2 (C) and GFAP (D), consistent with glioblastoma.
Funding statement:
This research was in part funded by the grant number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS).
Statement of authorship:
L.W. performed a literature review and drafted the manuscript, J.L.C. cared for the patient and critically reviewed the manuscript, S.J.C. cared for the patient and critically reviewed the manuscript, M.R. reviewed the pathology, provided pathology imaging, and critically reviewed the manuscript, U.S. conceptualized and supervised the work, cared for the patient, and critically reviewed the manuscript.
Footnotes
Conflicts of interest: The authors do not have any conflicts of interest to disclose.
References
- 1.Kalokhe G, Grimm SA, Chandler JP, Helenowski I, Rademaker A, Raizer JJ. Metastatic glioblastoma: case presentations and a review of the literature. J Neurooncol. 2012;107(1):21–27. doi: 10.1007/s11060-011-0731-1 [DOI] [PubMed] [Google Scholar]
- 2.Lah TT, Novak M, Breznik B. Brain malignancies: Glioblastoma and brain metastases. Semin Cancer Biol. 2020;60:262–273. doi: 10.1016/j.semcancer.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 3.Kumaria A, Teale A, Kulkarni GV, Ingale HA, Macarthur DC, Robertson IJA. Glioblastoma multiforme metastatic to lung in the absence of intracranial recurrence: case report. Br J Neurosurg. 2022;36(2):290–292. doi: 10.1080/02688697.2018.1529296 [DOI] [PubMed] [Google Scholar]
- 4.Müller C, Holtschmidt J, Auer M, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6(247):247ra101. doi: 10.1126/scitranslmed.3009095 [DOI] [PubMed] [Google Scholar]
- 5.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirsching HG, Galanis E, Weller M. Glioblastoma. Handb Clin Neurol. 2016;134:381–397. doi: 10.1016/B978-0-12-802997-8.00023-2 [DOI] [PubMed] [Google Scholar]
- 7.Munjapara V, Heumann T, Schreck KC, et al. BRAF V600E-Mutant Glioblastoma with Extracranial Metastases Responsive to Combined BRAF and MEK Targeted Inhibition: A Case Report. Case Rep Oncol. 2022;15(3):909–917. Published 2022 Oct 7. doi: 10.1159/000525660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong MJ, Koduri S, Allison JA, et al. Bone metastasis from glioblastoma: a systematic review. J Neurooncol. 2022;158(3):379–392. doi: 10.1007/s11060-022-04025-4 [DOI] [PubMed] [Google Scholar]


