Abstract
Rationale
Several lines of evidence indicate that the neurosteroid dehydroepiandrosterone (DHEA) is involved in anxiety. BNN27 is a new DHEA derivative lacking steroidogenic effects. The beneficial effects exerted by BNN27 in preclinical models of schizophrenia and memory disorders have been recently reported.
Objectives
The present study was designed to investigate the effects of this DHEA novel analog on anxiety-like behavior in rats.
Methods
To this end, the light/dark box, the open field, the contextual fear conditioning, and the excessive self-grooming induced by the serotonin 5-HT2c receptor agonist mCPP tests were utilized.
Results
Animals treated acutely with BNN27 (1, 3, and 6 mg/kg) dose dependently spent more time in the bright compartment of the light/dark box and in the central zone of the open field with respect to their vehicle-treated cohorts. Further, BNN27 reduced freezing behavior and weakened the mCPP-induced excessive self-grooming.
Conclusions
Our data indicate that BNN27 is a highly potent anxiolytic agent, as in all studied paradigms it showed anxiolytic-like effects in male rats.
Keywords: DHEA, BNN27, Anxiety, Rat
Introduction
Anxiety is characterized by a flexible psychological and behavioral status that promote coping when encountered with a potential threat. Anxiety may be turn into a pathological state and interfere with coping. Anxiety disorders comprising generalized anxiety disorder (GAD), phobias, post-traumatic stress disorder (PTSD), and panic disorder are a major public health issue worldwide (Steimer 2002).
So far, molecules acting on the γ-aminobutyric acid (GABA) and serotonergic system, like benzodiazepines, partial agonists of the serotonergic 5-HT1A receptor, and selective serotonin reuptake inhibitors (SSRIs), are widely used for the alleviation of anxiety symptoms. Nonetheless, some forms of anxiety do not respond to pharmacological treatment (Hammer et al. 2004; Van Ameringen et al. 2004).
Further, serious undesired side effects (i.e., sedation, memory impairments, dependence and withdrawal, sexual dysfunction, and increase of body weight) are revealed following treatment with either benzodiazepines or SSRIs. In addition, buspirone, a 5-HT1A receptor partial agonist, although is not associated with appreciable toxicity is scarcely utilized since its efficacy is low (Cryan and Sweeney 2011). Therefore, there is a pressing need for new medications with high efficacy and low toxicity for the treatment of the various forms of the anxiety disorders (Gorman 2003).
It is well documented that steroid hormones exert a regulatory action in growth maturation and differentiation of the brain. Dehydroepiandrosterone (DHEA) and its metabolite dehydroepiandrosterone sulfate (DHEAS) are synthesized in the adrenal glands and in the brain (Baulieu and Robel 1998). In a series of studies, the neuroprotective, antioxidant, and anti-inflammatory profile of DHEA and DHEAS has been emerged (Maninger et al. 2009). These neuroprotective properties expressed by DHEA seem to be dependent on their ability to bind and activate both tyrosine kinase (Trk) and pan-neurotrophin p75 (p75NTR) receptors (Charalampopoulos et al. 2004; Lazaridis et al. 2011; Pediaditakis et al. 2015).
The implication of the DHEA in anxiety has been suggested (Eser et al. 2006). High serum DHEA levels have been observed in patients suffering from panic (Tait et al. 2002) and PTSD (Rasmusson et al. 2004; Spivak et al. 2000). Interestingly, it has been proposed that this increase of DHEA and DHEAS concentrations, in response to adrenocorticotropic hormone (ACTH) stimulation, appears to be critical for the attenuation of the anxiety symptom and may be regarded as a compensatory response to stress (Rasmusson et al. 2010). In addition, treatment with DHEA was found to improve anxiety symptoms in schizophrenia patients (Stros et al. 2003). Preclinical findings also corroborate for a role of DHEA in anxiety. It has been reported that administration of DHEA induced an anti-anxiety-like behavior in rodents revealed in unconditioned exploration-driven anxiety tests such as the elevated plus maze (EPM) and the open field (OF) tests (Fedotova and Sapronov 2004; Maayan et al. 2006; Melchior and Ritzmann 1994). There is scarce evidence, however, whether DHEA could display an anti-anxiety-like effect in a conditioned non-exploration driven model of anxiety. Finally, it cannot be underestimated the serious undesired endocrine effects (hormone-dependent neoplasias) due to DHEA’s ability to be metabolized into estrogens, androgens, and progestins (Klinge et al. 2018; Webb et al. 2006). Based on the above, the potential clinical utilization of DHEA appears problematic.
BNN27 is a new synthetic derivative of DHEA, which, unlike DHEA, lacks androgenic or estrogenic undesired action since it does not activate steroid hormone receptor (Calogeropoulou et al. 2009). BNN27 is a small lipophilic compound, well tolerated, which crosses the blood brain barrier (BBB) (Bennet et al. 2016). In contrast to DHEA, BNN27 presents a high affinity for the TrkA and p75NTR receptors of nerve growth factor (NGF) but does not affect pain thresholds (Pediaditakis et al. 2015; 2016a, b).
In a series of studies, BNN27’s antiapoptotic, anti-inflammatory, and antioxidant properties have been evidenced. Specifically, BNN27 was found to protect the PC12 cell line against serum deprivation–induced apoptosis at nanomolar concentrations (Calogeropoulou et al. 2009) and rescued from apoptosis TrkA-positive sympathetic sensory neurons and p75NTR-expressing TrkA-negative cerebellar granule neurons (Pediaditakis et al. 2016a). In addition, BNN27 reduced the pro-inflammatory factors, tumor necrosis factor alpha (TNFα), and interleukin-1 beta (IL-1β) while it increased the anti-inflammatory (IL-10 and Il-4) cytokine levels (Glajch et al. 2016). BNN27 was shown to attenuate the loss of motor neurons co-cultured with astrocytes derived from amyotrophic lateral sclerosis (ALS) patients with superoxide dismutase (SOD) mutations via the reduction of oxidative stress (Iban-Arias et al. 2018). BNN27 (30 and 90 mg/kg) reduced locomotor activity and exploration in rats but, when it administered at 30 mg/kg, did not affect animals’ performance in the light/dark (L/D) and forced swimming (FS) tests which are procedures measuring anxiety-like and depression-like behavior, respectively, in rodents (Kokras et al. 2020). On the contrary, acute challenge with a low dose range (3 and 6 mg/kg) of BNN 27 counteracted behavioral deficits, including cognition impairments, revealed either in glutamatergic or dopaminergic models of schizophrenia in rats (Pitsikas et al. 2021; Zoupa et al. 2019).
In this context, it is important to emphasize that the outcome of clinical and preclinical research suggests that oxidative stress and inflammation are involved in the pathogenesis of anxiety disorders (Michopoulos et al. 2017; Salim 2014; Smaga et al. 2015). Cognitive impairments are also observed in anxiety patients (Gkintoni and Ortiz 2023; Gulpers et al. 2022; Yang et al. 2015). Moreover, it is well documented that anxiety disorders are a typical feature in schizophrenia patients (for review see Braga et al. 2013).
Up to now, there is no information whether acute exposure to a low dosage of BNN27 (3 and 6 mg/kg) which exerted a beneficial effect in animal models resembling schizophrenia could induce anti-anxiety-like behavior in rats. The present study was designed aiming to elucidate this issue. To this end, the L/D (Crawley and Goodwin 1980); the OF (Prut and Belzung 2003), which are both unconditioned exploration-driven models of anxiety (Bouwknecht and Paylor 2008); and the contextual fear conditioning (CFC), which is a conditioned non-exploration driven model of anxiety (Resstel et al. 2006), tests were used. Finally, the ability of BNN27 to attenuate compulsive behavior (excessive self-grooming) induced by the serotonin 5-HT2c receptor agonist (mCPP) (Bagdy et al. 1992) was also assessed.
Materials and methods
Subjects
Independent groups of naïve 3-month-old male Wistar rats (144 animals) (Hellenic Pasteur Institute, Athens, Greece) weighing 250–300 g were used. The animals were housed in Makrolon cages (47.5 cm length × 20.5 cm height × 27 cm width), three per cage, in a regulated environment (21 ± 1 °C; 50–55% relative humidity; 12-h/12-h light/dark cycle, lights on at 07.00 h) with free access to standard laboratory diet (pellets) for rats and water.
The procedures that involved animals and their care were conducted in conformity with the international guidelines in compliance with international guidelines and national (Animal Act, P.D. 160/91) and international laws and policies (EU Directive 2010/63). Experiments were approved by the local committee (Prefecture of Larissa, Greece, protocol number 386501/2023). Every effort was made to minimize the number of animals used and their suffering.
Behavior
Experimental protocol
Experiments were conducted between 10.00 and 14.00 h in a room where only these animals were housed. Different populations of rats were used across different experiments. Each rat was tested only once. On the test day, the rats were transported to the test room and left in their home cages undisturbed for 2 h. To avoid the presence of olfactory cues, all the apparatuses were thoroughly cleaned with 20% ethanol and then wiped with dry paper after each trial. Animals’ behavior was video recorded. Data evaluation (of all four experiments except motility data of experiment 4) was subsequently performed using a stopwatch by experimenters who were unaware of the pharmacological treatment of each subject. Motor activity data evaluation of the experiment 4 was provided automatically from the test apparatus.
Drugs
All solutions were freshly prepared on the day of testing and were administered intraperitoneally (i.p.) in a volume of 1 ml/kg. BNN27 [(20R)-3β,21-dihydroxy-17R,20-epoxy-5-pregnene] was synthesized at the National Research Foundation (Calogeropoulou et al. 2009). BNN27 was suspended in saline (NaCl 0.9%) containing 0.1% Tween 80 and was sonicated for 5 min. mCPP (1-(3-chlorophenyl)piperazine) (Sigma, St. Louis, MO, USA) was dissolved in saline. Control animals received isovolumetric amounts of the specific vehicle solution used in each study. mCPP dose (0.6 mg/kg) was selected based on prior findings which this dose was found to cause excessive self-grooming (Graf et al. 2003; Peristeri and Pitsikas 2022).
Light/dark (L/D) test
The L/D box apparatus consisted of a wooden box (48 cm length × 24 cm height × 27 cm width) divided into two equal size compartments by a barrier that contained a doorway (10 cm height × 10 cm width). One of the compartments was painted black and was covered with a lid, and the other compartment was painted white and illuminated with a 60-W light bulb (Merlo Pich and Samanin 1989) positioned 40 cm above the upper edge of the box. The test was performed as described previously (Grivas et al. 2013). The animals were placed in the middle of the lit compartment, facing away from the dark chamber. The rats were allowed to freely explore the apparatus for 5 min. The observed variables were (a) the latency to enter (with all four paws) the dark compartment, (b) the number of transitions between the two compartments, and (c) the time spent in the light and dark compartments.
Open field (OF) test
The test apparatus consisted of an open box made of PPLEXIGLAS (70 cm length × 50 cm height × 70 cm width). The open field arena was divided by black lines into 16 squares of 17.5 × 17.5 cm2. The central four squares were defined as the central zone, in which animals’ activity was regarded as a measure of anxiety (Prut and Belzung 2003). The test was performed as described previously (Grivas et al. 2013). On the test day, each animal was then placed in the same corner of the open field arena and its behavior was recorded for 5 min. The observed variables were (a) the amount of the time spent in the central zone of the open field arena as defined by all forepaws being in the central four squares of the apparatus, (b) the number of squares crossed (i.e., horizontal activity), (c) the number of rearing behaviors (i.e., vertical activity, defined as raising both forepaws above the floor while balancing on hind limbs), and (d) the duration of grooming events.
Contextual fear conditioning (CFC) test
The apparatus consisted of a box made of PLEXIGLAS (50 cm length × 50 cm height × 50 cm width) with a grid floor composed of 17 stainless steel rods (3 mm in diameter). Electric shocks were delivered to the grid floor by an isolated electric shock generator.
For assessing in animals’ freezing behavior, a procedure adapted from a previous study was utilized (Gravious et al. 2006). On day 1, rats were placed individually into the chamber and received a single 2-min habituation trial. On day 2, the contextual conditioning trial was conducted. Rats were placed again individually into the apparatus, and after a 5-min period of acclimatization, three electric foot shocks (0.5 mA, 1 s) (Gravious et al. 2006) were delivered. The interval between the electric shocks was 1 min. One minute following the last foot shock, animals were removed from the apparatus and returned to their home cages. Testing was carried out 24 h after contextual conditioning, on day 3. The animals were again placed individually into the apparatus, and their freezing behavior (total amount of time) was recorded for 5 min. Freezing was defined as the total absence of body movements except for movement related to respiration.
Self-grooming behavior
Rats’ grooming behavior was assessed in an activity cage (catalog number 7420, Ugo Basile, Varese, Italy). The apparatus consisted of a box made of PLEXIGLAS (41 cm length × 33 cm height × 41 cm width). Every movement of the rat produced a signal caused by vibrations in the inductance and capacitance of resonance circuitry of the apparatus. The signals were then automatically converted into numbers that reflected horizontal activity counts. Changes in activity counts represent a standard behavioral assay for testing the motoric effects of drugs. For evaluating in rats’ grooming behavior, a procedure modified from previous studies was used (Graf et al. 2002; Peristeri and Pitsikas 2022). On day 1, rats received a single 10-min habituation session in the apparatus. On day 2, following appropriate treatment, animals were placed again into the apparatus, and the duration of grooming events was recorded for 20 min. Vibrations (unusual spontaneous behaviors); the nose, face, and head wash; body grooming; scratching; paw licking; head shaking; tail and genital grooming were considered components of grooming behavior (Graf et al. 2003). Further, locomotor activity, expressed as total counts over 20 min and number of rearing episodes (i.e., defined as raising both forepaws above the floor while balancing on hind limbs), were recorded.
Experiments 1, 2, and 3: effects of acute administration of low doses of BNN27 on rats’ performance in the L/D OF and CFC tests
Animals were randomly divided into four experimental groups with eight rats per group as follows: vehicle, BNN27 1 mg/kg, BNN27 3 mg/kg, and BNN27 6 mg/kg. To examine the effects of acute treatment with BNN27 on rats’ performance in the L/D, OF, and CFC tests, rats received a single injection of different doses of BNN27 or vehicle 40 min before testing (Pitsikas et al. 2021). Concerning the CFC test, vehicle and the different doses of BNN27 were injected on day 3, 40 min before testing (Jacob et al. 2009).
Experiment 4: effects of acute administration of low doses of BNN27 in counteracting mCPP-induced excessive self-grooming
Rats were randomly divided into six experimental groups (eight rats per group) as follows: vehicle + vehicle, vehicle + BNN27 3 mg/kg, vehicle + BNN27 6 mg/kg, mCPP 0.6 mg/kg + vehicle, mCPP 0.6 mg/kg + BNN27 3 mg/kg, and mCPP 0.6 mg/kg + BNN27 6 mg/kg. BNN27 and mCPP were administered 40 and 10 min, respectively, before testing (Graf et al. 2003; Pitsikas et al. 2021). Control animals received the respective vehicles 40 and 10 min, respectively, before testing.
Statistical analysis
Data from experiments 1, 2, and 3 were expressed as mean ± SEM and were analyzed using one-way analysis of variance (ANOVA) test. The factor was treatment. Post hoc comparisons between treatment means were made using the Tukey’s t test. Self-grooming duration data from experiment 4 were not normally distributed (Shapiro–Wilk normality test failed, p < 0.05). Therefore, these data were expressed as medians and interquartile ranges and were analyzed using the Kruskal–Wallis non-parametric test. Post hoc pairwise multiple comparisons were made using the Newman-Keuls test. The other data from experiment 4 (locomotor activity and number of rearings) were expressed as mean ± SEM and were analyzed utilizing the two-way ANOVA test. The factors were mCPP and BNN27. A p value of < 0.05 was considered significant (Kirk 1968).
In all experiments, variances were homogeneous, and data were normally distributed (except self-grooming duration data of experiment 4).
Results
Experiment 1: effects of acute administration of low doses of BNN27 on rats’ performance in the L/D test
Treatment with BNN27 did not affect the first entry into to the dark chamber (F3, 31 = 0.433, p = 0.702, not significant (n.s)) (Fig. 1A) and the number of transitions between the two compartments (F3, 31 = 2.187, p = 0.112, n.s) (Fig. 1B). Interestingly, BNN27 significantly increased the total time spent in the light chamber as revealed by a statistically significant effect of treatment (F3, 31 = 4.640, p = 0.009). The post hoc analysis conducted on these data showed that the vehicle-treated rats spent significantly less time in the lit chamber compared to their counterparts treated either with 3 or 6 mg/kg BNN27 (p < 0.05; Fig. 1C).
Fig. 1.
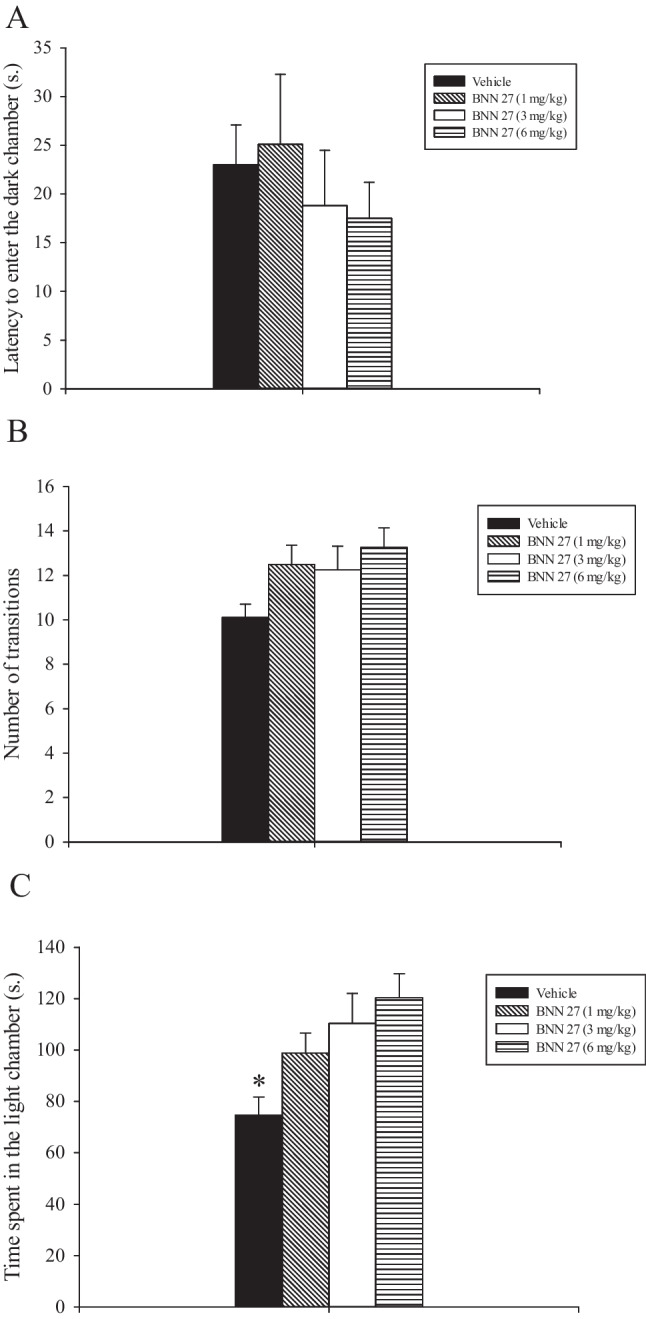
Light/dark test. Vehicle and BNN27 were injected i.p., 40 min before testing. Results are expressed as mean ± SEM. A Latency to enter the dark chamber. B Number of transitions. C Time spent in the light chamber. *p < 0.05 vs. all the other groups
Experiment 2: effects of acute administration of low doses of BNN27 on rats’ performance in the OF test
The effects of treatment with BNN27 on animals’ performance in the OF test are illustrated in Table 1. BNN27 did not affect the number of squares crossed (F3, 31 = 1.723, p = 0.185; n.s) and the rearing episodes (F3, 31 = 2.557, p = 0.075; n.s). BNN27 appeared to reduce grooming duration, but this effect did not reach a statistical significance (F3, 31 = 2.727, p = 0.063, n.s). By contrast, treatment with BNN27 increased the time spent in the central zone of the apparatus (F3, 31 = 3.638, p = 0.025). The post hoc comparisons showed that rats treated with 3 and 6 mg/kg BNN27 spent more time in the central zone of the OF apparatus compared to their vehicle-treated cohorts (p < 0.05).
Table 1.
Effects of acute treatment with BNN 27 on rats’ performance in the open field test
| Treatment | Number of squares crossed | Number of rearings | Time spent in the central zone (s) | Grooming duration (s) |
|---|---|---|---|---|
| Vehicle | 86.1 ± 3.3 | 32.3 ± 0.6 | 6.4 ± 2* | 12.4 ± 2.7 |
| BNN 27 (1 mg/kg) | 98.4 ± 2.9 | 32.5 ± 0.7 | 9 ± 1.1 | 6.8 ± 2.3 |
| BNN 27 (3 mg/kg) | 94 ± 5.4 | 35.6 ± 1.1 | 14.5 ± 2.4 | 7.3 ± 1.3 |
| BNN 27 (6 mg/kg) | 99 ± 5.7 | 35.3 ± 1.7 | 14.3 ± 2.6 | 4.9 ± 1.1 |
Data are expressed as mean ± SEM. of eight rats per treatment group. Vehicle and BNN 27 were injected intraperitoneally 40 min before testing
*p < 0.05 vs. the BNN27 3 and 6 mg/kg groups
Experiments 3: effects of acute administration of low doses of BNN27 on rats’ performance in the CFC test
The effects of acute challenge with BNN27 on animals’ performance in the CFC test are depicted in Fig. 2. Treatment with BNN27 significantly reduced freezing duration (F3, 31 = 4.787, p = 0.008). The post hoc analysis conducted on these data showed that freezing levels of rats treated with 3 and 6 mg/kg BNN27 were significantly lower as compared to those expressed by the vehicle-treated animals (p < 0.05).
Fig. 2.
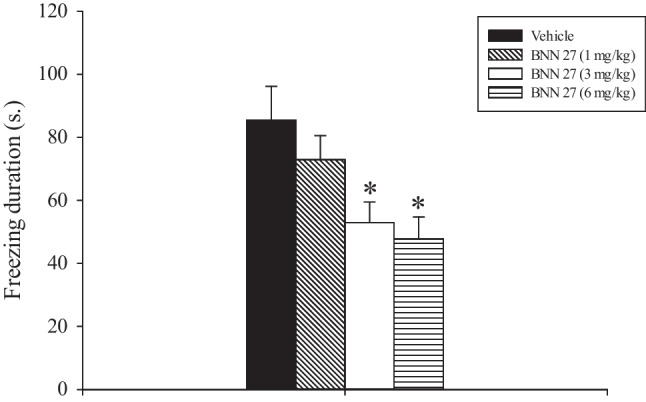
Contextual fear conditioning test. Vehicle and BNN27 were injected i.p., 40 min before testing. Results are expressed as mean ± SEM. *p < 0.05 vs. all the other groups
Experiment 4: effects of acute administration of low doses of BNN27 in counteracting mCPP-induced excessive self-grooming
Statistical analyses of self-grooming duration data showed an statistically significant effect of treatment: H(5) = 19.977, p = 0.001. These results indicate that rats receiving mCPP plus vehicle displayed higher self-grooming in comparison to all the other experimental groups including the mCPP + BNN27 3 mg/kg and the mCPP + BNN27 6 mg/kg-treated animals (p < 0.05; Table 2).
Table 2.
Effects of acute treatment with BNN27 on excessive self-grooming induced by mCPP
| Treatment | Grooming duration (s) |
|---|---|
| Vehicle + vehicle | 116.5 (99.75–146.25) |
| Vehicle + BNN27 (3 mg/kg) | 111.5 (91.75–146) |
| Vehicle + BNN27 (6 mg/kg) | 104 (65.25–136.5) |
| mCPP (0.6 mg/kg) + vehicle | 236.5 (174.75–282.5)* |
| mCPP + BNN27 (3 mg/kg) | 174.5 (147.75–197.5) |
| mCPP + BNN27 (6 mg/kg) | 189.5 (122.75–210.5) |
Data are expressed as medians and interquartile ranges of eight rats per treatment group. mCPP and BNN 27 were injected intraperitoneally 40 and 10 min, respectively, before testing
* p < 0.05 vs. all the other groups
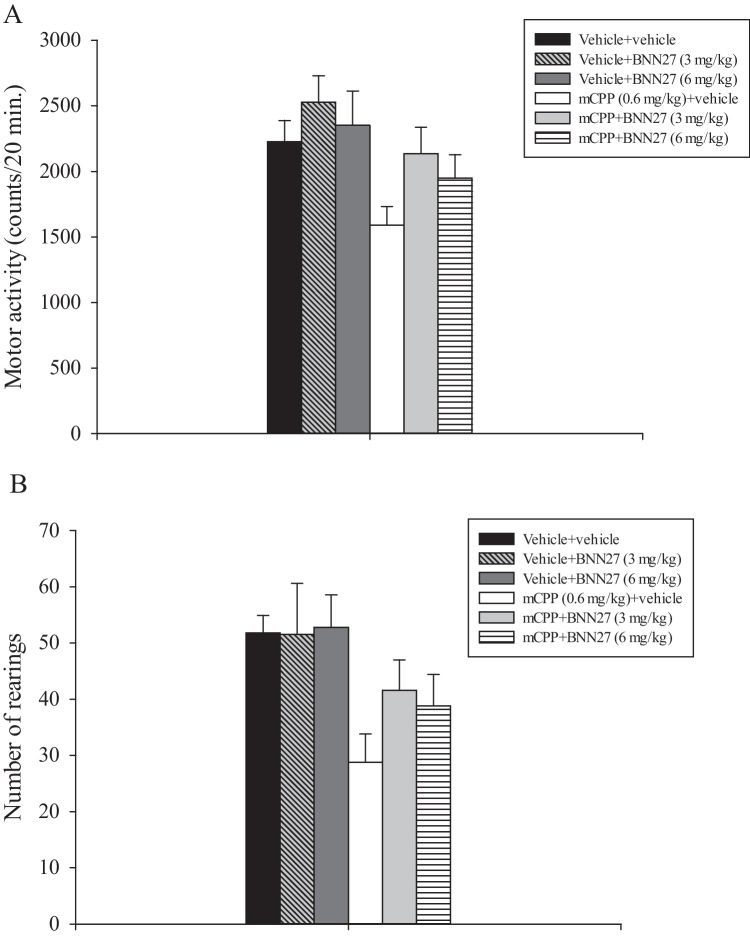
A two-way ANOVA conducted on motility data revealed a significant main effect of mCPP (F1, 47 = 8.962, p < 0.005), but not of BNN27 (F2, 47 = 2.369, p < 0.106, n.s.), or a significant interaction between mCPP and BNN27 (F2, 47 = 0.247, p < 0.783, n.s.; Fig. 3A). These results indicate that all groups of rats receiving mCPP displayed lower motility levels respect to all the other experimental groups. Post hoc comparisons between treatment means were not conducted since a significant interaction between mCPP and BNN27 was not reached (Kirk 1968).
Fig. 3.
Self-grooming test. Vehicle, mCPP, and BNN27 were injected i.p., 40 min and 10 min, respectively, before testing. Results are expressed as mean ± SEM. A Locomotor activity. B Number of rearings
Analysis of rearing episodes’ data evidenced a main effect of mCPP (F1, 47 = 10.456, p < 0.002), but not of BNN27 (F2, 47 = 0.673, p = 0.515, n.s.), or a significant interaction between mCPP and BNN27 (F2, 47 = 0.643, p = 0.531, n.s.; Fig. 3B). These findings suggest that all groups of rats receiving mCPP displayed lower number of rearing episodes as compared to all the other experimental groups. Post hoc pairwise multiple comparisons between treatment means were not performed since a significant interaction between mCPP and BNN27 was not achieved (Kirk 1968).
Discussion
L/D test has been shown to reliably predict the anxiolytic- and anxiogenic-like effects of drugs in rodents. L/D test is a procedure that is based on the innate aversion of rodents for highly illuminated areas and on their spontaneous exploratory behavior in response to mild stressors that is a novel environment and light (Crawley and Goodwin 1980). This test has the advantages of being quick and easy to use without prior training of the animals and neither food nor water deprivation is required (Bourin and Hascoet 2003). Transitions in this test are considered an index of activity/exploration because habituation over time is seen with this measure, whereas the time spent in each chamber reflects aversion/attraction (Belzung et al. 1987). Acute administration of 3 and 6 but not 1 mg/kg BNN27 to rats increased the time spent in the light chamber of the L/D box compared to their vehicle-treated cohorts. Further, BNN27 did not influence both the latency of the first entry into the dark chamber of the apparatus and the number of transitions between the two compartments of the apparatus.
OF test is a standard neophobic test of anxiety. It involves encounter with a novel environment and give rise to behavioral and physiological reactions related to anxiety. In this test, rodents usually tend to avoid open spaces. Thus, the time spent in the central area of an open field arena is a measure of an anxiety state (Prut and Belzung 2003). A single injection of BNN27 (3 and 6 but not 1 mg/kg) significantly augmented the time spent by rats in the central zone of the OF apparatus.
Exposure to a novel environment increases also self-grooming tendencies (Jolles et al. 1979). Self-grooming is a congenital rodent behavior characterized by a sequential pattern of movements, reflects compulsive behavior, and is considered an anxiety index (Estanislau et al. 2019). BNN27 at 6 mg/kg seemed to reduce grooming activity, but this effect did not reach a statistical significance.
CFC test is considered a preclinical model of anxiety (e.g., Biojone et al. 2011; Jacobs et al. 2009; Krystal et al. 2012; Yan et al. 2016) and might resemble PTSD symptoms (e.g., freezing) (Bertaina-Anglade et al. 2017; Hooversmith et al. 2019; Torok et al. 2019). PTSD is a major anxiety disorder that may develop after an individual has experienced or witnessed a severe traumatic event (Torok et al. 2019). CFC measures fear, in terms of freezing, linked to a context where foot shock occurred (Pain et al. 2002; Resstel et al. 2006). BNN27 (3 and 6 but not 1 mg/kg) was found efficacious in attenuating freezing behavior.
The above reported findings cannot be attributed to a potential effect of BNN27 on motility since the number of transitions between the two different chambers of the L/D box, the number of squares crossed and the number of rearings recorded in the OF were not dissimilar among the different treatment groups. An unspecific motoric effect of BNN27 on rats’ performance in the CFC can also be ruled out since at this low dose range, BNN27 did not modify animals’ motor activity (Pitsikas et al. 2021) while reduction of it has been observed at a high dose of BNN27 (90 mg/kg) (Kokras et al. 2020).
The anxiogenic properties of the selective 5-HT2c receptor agonist mCPP are evidenced in either preclinical or clinical studies (e.g., Charney et al. 1987; Singewald et al. 2003). Further, it has been reported that mCPP exaggerates self-grooming in rats (Bagdy et al. 1992; Graf et al. 2003; Peristeri and Pitsikas 2022).
In agreement with prior results (Bagdy et al. 1992; Graf et al. 2003; Peristeri and Pitsikas 2022), acute exposure to mCPP (0.6 mg/kg) significantly raised up self-grooming activity in rats and reduced both horizontal motor activity and number of rearings. Acute administration of BNN27 (3 and 6 mg/kg) attenuated excessive self-grooming caused by mCPP. BNN27, at any dose tested, did not affect grooming activity in control animals. BNN27 appeared to counteract the effects of mCPP on horizontal (hypomotility) and vertical activity (decrease of rearings), but this action did not achieve a statistical significance. These latter results suggest that the effects of BNN27 on self-grooming might be unrelated to its action on parameters reflecting physical activity.
Although mCPP displays affinity for the family of the 5-HT2 receptors, its action on self-grooming has been shown to be mediated by the 5HT2c receptor (Graf et al. 2003). The 5-HT2c receptor is located in brain regions critically involved in anxiety and OCD, including the basal ganglia and orbitofrontal and cingulate cortices (Clemmet et al. 2000; Pasqualetti et al. 1999; Pompeiano et al. 1994; Santana and Artigas 2017). Accordingly, we hypothesize that BNN27 might counteract the effects of mCPP on self-grooming by an antagonistic action at the 5-HT2c receptor site. Additional research is required to elucidate this point.
Summarizing, the present results indicate that BNN27, like DHEA, expressed an anti-anxiety action in unconditioned exploration-driven procedures that are based on the conflict between the desire to explore and avoidance of novel spaces as are the L/D and OF tests. Moreover, BNN27 displayed an anxiolytic effect in a conditioned non-exploration driven model of anxiety (Bouwknecht and Paylor 2008) such as the CFC test. Interestingly, the effective anti-anxiety doses of BNN27 (3 and 6 mg/kg) are the same that exerted beneficial actions in animal models of cognition (Pitsikas and Gravanis 2017) and schizophrenia (Pitsikas et al. 2021; Zoupa et al. 2019). BNN27 administered acutely at 1 mg/kg did not express any appreciable biological activity.
Results of the present study are in partial contrast with previous work in which a higher dose range of BNN27 than that used in our study was found to suppress motility (90 mg/kg) and exploration (30 and 90 mg/kg). Additionally, BNN27 (30 mg/kg) did not affect rats’ performance in procedures reflecting anxiety (L/D test) or depression (FS test). Further, a lower dose of BNN27 (10 mg/kg) did not influence motility, exploration, and did not have an impact on rats’ performance in the L/D and FST tests (Kokras et al. 2020).
Overall, the anxiolytic effects of BNN27 were observed at 3 and 6 mg/kg but not at the “side” doses of 1, 10, 30, and 90 mg/kg. This pattern of results suggests that a bell-shaped dose–effect curve might underlie BNN27’s biological effects. At present, the biological bases of the bell-shaped dose–response curves are unknown, although receptor fatigue or tachyphylaxis (Day 1979) has been proposed as potential mechanisms (Martinez 1986). Higher doses of BNN27 may have two repercussions: (a) activate other receptors with lower affinity compared to TrkA/p75NTR receptors, which might counteract its anti-anxiety effects, and (b) provoke desensitization through internalization of its TrkA/p75NTR receptors and their known long-lasting turnover and their intracellular trapping (Pediaditakis et al. 2016a).
The mechanism(s) of action by which BNN27 might exert its anti-anxiety effects is still under investigation. Research is required to elucidate the exact role of BNN27 in anxiety. In this context, it has been recently shown that a low dose (10 mg/kg) of BNN27 was able to increase GABA concentrations in the hippocampus in either male or female rats. It has been suggested that an increase of GABA levels in hippocampus might be correlated with a potential anxiolytic activity (Holm et al. 2011). This latter observation might provide a support for the anti-anxiety-like behavior expressed by BNN27 revealed in the present study. Recent evidence indicates that deletion of p75NTR receptors in mice leads to physiological and morphological changes in the amygdala and altered anxiety behavior that is linked to the limbic system (Busch et al. 2017; Puschban et al. 2016). It is thus possible that BNN27 might affect anxiety through its interaction with p75NTR receptors and cross talk with the 5-HT2 or GABAA receptors strongly involved in anxiety circuits (Pediaditakis et al. 2016b).
Moreover, the potent antioxidant and anti-inflammatory properties of BNN27 evidenced in different studies and described above might also provide an alternative explanation of the present findings.
The current study presents some limitations. The effects of BNN27 were shown following acute treatment solely in behavioral studies conducted exclusively in male rats. Importantly, it is well documented that the prevalence of anxiety for females is roughly twice that for males (Kessler et al. 2012).
Additional research, therefore, is mandatory to definitively establish the efficacy of BNN27 as an anxiolytic agent. The investigation of the potential anti-anxiety-like action of BNN27 on both male and female rodents across a large variety of behavioral paradigms might be of high translational value. Further, treatment strategies should include either acute or prolonged administration of the compound. Biochemical, molecular, and electrophysiological studies should also be conducted aiming to provide a solid support to the here presented behavioral results.
In summary, the present findings indicate that the DHEA-synthetic derivative BNN27 which devoid of the undesired endocrine effects of DHEA expressed an anti-anxiety-like behavior revealed in a battery of behavioral procedures resembling different subtypes of anxiety disorders. The current results, although preliminary, offer a new lead molecule, BNN27, to develop new therapeutic agents for the treatment of anxiety.
Funding
Open access funding provided by HEAL-Link Greece.
Data Availability
The data that support the findings of this study are available upon request from the corresponding author.
Declarations
The authors declare that the experiments comply with the current laws of Greece.
Conflict of interest
All authors, except Achille Gravanis, declare that they have not any competing financial interests in relation to the work described. Dr. Achille Gravanis is the co-founder of spin-off Bionature EA LTD, proprietary of compound BNN27 (patented with the WO 2008/1555 34 A2 number at the World Intellectual Property Organization).
Footnotes
Highlights
• BNN27 is a novel DHEA derivative.
• BNN27 displays an anti-anxiety-like behavior in preclinical models.
• BNN27 might be a potential candidate for the treatment of anxiety disorders.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bagdy G, Kalogeras KT, Szemeredi K. Effect of 5-HT1C and 5-HT2 receptor stimulation on excessive grooming, penile erection and plasma oxytocin concentrations. Eur J Pharmacol. 1992;229:9–14. doi: 10.1016/0014-2999(92)90279-D. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci USA. 1998;95:4089–4091. doi: 10.1073/pnas.95.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Misslin R, Vogel E, Dodd RH, Chapouthier G. Anxiogenic effects of methyl-β-carboline-carboxylate in a light/dark choice situation. Pharmacol Biochem Behav. 1987;28:29–33. doi: 10.1016/0091-3057(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, O’Brien LC, Brohawn DG. Pharmacological properties of microneurotrophin drugs developed for the treatment of amyotrophic lateral sclerosis. Biochem Pharmacol. 2016;117:68–77. doi: 10.1016/j.bcp.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Bertaina-Anglade V, O’Connor SM, Andriambeloson E. A perspective on the contribution of animal models to the pharmacological treatment of posttraumatic stress disorder. Austral Psychiatry. 2017;25:342–347. doi: 10.1177/1039856217716288. [DOI] [PubMed] [Google Scholar]
- Biojone C, Casarotto PC, Resstel LB, Zangrossi H, Jr, Guimaraes FS. Anti-aversive effects of the atypical antipsychotic, aripiprazole, in animal models of anxiety. J Psychopharmacol. 2011;25:801–807. doi: 10.1177/0269881110376690. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/S0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Pitfalls in the interpretation of genetic and pharmacological effects of anxiety-like behaviour in rodents. Behav Pharmacol. 2008;19:385–402. doi: 10.1097/FBP.0b013e32830c3658. [DOI] [PubMed] [Google Scholar]
- Braga RJ, Reynolds GP, Siris SG. Anxiety comorbidity in schizophrenia. Psychiatry Res. 2013;210:1–7. doi: 10.1016/j.psychres.2013.07.030. [DOI] [PubMed] [Google Scholar]
- Busch R, Baldus M, Vogt MA, Berger SM, Bartsch D, Gass P, von Bohlen und Halbach O. Effects of p75NTR deficiency on cholinergic innervation of the amygdala and anxiety-like behavior. J Neurochem. 2017;141:461–471. doi: 10.1111/jnc.14006. [DOI] [PubMed] [Google Scholar]
- Calogeropoulou T, Avlonitis N, Minas V, Alexi X, Pantzou A, Charalampopoulos I, Zervou M, Vergou V, Katsanou ES, Lazaridis I, Alexis MN, Gravanis A. Novel dehydroepiandrosterone derivatives with antiapoptotic activity. J Med Chem. 2009;52:6569–6587. doi: 10.1021/jm900468p. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Tsatsanis C, Dermitzaki E, Alexaki VI, Castanas E, Margioris AN, Gravanis A. Dehydroepiandrosterone and allopregnolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc Natl Acad Sci USA. 2004;101:8209–8214. doi: 10.1073/pnas.0306631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodman WK, Heninger GR. Serotonin function in anxiety. II: effects of the serotonin agonist mCPP in panic disorder patients and healthy subjects. Psychopharmacology. 1987;92:14–24. doi: 10.1007/BF00215473. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/S0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Goodwin FK. Preliminary report of a simple animal behaviour for the anxiolytic effect of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day MD. Autonomic Pharmacology. Churchill Livingston, New York, USA: Experimental and Clinical Aspects; 1979. [Google Scholar]
- Eser D, Schule C, Romeo E, Baghai TC, Di Michele F, Pasini A, Zwager P, Padberg F, Rupprecht R. Neuropsychopharmacological properties of neuroactive steroids in depression and anxiety disorders. Psychopharmacology. 2006;186:373–387. doi: 10.1007/s00213-005-0188-z. [DOI] [PubMed] [Google Scholar]
- Estanislau C, Veloso AWN, Filgueiras GB, Maio TP, Dal-Col MLC, Cunha DC, Klein R, Carmona LF, Fernandez-Teruel A. Rat self-grooming and its relationship with anxiety, dearousal and perseveration: Evidence for a self-grooming trait. Physiol Behav. 2019;209:112585. doi: 10.1016/j.physbeh.2019.112585. [DOI] [PubMed] [Google Scholar]
- Fedotova J, Sapronov N. Behavioral effects of dehydroepiandrosterone in adult rats. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1023–1027. doi: 10.1016/j.pnpbp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Gkintoni E, Ortiz PS. Neuropsychology of generalized anxiety disorder in clinical setting: a systematic evaluation. Health Care. 2023;11:2446. doi: 10.3390/healthcare11172446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajch KE, Ferraiuolo L, Mueller KA, Stopford MJ, Prabhkar V, Gravanis A, Shaw PJ, Sadri-Vakili G. Microneurotrophins improve survival in motor neuron-astrocyte co cultures but do not improve disease phenotypes in a mutant SOD1 mouse model of amyotrophic lateral sclerosis. Plos One. 2016;11:e0164103. doi: 10.1371/journal.pone.0164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM. New molecule targets for antianxiety interventions. J Clin Psychiatry. 2003;64:28–35. [PubMed] [Google Scholar]
- Graf M, Kantor S, Anheuer ZE, Modos EA, Bagdy G. m-CPP-induced self-grooming is mediated by 5-HT2c receptors. Behav Brain Res. 2003;142:175–179. doi: 10.1016/S0166-4328(02)00404-7. [DOI] [PubMed] [Google Scholar]
- Gravius A, Barberi C, Schafer D, Schmidt WJ, Danysz W. The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats- a comparison. Neuropharmacology. 2006;51:1146–1155. doi: 10.1016/j.neuropharm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Grivas V, Markou A, Pitsikas N. The metabotropic glutamate 2/3 receptor agonist LY379268 induces anxiety-like behavior at the highest dose tested in two rat models of anxiety. Eur J Pharmacol. 2013;715:105–110. doi: 10.1016/j.ejphar.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulpers BJA, Verhey FRJ, Eussen SJPM, Scham MT, de Galan BE, van Boxtel MPJ, Stehouwer CDA, Kohler S. Anxiety and cognitive functioning in the Maastricht study: a cross-sectional population study. J Affect Disord. 2022;319:670–579. doi: 10.1016/j.jad.2022.09.072. [DOI] [PubMed] [Google Scholar]
- Hammer M, Robert S, Fruech BS. Treatment-resistant posttraumatic stress disorder: strategies for intervention. CNS Spectr. 2004;9:740–752. doi: 10.1017/S1092852900022380. [DOI] [PubMed] [Google Scholar]
- Holm MM, Nieto-Gonzalez JL, Vardya I, Henningsen K, Jayatissa MN, Wiborg O, Jensen K. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2011;21:422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- Ibán-Arias R, Lisa S, Mastrodimou N, Kokona D, Koulakis E, Iordanidou P, Kouvarakis A, Fothiadaki M, Papadogkonaki S, Sotiriou A, Katerinopoulos HE, Gravanis A, Charalampopoulos I, Thermos K. The synthetic microneurotrophin BNN27 affects retinal function in rats with streptozotocin-induced diabetes. Diabetes. 2017;67:321–333. doi: 10.2337/db17-0391. [DOI] [PubMed] [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009;57:97–108. doi: 10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Jolles J, Rompa-Barendregt J, Gispen WH. Novelty and grooming behavior in the rat. Behav Neural Biol. 1979;25:563–572. doi: 10.1016/S0163-1047(79)90362-5. [DOI] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: procedures for the behavioral science. Belmont, CA, USA: Brooks/Cole; 1968. [Google Scholar]
- Klinge CM, Clark BJ, Prough RA. Dehydroepiandrosterone research: past, current and future. Vitam Horm. 2018;108:1–28. doi: 10.1016/bs.vh.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dioli C, Paravatou R, Sotiropoulos MG, Delis F, Antoniou K, Calogeropoulou T, Charalampopoulos I, Gravanis A. Psychoactive properties of BNN27, a novel neurosteroid derivative, in male and female rats. Psychopharmacology. 2020;237:2435–2449. doi: 10.1007/s00213-020-05545-5. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Sutherland J, Hochman DW. Loop diuretics have anxiolytic effects in rat models of conditioned anxiety. Plos One. 2012;7:e15417. doi: 10.1371/journal.pone.0035417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis I, Charalamopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. Plos Biol. 2011;9:e1001051. doi: 10.1371/journal.pbio.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan R, Touati-Werner D, Ram E, Strous R, Keren O, Weizman A. The protective effect of frontal cortex dehydroepiandrosterone in anxiety and depressive models in mice. Pharmacol Biochem Behav. 2006;85:415–421. doi: 10.1016/j.pbb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VL, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL. Memory: drugs and hormones. In: Martinez JL, Kessner RP, editors. Learning and memory: a biological view. San Diego, USA: Academic Press; 1986. pp. 127–163. [Google Scholar]
- Melchior CL, Ritzmann RF. Dehydroepiandrosterone is an anxiolytic in mice on the plus maze. Pharmacol Biochem Behav. 1994;47:437–441. doi: 10.1016/0091-3057(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Samanin R. A two-compartment exploratory model to study anxiolytic/anxiogenic effects of drugs in rats. Pharmacol Res. 1989;21:595–602. doi: 10.1016/1043-6618(89)90201-6. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD and beyond. Neuropsychopharmacology. 2017;42:254–270. doi: 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain L, Launoy A, Fouquet N, Oberling P. Mechanisms of action of midazolam on expression of contextual fear in rats. Br J Anesth. 2002;89:614–621. doi: 10.1093/bja/aef228. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Castagna M, Marazziti D, Cassano GB, Nardi I. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92:601–611. doi: 10.1016/S0306-4522(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Pediaditakis I, Iliopoulos I, Theologidis I, Delivanoglou N, Margioris AN, Charalampopoulos I, Gravanis A. Dehydroepiandrosterone: an ancestral ligand of neurotrophin receptors. Endocrinology. 2015;156:16–23. doi: 10.1210/en.2014-1596. [DOI] [PubMed] [Google Scholar]
- Pediaditakis I, Efstathopoulos P, Prousis KC, Zervou M, Arevalo JC, Alexaki VI, Nikoletopoulou V, Karagianni E, Potamitis C, Tavernarakis N, Chavakis T, Margioris AN, Venihaki M, Calogeropoulou T, Charalampopoulos I, Gravanis A. Selective and differential interactions of BNN27, a novel C17-spiroepoxy steroid derivative, with TrkA receptors, regulating neuronal survival and differentiation. Neuropharmacology. 2016;11:266–282. doi: 10.1016/j.neuropharm.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Pediaditakis I, Kourtgiantaki A, Prousis KC, Potamitis C, Xanthopoulos KP, Zervou M, Calogeropoulou T, Charalampopoulos I, Gravanis A. BNN27, a 17-spiroepoxy steroid derivative, interacts with and activates p75 neurotrophin receptor, rescuing cerebellar granule neurons from apoptosis. Front Pharmacol. 2016;7:512. doi: 10.3389/fphar.2016.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peristeri E, Pitsikas N. Effects of low doses of different nitric oxide (NO) donors in rat models of obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) Nitric Oxide. 2022;129:1–7. doi: 10.1016/j.niox.2022.09.001. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Gravanis A. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts delay-dependent and scopolamine-induced recognition memory deficits in rats. Neurobiol Learn Mem. 2017;140:145–153. doi: 10.1016/j.nlm.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Zoupa E, Gravanis A. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts cognitive deficits induced by the D1/D2 dopaminergic receptor agonist apomorphine in rats. Psychopharmacology. 2021;238:227–237. doi: 10.1007/s00213-020-05672-z. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328X(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviours: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- Puschban Z, Sah A, Grutsch I, Singewald N, Dechant G. Reduced anxiety-like behavior and altered hippocampal morphology in female p75NTR (exon IV-/-) mice. Front Behav Neurosci. 2016;10:103. doi: 10.3389/fnbeh.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Vasek J, Lipschitz DS, Vojvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Schnurrm PP, Zakowska Z, Scioli E, Forman DE. Adaption to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med. 2010;235:1150–1162. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]
- Resstel LBM, Joca SRL, Moreira FA, Guimaraes FS. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res. 2006;172:294–298. doi: 10.1016/j.bbr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. 2014;12:140–147. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Artigas F. Expression of serotonin2C receptors in pyramidal and GABAergic neurons of rat prefrontal cortex: a comparison with striatum. Cereb Cortex. 2017;27:3125–3139. doi: 10.1093/cercor/bhw148. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/S0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek I, Przegalinski E, Pera J, Filip M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep. 2015;67:569–580. doi: 10.1016/j.pharep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Spivak B, Maayan R, Kotler M, Mester R, Gil-Ad I, Shtaif B, Weizman A. Elevated circulatory level of GABA(A): antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychol Med. 2000;30:1227–1231. doi: 10.1017/S0033291799002731. [DOI] [PubMed] [Google Scholar]
- Steimer T. The biology of fear-and anxiety-related behaviors. Dialogues Clin Neurosci. 2002;28:123–137. doi: 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–141. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- Tait GR, McManus K, Bellavance F, Lara N, Chrapko W, Le Melledo JM. Neuractive steroid changes in response to challenge with the panicogenic agent pentagastrin. Psychoneuroendocrinology. 2002;27:417–429. doi: 10.1016/S0306-4530(01)00051-8. [DOI] [PubMed] [Google Scholar]
- Torok B, Sipos E, Pivac N, Zelena D. Modelling posttraumatic stress disorder in animals. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:117–133. doi: 10.1016/j.pnpbp.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Pipe B, Bennett M. Optimizing treatment in social phobia: a review of treatment resistance. CNS Spectr. 2004;9:753–762. doi: 10.1017/S1092852900022392. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehydroepiandrosterone involved multiple receptors. Drug Metab Res. 2006;38:89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan A, Chen C, Inoue T, Nakagawa S, Kitaichi Y, Wang C, Izumi T, Kusumi I. Mirtazapine exerts an anxiolytic-like effect through activation of the median raphe nucleus-dorsal hippocampal 5-HT pathway in contextual fear conditioning in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2016;20:17–23. doi: 10.1016/j.pnpbp.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang X, Zhu Y, Dai Y, Liu T, Wang Y. Cognitive impairment in generalized anxiety disorder revealed by event-related potential N270. Neuropsychiatr Dis Treat. 2015;11:1405–1411. doi: 10.2147/NDT.S84666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoupa E, Gravanis A, Pitsikas N. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts behavioural deficits induced by the NMDA receptor antagonist ketamine in rats. Neuropharmacology. 2019;151:74–83. doi: 10.1016/j.neuropharm.2019.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.