Abstract
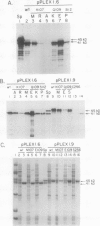
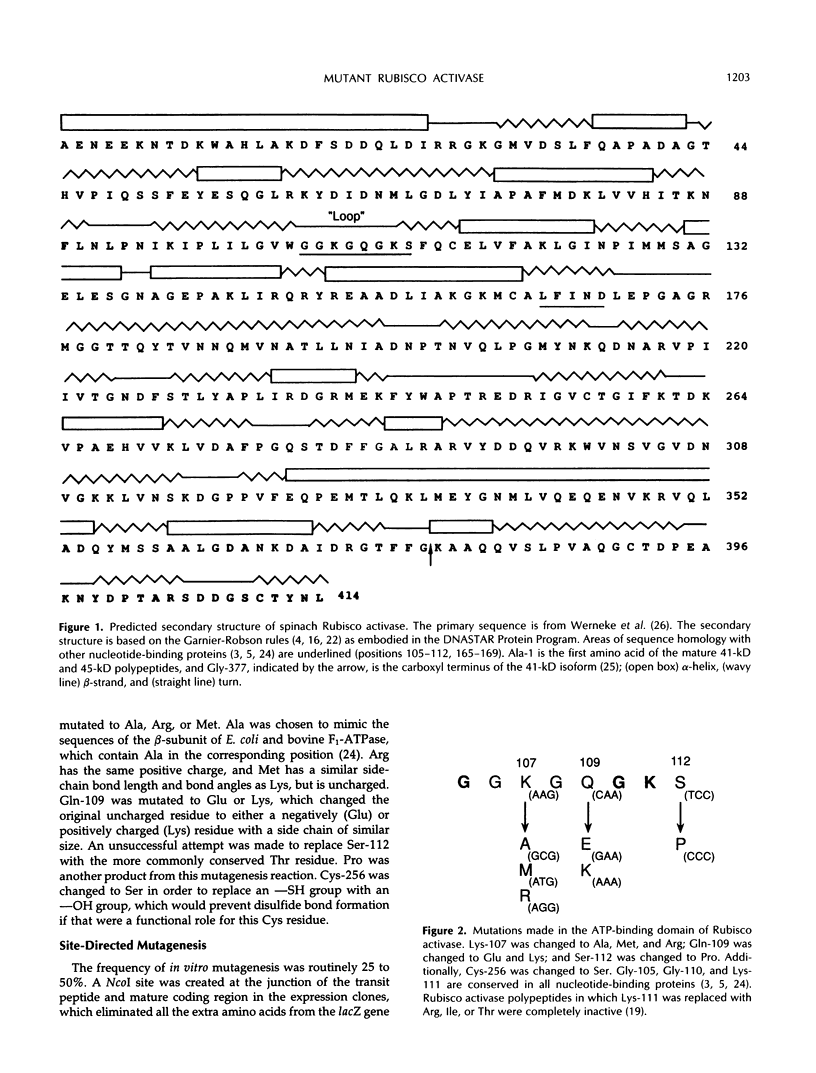
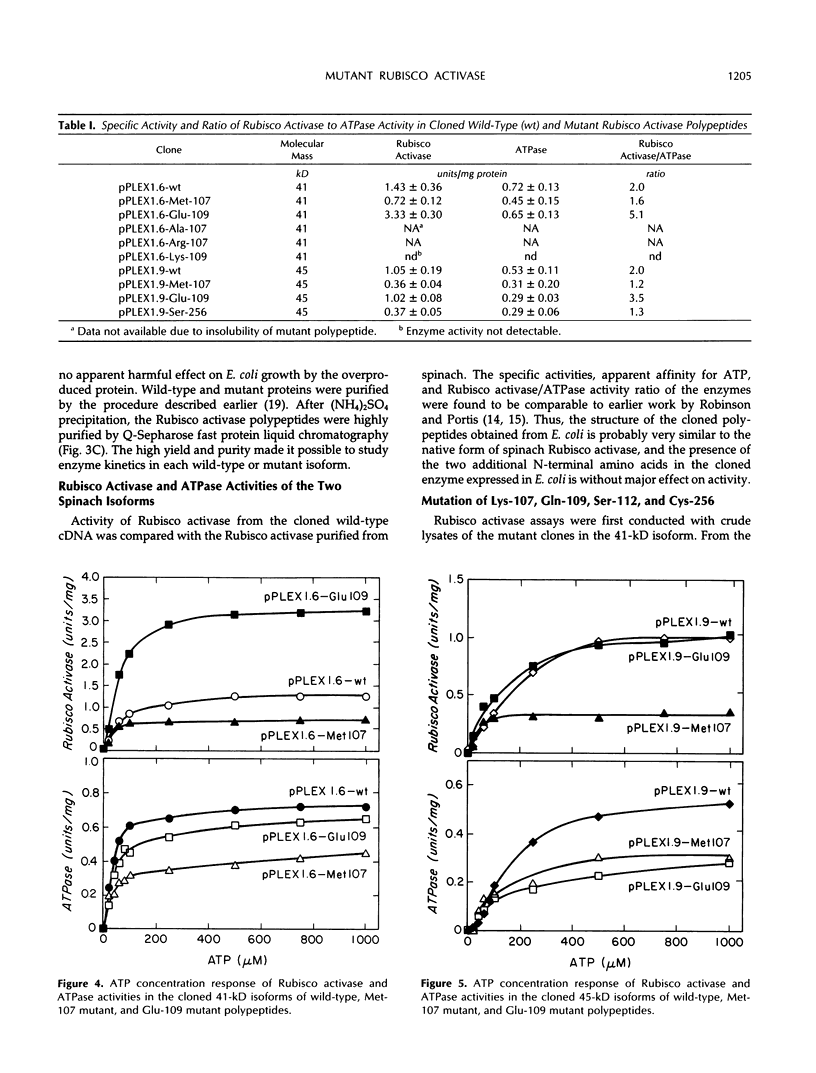
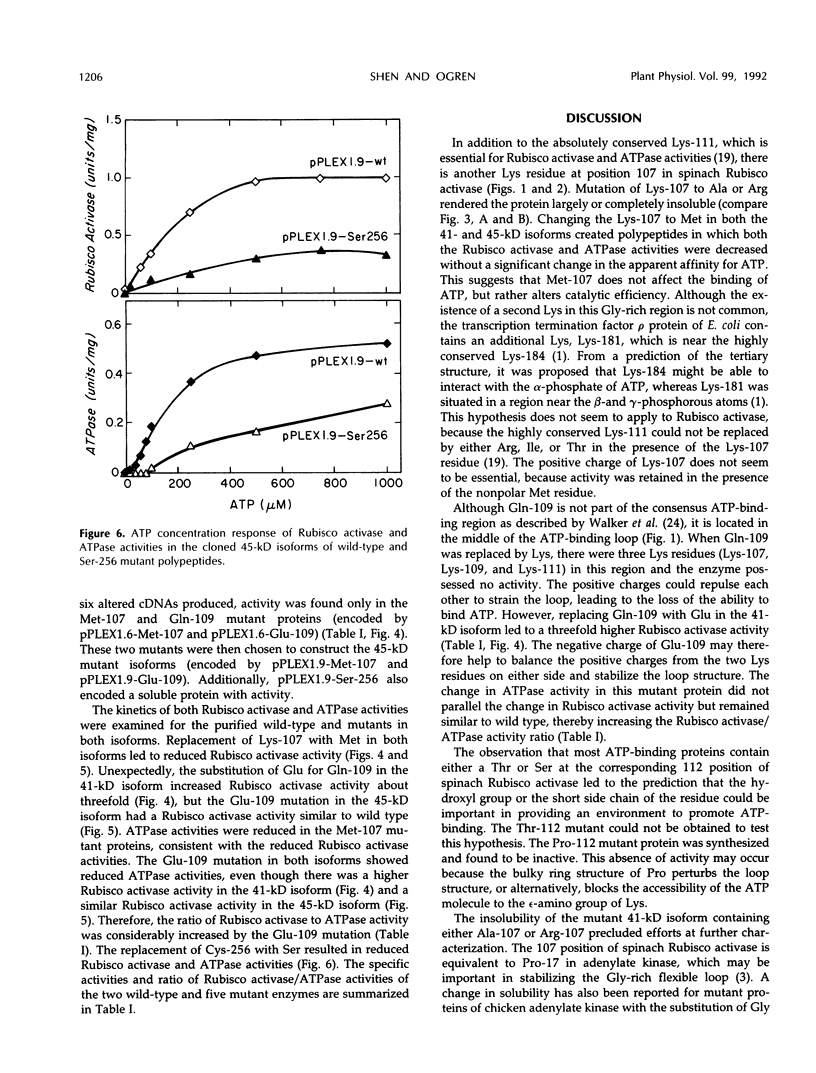
Site-directed mutagenesis was performed on the 1.6 and 1.9 kilobase spinach (Spinacea oleracea) ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase cDNAs, encoding the 41 and 45 kilodalton (kD) isoforms of the enzyme, to create single amino acid changes in the putative ATP-binding site of Rubisco activase (Lys-107, Gln-109, and Ser-112) and in an unrelated cysteine residue (Cys-256). Replacement of Lys-107 with Met produced soluble protein with reduced Rubisco activase and ATPase activities in both isoforms. Substituting Ala or Arg for Lys-107 produced insoluble proteins. Rubisco activase activity increased in the 41-kD isoform when Gln-109 was changed to Glu, but activity in the 45-kD isoform was similar to the wild-type enzyme. ATPase activity in the Glu-109 mutations did not parallel the changes in Rubisco activase activity. Rather, a higher ratio of Rubisco activase to ATPase activity occurred in both isoforms. The mutation of Gln-109 to Lys inactivated Rubisco activase activity. Replacement of Ser-112 with Pro created an inactive protein, whereas attempts to replace Ser-112 with Thr were not successful. The mutation of Cys-256 to Ser in the 45-kD isoform reduced both Rubisco activase and ATPase activities. The results indicate that the two activities of Rubisco activase are not tightly coupled and that variations in photosynthetic efficiency may occur in vivo by replacing the wild-type enzyme with mutant enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dombroski A. J., Brennan C. A., Spear P., Platt T. Site-directed alterations in the ATP-binding domain of rho protein affect its activities as a termination factor. J Biol Chem. 1988 Dec 15;263(35):18802–18809. [PubMed] [Google Scholar]
- Duncan T. M., Parsonage D., Senior A. E. Structure of the nucleotide-binding domain in the beta-subunit of Escherichia coli F1-ATPase. FEBS Lett. 1986 Nov 10;208(1):1–6. doi: 10.1016/0014-5793(86)81519-8. [DOI] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power and the environment. Nature. 1970 Aug 22;227(5260):777–777. doi: 10.1038/227777a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R., Jr Adenosine triphosphate hydrolysis by purified rubisco activase. Arch Biochem Biophys. 1989 Jan;268(1):93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Ogren W. L. Light and CO(2) Response of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Activation in Arabidopsis Leaves. Plant Physiol. 1986 Mar;80(3):655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. B., Orozco E. M., Jr, Ogren W. L. Expression of the two isoforms of spinach ribulose 1,5-bisphosphate carboxylase activase and essentiality of the conserved lysine in the consensus nucleotide-binding domain. J Biol Chem. 1991 May 15;266(14):8963–8968. [PubMed] [Google Scholar]
- Somerville C. R., Portis A. R., Ogren W. L. A Mutant of Arabidopsis thaliana Which Lacks Activation of RuBP Carboxylase In Vivo. Plant Physiol. 1982 Aug;70(2):381–387. doi: 10.1104/pp.70.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streusand V. J., Portis A. R. Rubisco Activase Mediates ATP-Dependent Activation of Ribulose Bisphosphate Carboxylase. Plant Physiol. 1987 Sep;85(1):152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Robson B. Relationship between helix-coil transition parameters for synthetic polypeptides and helix conformation parameters for globular proteins. A simple model. J Mol Biol. 1976 Nov 5;107(3):357–367. doi: 10.1016/s0022-2836(76)80009-5. [DOI] [PubMed] [Google Scholar]
- Tagaya M., Yagami T., Noumi T., Futai M., Kishi F., Nakazawa A., Fukui T. Site-directed mutagenesis of Pro-17 located in the glycine-rich region of adenylate kinase. J Biol Chem. 1989 Jan 15;264(2):990–994. [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Chatfield J. M., Ogren W. L. Alternative mRNA splicing generates the two ribulosebisphosphate carboxylase/oxygenase activase polypeptides in spinach and Arabidopsis. Plant Cell. 1989 Aug;1(8):815–825. doi: 10.1105/tpc.1.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Zielinski R. E., Ogren W. L. Structure and expression of spinach leaf cDNA encoding ribulosebisphosphate carboxylase/oxygenase activase. Proc Natl Acad Sci U S A. 1988 Feb;85(3):787–791. doi: 10.1073/pnas.85.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]