Abstract
Numerous pathogens can infect the olfactory tract, yet the pandemic caused by SARS-CoV-2 has strongly emphasized the importance of the olfactory mucosa as an immune barrier. Situated in the nasal passages, the olfactory mucosa is directly exposed to the environment to sense airborne odorants; however, this also means it can serve as a direct route of entry from the outside world into the brain. As a result, olfactotropic infections can have serious consequences, including dysfunction of the olfactory system, CNS invasion, dissemination to the lower respiratory tract, and transmission between individuals. Recent research has shown that a distinctive immune response is needed to protect this neuronal and mucosal tissue. A better understanding of innate, adaptive, and structural immune barriers in the olfactory mucosa is needed to develop effective therapeutics and vaccines against olfactotropic microbes such as SARS-CoV-2. Here, we summarize the ramifications of SARS-CoV-2 infection of the olfactory mucosa, review the subsequent immune response, and discuss important areas of future research for olfactory immunity to infectious disease.
Keywords: Mucosal immunology, Neuroimmunology, Infectious Disease, Sars-CoV-2
Subject terms: Mucosal immunology, Infectious diseases
Introduction
Remarking upon the intoxicating power that odors hold over our memories, Hellen Keller once said “smell is a potent wizard that transports you across thousands of miles and all the years you have lived”. Unfortunately, the pathway for smell may also transport neuroinvasive pathogens directly into our brains. Nature has exquisitely designed the olfactory system to achieve the sense of smell through a unique and complex anatomical arrangement. The olfactory mucosa (OM), consisting of a pseudostratified epithelium and lamina propria, lines the upper portion of the nasal cavity in the upper respiratory tract (URT). Neurobiologists have studied the biology of olfaction, including the life-long neurogenesis and unique patterning that characterize the olfactory system, for decades. However, very little effort has been made to elucidate how this sensory tissue is protected from pathogens. The pandemic caused by SARS-CoV-2 led to millions of short- and long-term smell loss cases. Indeed, for many people, loss of smell was the most striking first symptom of infection. The olfactory neuroepithelium is home to several unique cell types, but most importantly, it contains numerous olfactory sensory neurons (OSNs). The cell bodies of these neurons reside within the neuroepithelium, where they extend their dendrites with extensive cilia directly into the mucosal airway. If these cilia encounter an odorant, the OSN can rapidly relay a signal along the length of its axon. OSN axons converge to form bundles that then traverse holes in the cribriform plate of the skull, terminating directly in the olfactory bulb of the brain [1]. As a result, olfaction is a sensitive and efficient process for communicating information about the external environment. However, precisely because the OM simultaneously straddles the airway and the brain, microbes can subvert this biology to directly invade the CNS, bypassing conventional brain barriers with disastrous consequences, including fatal meningitis and encephalitis. Even for nonneuroinvasive pathogens such as SARS-CoV-2, local olfactory infection can drive inflammatory reactions that impact the adjacent CNS.
The olfactory mucosa can therefore be considered a mucosal barrier for the brain. Moreover, it is an important component of the upper airway. The URT is frequently thought to be a relatively homogeneous tissue, but unlike the respiratory lining of the URT, the OM is a distinct neuronal tissue with vastly different cell types and immune considerations. The OM plays an important role in the pathogenesis of respiratory diseases, even if CNS symptoms do not occur. The nose is the entry site for most respiratory pathogens, many of which have known olfactotropism; others have undefined olfactotropism. The immune response in the OM can therefore be critically important for preventing pathogen dissemination to other tissues in the body. Because OM pathogen replication may also serve as a pathogen reservoir, the OM immune response likely also serves to reduce transmission between individuals. In addition, infection-related OM damage impairs the ability to smell, an outcome that negatively impacts quality of life and the ability to sense environmental danger. Immunity against olfactotropic infections is therefore essential to prevent CNS neuroinvasion and respiratory pathogen dissemination and transmission and to protect the sense of smell itself.
Anosmia, smell loss without acute airflow blockage, was quickly recognized as a negative effect of COVID-19 but also indicated that the virus distinctly impacted the olfactory mucosa. The ubiquity of olfactory infections during the COVID-19 pandemic has stimulated interest in the OM as an immunological tissue. Unfortunately, this interest has served to highlight how poor our understanding of immune responses in the olfactory system is. As an additional side effect of the pandemic, researchers and the public are beginning to pay more attention to other potentially olfactotropic pathogens. Indeed, many respiratory and neurotropic pathogens are able to infect the OM, but the attention that the pandemic brought to olfactory infection has focused an important spotlight on this understudied URT region. Nevertheless, due to difficulty in obtaining olfactory biopsies and how infrequently the olfactory system is considered clinically, many common infections may impact the OM in ways we do not yet understand. Furthermore, many animal models of infection fail to establish olfactotropism in airway diseases. We currently are at an inflection point in the study of olfactory disease pathogenesis, as recent studies in animals and in humans have begun to emphasize the importance of the OM immune response.
Olfactory immunology has implications for several topics of clinical importance, including vaccination, encephalitis and meningitis, postviral olfactory dysfunction, microbial transmission, innate and adaptive immunity, and neuroimmunology. This review summarizes infectious diseases and subsequent immune responses in the olfactory mucosa. We directly address SARS-CoV-2 and similar viral pathogens that infect the olfactory mucosa. Olfactory immunity against these microbes is also discussed, and future directions for the nascent field of olfactory immunology are considered.
Olfactory SARS-CoV-2 infection
Human pathogenesis
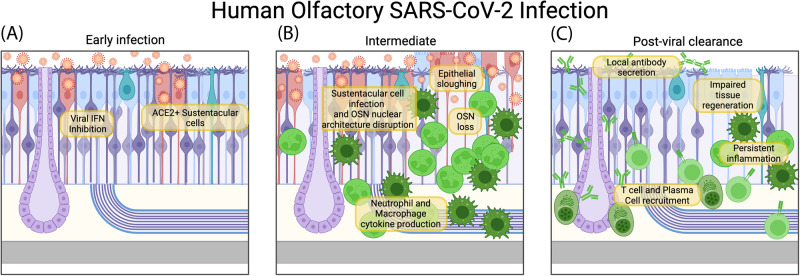
At the onset of the COVID-19 pandemic, as cases spread across the globe, there was fear that the pathogen, similar to other coronaviruses, may have neurotropic tendencies. While pulmonary infection can drive severe disease and death, concern for neurotropism was sparked after olfactory dysfunction emerged as a very commonly reported symptom of COVID-19 [2–4], combined with widespread reports of neurological consequences such as “brain fog”. As a result, several groups have sought to ascertain whether SARS-CoV-2 can invade the CNS through the olfactory nerve. Initially, postmortem samples of human brain and olfactory tissue provided concerning but uncertain evidence regarding the occurrence and relevance of SARS-CoV-2 olfactory neuroinvasion, with the virus occasionally being present in the brain [5–7] of individuals who had died of COVID-19. As expression of ACE2 and TMPRSS2, the host proteins needed for SARS-CoV-2 cell entry, was largely uncharacterized in the olfactory system, it was difficult to determine whether direct OSN infection was possible. Furthermore, Nrp1 was suggested as an alternative entry receptor that may facilitate olfactory infection [8]. Subsequent studies indicated that while these entry proteins are present in the OM, they are primarily expressed on sustentacular cells [8–14]. Of note, ACE2 was found to be expressed more highly in the URT than in the lungs [15]. Infection or inflammation can upregulate ACE2 in the epithelium [10], and induced ACE2 is expressed as the isoform dACE2, which is incapable of binding the SARS-CoV-2 spike protein [16]. Additional analyses of olfactory biopsies have painted a clearer picture of SARS-CoV-2 infection in the olfactory system (Fig. 1). In humans, the virus primarily infects the sustentacular cells of the olfactory mucosa [17–21], which fits with the observed distribution of the ACE2 entry receptor. These cells are the primary structural cell type in the olfactory epithelium, providing support for OSNs and their dendritic projections to detect odors at the air interface. SARS-CoV-2 sustentacular cell infection leads to massive inflammation leading to sustentacular cell death, loss of epithelial tissue structure, and subsequent disruption of OSN nuclear architecture and function [17, 21]. Consequently, without structural support, OSNs can be lost, and the sense of smell is either diminished or completely ablated. Fortunately, direct SARS-CoV-2 olfactory neuron infection rarely occurs, and subsequent neuroinvasion seems unlikely. A critical analysis of studies claiming olfactory neuroinvasion was conducted by Butowt et al. [20]. In relatively rare cases in which SARS-CoV-2 infection of the CNS is identified [6, 7], olfactory neuroinvasion should be considered as one possible route of entry, along with spread across the inflamed blood‒brain barrier and infection of other neuronal cells in epithelial tissues [20, 22–24].
Fig. 1. Olfactory immune response to SARS-CoV-2 in Humans.
The stages of the immune response to SARS-CoV-2 in the olfactory mucosa. A SARS-CoV-2 uses ACE2 to enter sustentacular cells and antagonize the induction of interferons. B Infiltrating neutrophils and macrophages produce inflammatory cytokines. Sustentacular cells are lost, epithelial structure deteriorates, and olfactory sensory neurons undergo disruption of nuclear architecture and cell death. C After SARS-CoV-2 has been cleared by the immune response, T cells and plasma cells populate the tissue. Plasma cells produce locally protective mucosal antibody. T cells may contribute to sustained inflammation, preventing proper epithelial regeneration in some cases and preventing restoration of the sense of smell
Animal pathogenesis
Overall, SARS-CoV-2 olfactory pathogenesis varies across animal models. Mammalian studies have helped to shed light on SARS-CoV-2 human pathogenesis while also illustrating the dangers of olfactory neuroinvasion. In some organisms, SARS-CoV-2 invades the brain via the olfactory nerve (Fig. 2), raising the concern that future variants may have more neurovirulent tendencies. Indeed, experiments across mammalian models indicate that olfactotropism differs depending on the variant of SARS-CoV-2 [24–26].
Fig. 2. Olfactory neuroinvasion in mammalian SARS-CoV-2.
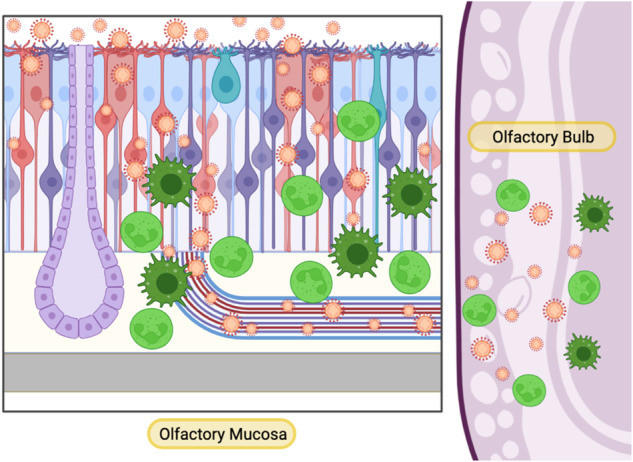
In some animal models, SARS-CoV-2 is able to invade the brain through the olfactory nerve. The virus infects sustentacular cells and olfactory sensory neurons in the mucosa, then travels through olfactory axons, reaching the olfactory bulb of the brain. Immune cells migrate to both the mucosa and the brain, producing inflammatory mediators that can both fight the virus and contribute to destructive neuroinflammation
Because parental SARS-CoV-2 cannot use murine ACE2 for cell entry, to study SARS-CoV-2 in mice, animals must be engineered to express human ACE2, or a murine-adapted virus must be used. The commonly used K18-hACE2 transgenic mouse was initially generated as a model for SARS-CoV-1 [27] and uses the human keratin-18 promoter to artificially express hACE2 in nearly every epithelial cell, including OSNs and sustentacular cells. As a result, direct OSN infection and consequent CNS pathology are observed following intranasal inoculation [28, 29]. Multiple SARS-CoV-2 variants often result in lethal disease [30]. In addition to olfactory transmucosal neuroinvasion, these mice lose their sense of smell. This smell loss persists for several weeks following infection and likely reflects widespread damage to the olfactory epithelium caused by sustentacular and OSN infection [31]. K18-hACE2 mice experience extensive olfactory tissue damage characterized by the “sloughing off” of the epithelial layer, consistent with other observations of aggressive olfactory infections [28, 32]. Interestingly, aerosolization of SARS-CoV-2 as opposed to intranasal droplet instillation changes its olfactory pathology in K18-hACE2 mice [33]. Aerosolized inoculation results in lower viral titers in the nasal turbinates, no viral spread to the olfactory bulb, and greater viral replication in the lungs and respiratory tract, more closely mirroring human infection. These mice still develop smell impairment, suggesting that nasal infection in the absence of CNS neuroinvasion can still drive olfactory damage and functional dysosmia [33]. The reasons why aerosolization does not result in neuroinvasion remain unclear but will be critical to understanding factors that may heighten neuroinvasive tendencies in other viruses.
Another mouse model in which hACE2 expression is driven by the endogenous mouse Ace2 promoter also shows infection of the olfactory epithelium and fatal neuroinvasion [34]. In this model, SARS-CoV-2 accesses the brain by infecting olfactory neurons, resulting in lethal cachexia, hypoxemia, and respiratory failure independent of lung infection. Selective expression of hACE2 in the olfactory epithelium and in neurons is sufficient for these phenotypes, illustrating that SARS-CoV-2 can cause severe illness even without lower respiratory tract infection [34]. Other murine models have been used to model different aspects of COVID-19, including mouse-adapted SARS-CoV-2 strains [31] and mice that express hACE2 via AAV-controlled expression [6]. Chimeric viruses, such as VSV-SARS-CoV-2 S, which expresses the SARS-CoV-2 spike protein on a vesicular stomatitis virus (VSV) backbone, have been useful for studies of the humoral immune response to SARS-CoV-2 in mice [35, 36].
A more “natural” model of SARS-CoV-2 olfactory neuroinvasion occurs in Syrian golden hamsters (Fig. 2). Similar to humans, SARS-CoV-2 was initially observed to infect sustentacular cells in the olfactory epithelium, causing massive immune infiltration and desquamation [37, 38]. However, olfactory neurotropism of SARS-CoV-2 in hamsters seems to be highly dependent on the viral isolate, as some have been shown to replicate in OSNs without CNS infection [39, 40], while others infect OSNs and invade the CNS [41] Some subsequent variants, such as Delta in one study [25] and D614G in another [42], cause even more OSN infection and CNS neuroinvasion, with variants such as Omicron having fewer of these tendencies [25, 42]. More recent data detected SARS-CoV-2 in the olfactory bulb for all five variants tested, though infection led to smell loss in only some variants [43]. Consistent with mouse data [33], this suggests that viral-induced anosmia may be independent of neuroinvasive capacity [43]. Indeed, hamsters frequently have lasting olfactory perturbations following SARS-CoV-2 infection, indicating that they may be useful models for post-COVID olfactory dysfunction [44, 45].
Nonhuman primates, especially macaques, have been used to test the immune response to SARS-CoV-2 and vaccine candidates, but direct examination of the olfactory mucosa has been infrequently conducted in these animals. SARS-CoV-2 infects the nasal passages of macaques [46–48] but does not seem to be neuroinvasive, though some studies have detected low levels of SARS-CoV-2 in olfactory CNS regions [49, 50]. SARS-CoV-2 infects many wildlife species and domesticated animal populations [51], but little is known about olfactory pathogenesis in other mammalian hosts. However, given the high fatality rate in wildlife populations such as deer and mink [52, 53], the frequency at which olfactory neuroinvasion occurs in these species should be investigated.
These animal data suggest that future variants of SARS-CoV-2 (or, more generally, future pandemic coronaviruses) may have increased olfactory neurotropism. How can we prepare for an emergent respiratory pathogen that may also cause catastrophic CNS infection? We must design vaccines that capably protect the OM. Critically, these immunizations should focus on generating mucosal antibodies since OSNs are directly exposed to the airway. We should also investigate therapeutics that target nasal viral replication shortly after exposure, as well as drugs that may effectively treat viral meningitis and encephalitis. Furthermore, SARS-CoV-2 has long-term neurological and olfactory consequences, for which there is little clinical recourse. A better understanding of olfactory immunity in general is needed to address all these challenges. To these ends, what lessons can we take from the COVID-19 pandemic? Below, we summarize what is known about the immune response to SARS-CoV-2 in the olfactory system.
Innate immune response
SARS-CoV-2 gains a foothold in the host due to its ability to limit the early innate immune response [54] (Fig. 1a). By dampening the interferon (IFN) response in nasal epithelial cells [55, 56], SARS-CoV-2 is able to replicate quickly, often becoming infectious before any symptoms are apparent. However, if innate immunity is activated quickly, it can significantly limit viral dissemination. Furthermore, one study in macaques showed that mild SARS-CoV-2 can be cleared from nasal tissue prior to the arrival of T cells, suggesting that once activated, the innate response can be quite effective [57]. Studies of human nasal samples indicate that a poised immune state, with higher basal levels of IFN and PRR expression, may protect children from severe COVID-19 [58, 59]. Intranasal administration of IFN-based therapeutics, either prophylactically or shortly after infection, has been shown to limit SARS-CoV-2 replication in nasal passages, but differences between respiratory and olfactory IFN responses and viral replication have not been directly measured [60, 61], making it difficult to interpret whether there may be tissue-specific effects of IFN in the URT. Once viral-induced inflammation begins to occur, circulating immune cells infiltrate the infected OM to combat the virus (Fig. 1b). These cells are predominantly neutrophils and macrophages, and their arrival into the tissue is accompanied by an increase in cytokine production [21, 31, 37, 38, 62]. These cytokines likely play important roles in both antiviral effector mechanisms and wound healing, but some evidence indicates that neutrophilic inflammation can actually exacerbate tissue damage and increase OM viral replication [38]. Nasopharyngeal swabs of humans primarily contain cells of respiratory origin while neglecting olfactory inflammation, but related studies have provided evidence to suggest that activated macrophages and neutrophils are involved in COVID-19 nasal inflammation and correlate with disease outcomes [56, 63–65]. Overall, neutrophils and macrophages seem to be important players in the olfactory COVID innate response, but whether they are productive or deleterious likely depends on the magnitude of the response and the specific cytokines expressed by these cells.
Adaptive immune response
An effective adaptive immune response in the olfactory mucosa is essential to clear SARS-CoV-2 and protect against future reinfection [66–69]. However, as the COVID-19 pandemic continued, it became apparent that prior infection or immunization provided significant protection from severe disease but did not prevent reinfection [70–73]. This can be partially explained by several factors, including limited neutralization capacity of anti-Spike antibodies, emergence of new variants, waning of the initial immune response, and incomplete herd immunity [74, 75]. However, breakthrough infections commonly present with upper respiratory symptoms and fewer symptoms in the lower respiratory tract [70]. Could immune protection in the nasal passages be incomplete, and if so, is this a phenomenon specific to the olfactory mucosa?
Several SARS-CoV-2 studies have suggested that blood-borne antibodies are incapable of protecting the upper respiratory tract from infection. Passive antibody transfer, while preventing lung SARS-CoV-2 infection, fails to protect the nasal passages, as virus can still be detected within nasal washes and within the nasal turbinates, including olfactory regions [36, 76]. Accordingly, many vaccines induce strong humoral protection of the lungs while insufficiently protecting nasal viral replication across animal models [36, 46, 76–83].
Our recent work has demonstrated why serum antibodies cannot extend protection to the entirety of the nasal airway [84]. We showed that circulating antibodies readily access and protect the respiratory mucosa within the nasal passages but are excluded from entering the olfactory tissue. This is due to the presence of the blood-olfactory barrier (BOB), which forms a tight endothelial barrier to segregate the olfactory mucosa from circulation [84]. As a result, even with high titers of blood-borne antibodies, the olfactory portions of the URT can be left exposed to infection.
Nonetheless, despite the presence of the BOB, the OM can still be protected from rechallenge. Our data and that of others have further shown that plasma cells can home to the OM, residing within the tissue to directly secrete antibodies to the mucosal surface, bypassing the BOB [80, 84] (Fig. 1c). Importantly, not all immunization approaches generate these olfactory plasma cells. Many conventional adjuvants, such as alum and TLR ligands, are incapable of driving plasma cell homing [84].
These data are consistent with studies that demonstrate protection of the upper respiratory tract from SARS-CoV-2 infection (Fig. 1c). Immunization strategies such as nonconventional adjuvants [77, 84], alternative antigen vectors [79, 80, 85–87], or intranasal administration [78, 88–90] have all shown promise in providing superior immune protection of the nasal passages, though careful differential analysis of olfactory and respiratory tissues has not been performed. The exact signals that dictate protective OM plasma cell homing and protection remain to be precisely identified, but the discovery of the BOB has important implications for the design of vaccines that aim to protect the olfactory mucosa. In addition, the relationship between tissue plasma cells and those in other mucosal tissues or bone marrow should be studied to identify how humoral immunity may be unique in the OM or reveal broadly applicable lessons.
Mucosal protection in the nasal turbinates is thought to be linked with the ability of vaccines to elicit antiviral IgA. Accordingly, mucosal IgA production is associated with SARS-CoV-2 protection following mRNA vaccination and prior SARS-CoV-2 infection [91–96]. Eliciting mucosal IgA is one reason intranasal vaccination approaches gained intense interest during the COVID-19 pandemic [97, 98], though parenteral immunizations have also been shown to induce mucosal antibodies in some cases [77, 91, 92]. While there are clearly important roles for secretory IgA in protection against mucosal pathogens, our recent work has shown that IgA production is not needed to protect the olfactory mucosa against viral rechallenge [84]. These data suggest that rather than a specific antibody isotype, the most important correlate for protecting the olfactory mucosa is local antibody production. This is in agreement with recent data that IgG antibodies can be detected in nasal secretions and may play a role in protection against SARS-CoV-2 [65, 83, 95, 99, 100].
Protection of the OM is not dependent on humoral immunity alone; T cells also play an important role in defense against COVID-19. T cells home to the OM following SARS-CoV-2 infection and remain in the tissue after viral clearance [101–103] (Fig. 1c). Similarly, after vaccination, antigen-specific CD8+ and CD4+ T cells can migrate to the nasal passages and reside long term [87, 103, 104], limiting viral replication upon SARS-CoV-2 challenge [105]. These T cells have a repertoire that differs from that of circulating T cells, suggesting an independent and functionally distinct nasal T-cell response [103]. Similar to the olfactory plasma cell response, T cells seem to differentially home to the nasal mucosa in response to infection or vaccination. Determining the signals that best elicit olfactory T cells is of great importance, especially for protection against future SARS-CoV-2 variants or other viruses that may have greater T-cell epitope conservation than B-cell epitope overlap [106]. While nasal, and especially olfactory, T cells have not been frequently analyzed, some studies in animals and humans offer clues for what immunizations may best stimulate these cells. In humans, infection generates the largest numbers of nasal T cells [101], particularly in combination with prior vaccination [103]. Parenteral immunization with mRNA vaccines, on the other hand, recruits very few T cells to the nasal mucosa [104], raising doubt as to whether parenteral vaccines can generate functional T-cell-mediated protection of the olfactory tissue [107]. As persistent antigen and inflammation in mucosal sites is known to greatly enhance T-cell recruitment and resident memory formation [108], it is logical that infection and local nasal immunization will be most effective at forming a robust nasal T-cell compartment. Consistent with this, viral-vectored intranasal vaccination approaches in mice have been shown to induce SARS-CoV-2-specific nasal T cells [87], and a intranasal vaccination approach led to a similar result in macaques [109]. However, as mentioned above for B cells, adjuvant signals and lymph node interactions can influence the ability of T cells to home to barrier sites [110]. It is possible that infection, unlike immunization, is also better able to induce signals that prime T cells for mucosal homing. Decoding these signals may inform adjuvant approaches that could improve the ability of parenteral vaccinations to drive T-cell homing to the olfactory tissue. Last, while olfactory T cells are likely important for viral clearance and protection during future infection, they may also have undesirable impacts. Indeed, evidence suggests that T-cell-driven inflammation can impair olfactory recovery, emphasizing that the T-cell response in the olfactory mucosa must be tightly regulated [102].
Consequences of olfactory infection
In many cases, COVID-19 has long-term effects following the acute disease stages. In the olfactory system, this most clearly manifests as intermediate or long-term smell loss [111–113], even following mild infection [114]. Clinical data show that while many patients quickly recover their ability to smell, others do not [2, 3], even up to two years after infection and in the apparent absence of infectious virus [115–117]. What could explain the differences in this regenerative capacity? Evidence suggests that sustained inflammation may prevent olfactory stem cells from repopulating the tissue with functional neurons [118] (Fig. 1c). While initial OM destruction is mediated by sustentacular cell death and infiltrating neutrophils [38], long-term dysosmia coincides with persistent inflammation from T cells and NK cells that express IFN-γ accompanied by changes in local myeloid populations. These immune cells appear to signal to sustentacular cells and olfactory stem cells, shifting them away from a regenerative state [102]. Host genetics also may impact the propensity for smell loss [119], and a variety of treatment options are being attempted clinically, from steroids to olfactory training to platelet-rich plasma [120–122]. However, given the role of sustained inflammation in dysosmia, immunomodulatory approaches may also be considered.
Moreover, sustained inflammation in the olfactory mucosa can alter the state of the CNS, even if the virus itself never reaches the brain parenchyma. In postmortem biopsies, COVID-19 patients showed elevated IFN and inflammatory gene expression in the olfactory bulb [44]. Consistent with this, patients with olfactory dysfunction have detectably larger olfactory bulbs measured by MRI [123, 124]. This prolonged inflammation is likely driven by local cytokines and resident immune cells, but it remains possible that as a neuronal tissue, the olfactory mucosa garners a degree of “immunoprivilege”, allowing it to serve as a long-term viral reservoir. Supporting this hypothesis, nasal swabs have identified SARS-CoV-2 RNA in “long-term viral shedders” several months after the initial infection has subsided [125, 126], and viral antigen has been reported in the OM of some, but not all, patients long term [41]. While replicating virus has not been detected in these patients, it is feasible that low levels of virus may be persistently harbored in the olfactory mucosa beyond the reach of typical nasal swabs. Incomplete viral clearance might explain why inflammation is often sustained in patients with olfactory dysfunction. As mentioned above, more recent variants of SARS-CoV-2 are differentially neurotropic and olfactotropic. Measuring smell loss, either through self-report surveys or through clinical testing, is often difficult on an individual basis, but population-level monitoring of anosmia may be useful for predicting future waves of SARS-CoV-2 and other pandemic viruses [127].
SARS-CoV-2 infection of the olfactory mucosa may contribute to other symptoms of long COVID. Patients report cognitive impairment, headache, brain fog, memory impairment, and anosmia as major symptoms [128–130]. These symptoms are now broadly included under the term “postacute sequelae of SARS-CoV-2” (PASC) [131], but early and continued reference to “NeuroCovid” highlights a core set of neurological consequences associated with SARS-CoV-2 infection [132, 133]. Animal models in which SARS-CoV-2 demonstrates olfactory neuroinvasion have shown inflammation of the olfactory bulb and other brain regions [42, 44] (Fig. 2). SARS-CoV-2 neuroinvasion can also induce gene signatures and pathologies associated with neurodegenerative disorders such as Alzheimer’s disease [134]. Neurological manifestations have been implicated in even mild cases of COVID-19 [135], suggesting that the absence of direct neuroinvasion does not prevent peripheral infection from impacting the CNS. While vascular inflammation [136] likely contributes to these symptoms, inflammation of the airway [137, 138], including the olfactory tissue, may contribute to neurologic disturbances. Accordingly, some studies have demonstrated olfactory bulb inflammation without direct neuroinvasion by SARS-CoV-2 [31]. It is important to note that anosmia itself might contribute to cognitive dysfunction, as the sense of smell contributes to neurological and psychological well-being [139].
Other olfactotropic viral infections
While SARS-CoV-2 has become the most high-profile pathogen with olfactory implications, many viral pathogens with pandemic potential have shown olfactotropic tendencies with and without clear CNS neurovirulence. In this section, we review known major olfactotropic pathogen threats and consider relevant aspects of the olfactory mucosal immune response to each.
Other SARS coronaviruses
Other coronaviruses have been shown to infect the olfactory mucosa, including SARS-CoV-1 [140], which does not penetrate the CNS in WT mice but is capable of olfactory neuroinvasion in the K18-hACE2 model [140]. An autopsy study of SARS patients detected the virus in the CNS, but the nasal mucosa was not sampled; thus, the method of neuroinvasion was unknown [141]. Human coronavirus (HCoV)-O43 is frequently detected in brain autopsies of deceased patients [142], and intranasal installation of HCoV-O43 in WT mice leads to infection of the CNS, seemingly spreading from the olfactory system [143, 144]. The deadly Middle East respiratory syndrome (MERS)-CoV outbreak was associated with numerous neurologic symptoms [145, 146], and a transgenic mouse model of MERS indicated the possibility of olfactory involvement in brain infection [147]. In addition, the betacoronavirus porcine hemagglutinating encephalomyelitis virus (PHEV) was recently found to invade the CNS via the olfactory and trigeminal nerves during intranasal murine infection [148]. PHEV infection caused inflammatory cell infiltration in the OM and upregulated expression of several inflammatory genes, such as Cxcl10 and Ccl5 [148]. Hepatitis viruses are also included in the family Coronaviridae, and two murine adapted strains, A59 and JHM, have been shown to invade the brain through the olfactory mucosa following intranasal challenge [149–151]. JHM causes extensive damage to the olfactory mucosa and OSN loss [152], making it a potentially useful model for postviral olfactory dysfunction. Given the frequency of coronavirus epidemic outbreaks in this century, more research on their potential for olfactory neuroinvasion should be conducted. COVID-19 has caused tremendous loss of human life and wellbeing, yet we are still not prepared to develop vaccines or therapies against future olfactotropic and neuroinvasive pandemic coronaviruses.
Influenza virus
Influenza viruses show variable olfactotropism but are likely the greatest contributor to postviral olfactory dysfunction. Supporting this, analysis of influenza patients shows an inverse relationship between vaccination rates and subjective olfactory dysfunction [153]. Olfactotropism seems to be dependent on the strain, even within the same family. In mice, influenza B/Malaysia/2506/2004 was found to infect OSNs, but these neurons were able to induce an antiviral gene expression program to nonlytically clear the virus before it could reach the CNS [154]. The recombinant R404BP derivative of influenza A/WSN/33 (H1N1) also infects OSNs, but apoptosis is induced in infected neurons to prevent spread to the brain [155]. Influenza A/Puerto Rico/8/34 (H1N1), also known as PR8, is the most widely used murine influenza model, and while some studies have reported PR8 antigen in the olfactory bulb of infected mice [156, 157], PR8 does not productively infect the CNS through the olfactory route [158, 159]. A related influenza model, H3N2 influenza A subtype X31, seems to infect sustentacular cells but does not spread to the CNS [160]. This infection causes CD8 T cells to adopt a resident memory phenotype in the OM where they are protected from rechallenge. Unlike CD8 T cells in the lung, OM CD8 T-cell residence is independent of TGF-β, suggesting that different factors are responsible for recruiting or retaining these cells between the lung and olfactory compartments. Importantly, olfactory T cells show limited dissemination to the lungs, protecting against severe disease. IFNs may be important factors for confining infection to the nasal passages, as one study found that Type I and Type III IFNs can prevent murine-adapted influenza A/seal/mass/1-SC35M/1980 (H7N7) and influenza A/duorn/307/1972 (H3N2) from reaching the lungs [161]. Ferret studies similarly indicated differing levels of OM infection between strains, observing no OM infection for A/H3N2/Netherlands/2008 but moderate OM infection in highly pathogenic A/H5N1/Indonesia/2005 and high levels of OM replication in the pandemic H1N1/Netherlands 2009 strain [162]. Hong Kong/H5N1/97 also replicated to high levels in ferret nasal turbinates without reaching the brain [163]. Interestingly, a study comparing nasal and lung infection across multiple influenza strains in ferrets indicated that only nasal infection allows for airborne transmission between organisms, while lung infections are not spread [164]. These data indicate that the URT immune response can not only prevent viral spread to the lung but also prevent propagation to other individuals in the population.
Influenza is frequently associated with neurologic symptoms and sequelae [165–167], and in many cases, influenza infection has coincided with meningitis or encephalitis [168–173]. Influenza antigen has been identified in the olfactory nerve of a postmortem sample, lending credibility to the olfactory route of CNS infection [174]. As mentioned above, many influenza strains have olfactory mucosal tropism, and direct olfactory neuroinvasion to the CNS has been reported in several mammalian influenza models. Influenza A/WSN/33 (H1N1) was shown to infect OSNs and spread to the olfactory bulb in immunocompetent mice, and mice lacking an adaptive immune response eventually died [175]. Highly pathogenic avian influenza virus A/Indonesia/5/05 (H5N1) is able to bind to OSN cilia, initiate massive OM infection, and likely uses the olfactory portal to cause CNS pathology in ferrets [162, 176]. Similarly, the pandemic Netherlands 2009 strain of H1N1 was able to infect the olfactory nerves and brains of ferrets. The influenza A/Vietnam/1203/2004 strain resulted in olfactory neuroinvasion and death in ferrets, although in mice, it does not seem to use the olfactory nerve to reach the CNS [163, 177]. In addition, a recent study identified two H3N8 isolates that infected the ferret brain and caused fatality, but the route of neuroinvasion was not analyzed [178]. Much remains to be learned about the viral and host factors that determine the neuroinvasive proclivities of various influenza strains, but the serious potential for olfactory mucosal involvement deserves consideration as new seasonal and pandemic strains emerge.
Conclusions
The COVID-19 pandemic has led to massive upheaval worldwide. Millions of people have lost their lives, and thousands continue to struggle with the long-term impacts of SARS-CoV-2 infection, including smell loss. When widespread reports surfaced that SARS-CoV-2 infection causes rapid onset smell loss, it signaled the unusual olfactotropic nature of SARS-CoV-2 and foreshadowed the importance of an overlooked and underserved topic of tissue-specific immunity.
Olfactotropic viruses such as SARS-CoV-2, both known and emergent, pose a threat to public health and are an open area of investigation for immunology. Coronaviruses and influenza viruses, the two families that pose the greatest threat for future pandemics, have been shown to variably impact the olfactory mucosa. OM immunity must be considered for microbes that invade the brain through the olfactory nerve but also for pathogens that replicate primarily within the nasal passages. Olfactotropism may be more common than currently believed, as few studies have examined OM infection in great detail for many airborne diseases.
What are the major outstanding questions about olfactory immunology, and what can we do to address these issues? First, we must overturn the dogma that the nasal passages are a single homogenous tissue. The nose contains two distinct epithelial tissues with divergent immune parameters: the respiratory mucosa and the olfactory mucosa. In both animal and human studies, we should increase efforts to delineate and distinguish the two since the immune response fundamentally differs between them. In conjunction with this concept, we should increase olfactory biopsy sampling, particularly in cases of upper respiratory infection. Conventional nasal swabs only capture respiratory tissue, and nasal washes either fail to distinguish respiratory from olfactory tissue or miss the latter entirely. It is likely that many pathogens other than those mentioned above infect the olfactory mucosa, and we have yet to recognize them.
Because the nasal passages are frequently the first to encounter airborne pathogens, the innate immune response in the olfactory mucosa is critical. As a neuronal tissue, the OM innate response may have several key differences when compared to the RM, which is dominated by more classical epithelial cells. Some evidence suggests that the IFN response in this tissue differs from that in the rest of the nasal airway. Immune cells in the OM must balance immune activity with maintaining the neurogenic potential of the olfactory mucosa while avoiding inflammation that may impact the CNS. Resident OM myeloid cells are the first responders to infection, but infiltrating cells likely play important roles in limiting replication.
Few studies have directly addressed adaptive immunity in the OM. One newly identified and critically important consideration for the innate and humoral immune response is the blood-olfactory barrier (BOB). This structure has yet to be directly identified in humans and remains to be further characterized in animal models, but by shaping access of serum proteins to the olfactory tissues, the BOB places limitations on OM immune responses. The presence of the BOB emphasizes the importance of mucosal resident plasma cells that produce locally protective antibodies, as well as the need for local production of large molecular weight molecules (such as complement factors). The dynamics that determine lymphocyte homing, residence, and retention in the OM remain to be fully described and will be important for both B and T-cell-mediated immunity.
Mounting evidence exists to link olfactory inflammation and neurodegenerative disorders, but the molecular and cellular mediators of such a connection remain to be understood. Inflammation of the olfactory mucosa may affect proximal brain structures and contribute to cognitive dysfunction in conditions such as long COVID. Neuroinvasive pathogens that use the olfactory nerve to enter the CNS and the subsequent immune response may breach the barrier to allow progressive infections and inflammation that eventually culminate in neurodegenerative disorders such as Alzheimer’s disease.
Vaccines against both respiratory disease and neurotropic microbes should consider OM protection as critical to their success, both in limiting disease severity and in halting transmission between individuals. To achieve high levels of mucosal antibodies at the olfactory surface, vaccine strategies that induce olfactory-homing plasma cells should be considered. Whether this is best achieved by certain adjuvants, particular antigen formulations, intranasal immunization, or prime-pull vaccination remains to be determined. Similarly, vaccines that induce T cells to reside in the nasal passages are also important for protection, especially against viruses that evolve to escape humoral pressure.
Knowledge of therapeutics that address olfactory infection, both during and after the acute phase of disease, is currently limited. General immunomodulatory approaches to interfere with viral replication, such as intranasal administration of IFN or other cytokines, have been thoroughly investigated only with respect to respiratory regions of the nasal mucosa. Similarly, anti-inflammatory drugs to limit olfactory inflammation may be appropriate for treatment of anosmia or other conditions, such as rhinitis. Steroids, olfactory training, and adoptive stem cell therapies have been investigated for loss of smell [179, 180], but drugs that target immune cells such as neutrophils, macrophages, or T cells might provide an alternative approach given the impact of sustained inflammation. There has been a dramatic increase in patients with long-term smell loss due to SARS-CoV-2 infection, and there is an urgent need for therapeutic interventions to help these individuals [181]. We are just beginning to understand the role of olfactory immunity in infectious disease, but the future promises more exciting advances.
Acknowledgements
E.A.M. and S.A.W. are supported by R01NS121067. E.A.M. is also supported by R21NS133561 and R21DC021260.
Author contributions
S.A.W. and E.A.M. conceived of and wrote the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Chen CR, Kachramanoglou C, Li D, Andrews P, Choi D. Anatomy and cellular constituents of the human olfactory mucosa: a review. J neurological Surg Part B, Skull Base. 2014;75:293–300. doi: 10.1055/s-0033-1361837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–90. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y, Min P, Lee S, Kim S-W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. (2020);35. [DOI] [PMC free article] [PubMed]
- 4.Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am J Otolaryngol - Head Neck Med Surg. 2020;41:102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–75. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 6.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–29. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–60. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6:5801–32. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016–35.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soni S, Jiang Y, Tesfaigzi Y, Hornick JL, Çataltepe S. Comparative analysis of ACE2 protein expression in rodent, non-human primate, and human respiratory tract at baseline and after injury: A conundrum for COVID-19 pathogenesis. PLOS ONE. 2021;16:e0247510. doi: 10.1371/journal.pone.0247510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. Iscience. (2020);23. [DOI] [PMC free article] [PubMed]
- 13.Gupta K, Mohanty SK, Mittal A, Kalra S, Kumar S, Mishra T, et al. The cellular basis of loss of smell in 2019-nCoV-infected individuals. Brief Bioinforma. 2021;22:873–81. doi: 10.1093/bib/bbaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klingenstein M, Klingenstein S, Neckel PH, Mack AF, Wagner AP, Kleger A, et al. Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs. 2021;209:155–64. doi: 10.1159/000513040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–46.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onabajo OO, Banday AR, Stanifer ML, Yan W, Obajemu A, Santer DM, et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat Genet. 2020;52:1283–93. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan M, Yoo S-J, Clijsters M, Backaert W, Vanstapel A, Speleman K, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932–49.e15. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan M, Clijsters M, Choi S, Backaert W, Claerhout M, Couvreur F, et al. Anatomical barriers against SARS-CoV-2 neuroinvasion at vulnerable interfaces visualized in deceased COVID-19 patients. Neuron. (2022):S0896-6273(22)01028-5. [DOI] [PMC free article] [PubMed]
- 19.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA neurology. (2020). [DOI] [PMC free article] [PubMed]
- 20.Butowt R, Meunier N, Bryche B, von Bartheld CS. The olfactory nerve is not a likely route to brain infection in COVID-19: a critical review of data from humans and animal models. Acta Neuropathol. 2021;141:809–22. doi: 10.1007/s00401-021-02314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185:1052–64.e12. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer L, Laksono BM, de Vrij FMS, Kushner SA, Harschnitz O, van Riel D The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. (2022):S0166-2236(22)00050-9. [DOI] [PMC free article] [PubMed]
- 23.Arunachalam JP, Anoop UR, Verma K, Rajendran R, Chidambaram S. SARS-CoV-2: The Road Less Traveled: The Respiratory Mucosa to the Brain. ACS Omega. 2021;6:7068. doi: 10.1021/acsomega.1c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proust A, Queval CJ, Harvey R, Adams L, Bennett M, Wilkinson RJ. Differential effects of SARS-CoV-2 variants on central nervous system cells and blood–brain barrier functions. J Neuroinflammation. 2023;20:184. doi: 10.1186/s12974-023-02861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Pekosz A, Villano JS, Shen W, Zhou R, Kulaga H, et al. Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. bioRxiv; (2022). [DOI] [PMC free article] [PubMed]
- 26.Seehusen F, Clark JJ, Sharma P, Bentley EG, Kirby A, Subramaniam K, et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. Viruses. 2022;14:1020. doi: 10.3390/v14051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCray PB, Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–21. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng J, Wong LYR, Li K, Verma AK, Ortiz M, Wohlford-Lenane C, et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. (2020). [DOI] [PMC free article] [PubMed]
- 29.Kumari P, Rothan HA, Natekar JP, Stone S, Pathak H, Strate PG, et al. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses. 2021;13:132. doi: 10.3390/v13010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seehusen F, Clark JJ, Sharma P, Bentley E, Kirby A, Subramaniam K, et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. bioRxiv; (2022). [DOI] [PMC free article] [PubMed]
- 31.Verma AK, Zheng J, Meyerholz DK, Perlman S SARS-CoV-2 infection of sustentacular cells disrupts olfactory signaling pathways. JCI Insight. (2022):e160277. [DOI] [PMC free article] [PubMed]
- 32.Yee KK, Pribitkin EA, Cowart BJ, Rosen D, Feng P, Rawson NE. Analysis of the Olfactory Mucosa in Chronic Rhinosinusitis. Ann N. Y Acad Sci. 2009;1170:590–5. doi: 10.1111/j.1749-6632.2009.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fumagalli V, Ravà M, Marotta D, Lucia PD, Laura C, Sala E, et al. Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Science Immunology. (2021). [DOI] [PMC free article] [PubMed]
- 34.Tang AT, Buchholz DW, Szigety KM, Imbiakha B, Gao S, Frankfurter M, et al. Cell-autonomous requirement for ACE2 across organs in lethal mouse SARS-CoV-2 infection. Plos Biol. 2023;21:e3001989. doi: 10.1371/journal.pbio.3001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieterle ME, Haslwanter D, Bortz RH, Wirchnianski AS, Lasso G, Vergnolle O, et al. A Replication-Competent Vesicular Stomatitis Virus for Studies of SARS-CoV-2 Spike-Mediated Cell Entry and Its Inhibition. Cell Host Microbe. 2020;28:486–96.e6. doi: 10.1016/j.chom.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Case JB, Rothlauf PW, Chen RE, Kafai NM, Fox JM, Smith BK, et al. Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice. Cell Host Microbe. 2020;28:465–74.e4. doi: 10.1016/j.chom.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Ar Gouilh M, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain, Behav, Immun. 2020;89:579–86. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgon C, Albin AS, Ando-Grard O, Da Costa B, Domain R, Korkmaz B, et al. Neutrophils play a major role in the destruction of the olfactory epithelium during SARS-CoV-2 infection in hamsters. Cell Mol Life Sci. 2022;79:616. doi: 10.1007/s00018-022-04643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang AJ, Lee A, Chu H, Chan J, Fan Z, Li C, et al. SARS-CoV-2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin Infect Dis. 2020;15:ciaa995. doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sia SF, Yan L-M, Chin AW, Fung K, Choy K-T, Wong AY, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–8. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13:eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer L, Rissmann M, Benavides FF, Leijten L, van Run P, Begeman L, et al. In vitro and in vivo differences in neurovirulence between D614G, delta and omicron BA. 1 SARS-CoV-2 variants. Acta Neuropathologica. Communications. 2022;10:1–13. doi: 10.1186/s40478-022-01426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Melo GD, Perraud V, Alvarez F, Vieites-Prado A, Kim S, Kergoat L, et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat Commun. 2023;14:4485. doi: 10.1038/s41467-023-40228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frere JJ, Serafini RA, Pryce KD, Zazhytska M, Oishi K, Golynker I, et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations post recovery. Sci Transl Med. 2022;0:eabq3059. doi: 10.1126/scitranslmed.abq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyna RA, Kishimoto-Urata M, Urata S, Makishima T, Paessler S, Maruyama J. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci Rep. 2022;12:628. doi: 10.1038/s41598-021-04622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–82. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Routhu NK, Cheedarla N, Gangadhara S, Bollimpelli VS, Boddapati AK, Shiferaw A, et al. A modified vaccinia Ankara vector-based vaccine protects macaques from SARS-CoV-2 infection, immune pathology, and dysfunction in the lungs. Immunity. 2021;54:542–56.e9. doi: 10.1016/j.immuni.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Doremalen N, Singh M, Saturday TA, Yinda CK, Perez-Perez L, Bohler WF, et al. SARS-CoV-2 Omicron BA. 1 and BA. 2 are attenuated in rhesus macaques as compared to Delta. Sci Adv. 2022;8:eade1860. doi: 10.1126/sciadv.ade1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckman D, Bonillas A, Diniz GB, Ott S, Roh JW, Elizaldi SR, et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Reports. (2022);41. [DOI] [PMC free article] [PubMed]
- 50.Jiao L, Yang Y, Yu W, Zhao Y, Long H, Gao J, et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct Target Ther. 2021;6:169. doi: 10.1038/s41392-021-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meekins DA, Gaudreault NN, Richt JA. Natural and experimental SARS-CoV-2 infection in domestic and wild animals. Viruses. 2021;13:1993. doi: 10.3390/v13101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martins M, Boggiatto PM, Buckley A, Cassmann ED, Falkenberg S, Caserta LC, et al. From Deer-to-Deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer. PLoS Pathog. 2022;18:e1010197. doi: 10.1371/journal.ppat.1010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pomorska-Mól M, Włodarek J, Gogulski M, Rybska M. SARS-CoV-2 infection in farmed minks–an overview of current knowledge on occurrence, disease and epidemiology. Animal. 2021;15:100272. doi: 10.1016/j.animal.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minkoff JM. tenOever B. Innate immune evasion strategies of SARS-CoV-2. Nat Rev Microbiol. 2023;21:178–94. doi: 10.1038/s41579-022-00839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatton CF, Botting RA, Dueñas ME, Haq IJ, Verdon B, Thompson BJ, et al. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat Commun. 2021;12:7092. doi: 10.1038/s41467-021-27318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegler CGK, Miao VN, Owings AH, Navia AW, Tang Y, Bromley JD, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 2021;184:4713–33.e22. doi: 10.1016/j.cell.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson CE, Namasivayam S, Foreman TW, Kauffman KD, Sakai S, Dorosky DE, et al. Mild SARS-CoV-2 infection in rhesus macaques is associated with viral control prior to antigen-specific T cell responses in tissues. Sci Immunol. 2022;7:eabo0535. doi: 10.1126/sciimmunol.abo0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loske J, Röhmel J, Lukassen S, Stricker S, Magalhães VG, Liebig J, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nature Biotechnology. (2021):1-6. [DOI] [PubMed]
- 59.Yoshida M, Worlock KB, Huang N, Lindeboom RGH, Butler CR, Kumasaka N, et al. The local and systemic response to SARS-CoV-2 infection in children and adults. Fernando J Calero-Nieto. 2021;11:3. [Google Scholar]
- 60.Bessière P, Wasniewski M, Picard-Meyer E, Servat A, Figueroa T, Foret-Lucas C, et al. Intranasal type I interferon treatment is beneficial only when administered before clinical signs onset in the SARS-CoV-2 hamster model. PLoS Pathog. 2021;17:e1009427. doi: 10.1371/journal.ppat.1009427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong Z, Karl CE, Halfmann PJ, Kawaoka Y, Winkler ES, Keeler SP, et al. Nasally delivered interferon-λ protects mice against infection by SARS-CoV-2 variants including Omicron. Cell Rep. 2022;39:110799. doi: 10.1016/j.celrep.2022.110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lupi L, Bordin A, Sales G, Colaianni D, Vitiello A, Biscontin A, et al. Persistent and transient olfactory deficits in COVID-19 are associated to inflammation and zinc homeostasis. Frontiers in Immunology. (2023);14. [DOI] [PMC free article] [PubMed]
- 63.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nature Biotechnology. (2020):1-10. [DOI] [PubMed]
- 64.Gao KM, Derr AG, Guo Z, Nundel K, Marshak-Rothstein A, Finberg RW, et al. Human nasal wash RNA-seq reveals distinct cell-specific innate immune responses between influenza and SARS-CoV-2. JCI Insight. (2021):e152288. [DOI] [PMC free article] [PubMed]
- 65.Smith N, Goncalves P, Charbit B, Grzelak L, Beretta M, Planchais C, et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol. 2021;22:1428–39. doi: 10.1038/s41590-021-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Israelow B, Mao T, Klein J, Song E, Menasche B, Omer SB, et al. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci Immunol. 2021;6:eabl4509. doi: 10.1126/sciimmunol.abl4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell. 2020;184:169–83. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine | NEJM. (2021). [DOI] [PMC free article] [PubMed]
- 70.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers | NEJM. (2021). [DOI] [PMC free article] [PubMed]
- 71.Eyre DW, Taylor D, Purver M, Chapman D, Fowler T, Pouwels KB, et al. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl J Med. 2022;386:744–56. doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl J Med. 2022;386:1207–20. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terreri S, Piano Mortari E, Vinci MR, Russo C, Alteri C, Albano C, et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe. 2022;30:400–8.e4. doi: 10.1016/j.chom.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipsitch M, Krammer F, Regev-Yochay G, Lustig Y, Balicer RD. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22:57–65. doi: 10.1038/s41577-021-00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ullah I, Prévost J, Ladinsky MS, Stone H, Lu M, Anand SP, et al. Live imaging of SARS-CoV-2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy. Immunity. 2021;54:2143–58.e15. doi: 10.1016/j.immuni.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou D, Chan JF-W, Zhou B, Zhou R, Li S, Shan S, et al. Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies. Cell Host Microbe. 2021;29:551–63.e5. doi: 10.1016/j.chom.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594:253–8. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
- 78.Bricker TL, Darling TL, Hassan AO, Harastani HH, Soung A, Jiang X, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36:109400. doi: 10.1016/j.celrep.2021.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DiPiazza AT, Leist SR, Abiona OM, Moliva JI, Werner A, Minai M, et al. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity. (2021);0. [DOI] [PMC free article] [PubMed]
- 80.Nouailles G, Adler JM, Pennitz P, Peidli S, Teixeira Alves LG, Baumgardt M, et al. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat Microbiol. 2023;8:860–74. doi: 10.1038/s41564-023-01352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schultz MD, Suschak JJ, Botta D, Silva-Sanchez A, King RG, Detchemendy TW, et al. A single intranasal administration of AdCOVID protects against SARS-CoV-2 infection in the upper and lower respiratory tracts. Hum Vaccines Immunotherapeutics. 2022;18:2127292. doi: 10.1080/21645515.2022.2127292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou R, Wang P, Wong Y-C, Xu H, Lau S-Y, Liu L, et al. Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models. EBioMedicine. 2022;75:103762. doi: 10.1016/j.ebiom.2021.103762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milligan EC, Olstad K, Williams CA, Mallory M, Cano P, Cross KA, et al. Infant rhesus macaques immunized against SARS-CoV-2 are protected against heterologous virus challenge 1 year later. Sci Transl Med. 2022;15:eadd6383. doi: 10.1126/scitranslmed.add6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wellford SA, Moseman AP, Dao K, Wright KE, Chen A, Plevin JE, et al. Mucosal plasma cells are required to protect the upper airway and brain from infection. Immunity. (2022). [DOI] [PMC free article] [PubMed]
- 85.Saunders KO, Lee E, Parks R, Martinez DR, Li D, Chen H, et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. (2021):1-9. [DOI] [PMC free article] [PubMed]
- 86.Furuyama W, Shifflett K, Pinski AN, Griffin AJ, Feldmann F, Okumura A, et al. Rapid Protection from COVID-19 in Nonhuman Primates Vaccinated Intramuscularly but Not Intranasally with a Single Dose of a Vesicular Stomatitis Virus-Based Vaccine. mBio. (2022):e0337921. [DOI] [PMC free article] [PubMed]
- 87.Diallo BK, Chasaide CN, Wong TY, Schmitt P, Lee KS, Weaver K, et al. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. npj Vaccines. 2023;8:68. doi: 10.1038/s41541-023-00665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, Harvey IB, et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell. 2020;183:169–84.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Doremalen N, Purushotham JN, Schulz JE, Holbrook MG, Bushmaker T, Carmody A, et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci Transl Med. 2021;13:eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li W, Wang T, Rajendrakumar AM, Acharya G, Miao Z, Varghese BP, et al. An FcRn-targeted mucosal vaccine against SARS-CoV-2 infection and transmission. bioRxiv; (2022). [DOI] [PMC free article] [PubMed]
- 91.Sheikh-Mohamed S, Chao GYC, Isho B, Zuo M, Nahass GR, Salomon-Shulman RE, et al. A mucosal antibody response is induced by intra-muscular SARS-CoV-2 mRNA vaccination. (2021).
- 92.Zuo F, Marcotte H, Hammarström L, Pan-Hammarström Q Mucosal IgA against SARS-CoV-2 Omicron Infection. N Engl J Med. (2022):e55-e. [DOI] [PubMed]
- 93.Havervall S, Marking U, Svensson J, Greilert-Norin N, Bacchus P, Nilsson P, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N. Engl J Med. 2022;387:1333–6. doi: 10.1056/NEJMc2209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fröberg J, Gillard J, Philipsen R, Lanke K, Rust J, van Tuijl D, et al. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat Commun. 2021;12:5621. doi: 10.1038/s41467-021-25949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren C, Gao Y, Zhang C, Zhou C, Hong Y, Qu M, et al. Respiratory mucosal immunity: kinetics of secretory immunoglobulin A in sputum and throat swabs from COVID-19 patients and vaccine recipients. Front Microbiol. 2022;13:782421. doi: 10.3389/fmicb.2022.782421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mao T, Israelow B, Peña-Hernández MA, Suberi A, Zhou L, Luyten S, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378:eabo2523. doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Russell MW, Mestecky J. Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front Immunol. 2022;13:957107. doi: 10.3389/fimmu.2022.957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mades A, Chellamathu P, Kojima N, Lopez L, MacMullan MA, Denny N, et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. Sci Rep. 2021;11:24448. doi: 10.1038/s41598-021-03931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, et al. Respiratory mucosal immunity against SARS-CoV-2 following mRNA vaccination. Sci Immunol. 2022;0:eadd4853. doi: 10.1126/sciimmunol.add4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roukens AHE, Pothast CR, König M, Huisman W, Dalebout T, Tak T, et al. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8+ T cell responses following COVID-19. Nat Immunol. 2022;23:23–32. doi: 10.1038/s41590-021-01095-w. [DOI] [PubMed] [Google Scholar]
- 102.Finlay JB, Brann DH, Abi Hachem R, Jang DW, Oliva AD, Ko T, et al. Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci Transl Med. 2022;14:eadd0484. doi: 10.1126/scitranslmed.add0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim JME, Tan AT, Le Bert N, Hang SK, Low JGH, Bertoletti A SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. Journal of Experimental Medicine. (2022);219. [DOI] [PMC free article] [PubMed]
- 104.Ssemaganda A, Nguyen HM, Nuhu F, Jahan N, Card CM, Kiazyk S, et al. Expansion of cytotoxic tissue-resident CD8+ T cells and CCR6+CD161+ CD4+ T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat Commun. 2022;13:3357. doi: 10.1038/s41467-022-30913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Yu J, McMahan K, Jacob-Dolan C, He X, Giffin V, et al. CD8 T cells contribute to vaccine protection against SARS-CoV-2 in macaques. Sci Immunol. 2022;7:eabq7647. doi: 10.1126/sciimmunol.abq7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Diniz MO, Mitsi E, Swadling L, Rylance J, Johnson M, Goldblatt D, et al. Airway-resident T cells from unexposed individuals cross-recognize SARS-CoV-2. Nat Immunol. 2022;23:1324–9. doi: 10.1038/s41590-022-01292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bertoletti A, Le Bert N, Tan AT SARS-CoV-2-specific T cells in the changing landscape of the COVID-19 pandemic. Immunity. (2022). [DOI] [PMC free article] [PubMed]
- 108.Zheng MZ, Wakim LM. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 2022;15:379–88. doi: 10.1038/s41385-021-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ishii H, Nomura T, Yamamoto H, Nishizawa M, Hau TTT, Harada S, et al. Neutralizing-antibody-independent SARS-CoV-2 control correlated with intranasal-vaccine-induced CD8+ T cell responses. CR Med. (2022);3. [DOI] [PMC free article] [PubMed]
- 110.Kok L, Masopust D, Schumacher TN. The precursors of CD8+ tissue resident memory T cells: from lymphoid organs to infected tissues. Nat Rev Immunol. 2022;22:283–93. doi: 10.1038/s41577-021-00590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA, et al. Post-viral effects of COVID-19 in the olfactory system and their implications. The Lancet Neurology. (2021);0. [DOI] [PMC free article] [PubMed]
- 112.Narayanan SN, Shivappa P, Padiyath S, Bhaskar A, Li YW, Merghani TH The Prevalence and Pathophysiology of Chemical Sense Disorder Caused by the Novel Coronavirus. Frontiers in Public Health. (2022);10. [DOI] [PMC free article] [PubMed]
- 113.Othman BA, Maulud SQ, Jalal PJ, Abdulkareem SM, Ahmed JQ, Dhawan M, et al. Olfactory dysfunction as a post-infectious symptom of SARS-CoV-2 infection. Ann Med Surg. 2022;75:103352. doi: 10.1016/j.amsu.2022.103352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cardoso CC, Rossi ÁD, Galliez RM, Faffe DS, Tanuri A, Castiñeiras TMPP. Olfactory Dysfunction in Patients With Mild COVID-19 During Gamma, Delta, and Omicron Waves in Rio de Janeiro, Brazil. JAMA. 2022;328:582–3. doi: 10.1001/jama.2022.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan HQM, Pendolino AL, Andrews PJ, Choi D. Prevalence of olfactory dysfunction and quality of life in hospitalised patients 1 year after SARS-CoV-2 infection: a cohort study. BMJ Open. 2022;12:e054598. doi: 10.1136/bmjopen-2021-054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lechien JR, Vaira LA, Saussez S. Prevalence and 24-month recovery of olfactory dysfunction in COVID-19 patients: A multicentre prospective study. J Intern Med. 2023;293:82–90. doi: 10.1111/joim.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Renaud M, Thibault C, Le Normand F, McDonald EG, Gallix B, Debry C, et al. Clinical Outcomes for Patients With Anosmia 1 Year After COVID-19 Diagnosis. JAMA Netw Open. 2021;4:e2115352. doi: 10.1001/jamanetworkopen.2021.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen S, Wang S The immune mechanism of the nasal epithelium in COVID-19–related olfactory dysfunction. Frontiers in Immunology. (2023);14. [DOI] [PMC free article] [PubMed]
- 119.Shelton JF, Shastri AJ, Fletez-Brant K, Aslibekyan S, Auton A. The UGT2A1/UGT2A2 locus is associated with COVID-19-related loss of smell or taste. Nat Genet. 2022;54:121–4. doi: 10.1038/s41588-021-00986-w. [DOI] [PubMed] [Google Scholar]
- 120.Le Bon S-D, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Oto-rhino-Laryngol. 2021;278:3113–7. doi: 10.1007/s00405-020-06520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pires IDA, Steffens ST, Mocelin AG, Shibukawa DE, Leahy L, Saito FL, et al. Intensive olfactory training in post-COVID-19 patients: a multicenter randomized clinical trial. Am J Rhinol Allergy. 2022;36:780–7. doi: 10.1177/19458924221113124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yan CH, Jang SS, Lin HFC, Ma Y, Khanwalkar AR, Thai A, et al., editors. Use of platelet‐rich plasma for COVID‐19–related olfactory loss: a randomized controlled trial. International Forum of Allergy & Rhinology; (2023): Wiley Online Library. [DOI] [PMC free article] [PubMed]
- 123.Tsivgoulis G, Fragkou PC, Lachanis S, Palaiodimou L, Lambadiari V, Papathanasiou M, et al. Olfactory bulb and mucosa abnormalities in persistent COVID‐19 induced anosmia: a Magnetic Resonance Imaging study. European Journal of Neurology. (2020):ene.14537-ene. [DOI] [PubMed]
- 124.Beigi-Khoozani A, Merajikhah A, Soleimani M Magnetic Resonance Imaging Findings of Olfactory Bulb in Anosmic Patients with COVID-19: A Systematic Review. Chin Med Sci J. (2022). [DOI] [PMC free article] [PubMed]
- 125.Hossain ME, Lister D, Bartolo C, Kinsella PM, Knox J, Aldrich R, et al. Prolonged Viral Shedding in Patients with Mild to Moderate COVID-19 Disease: A Regional Perspective. Infect Dis (Auckl) 2021;14:11786337211010428. doi: 10.1177/11786337211010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Long H, Zhao J, Zeng H-L, Lu Q-B, Fang L-Q, Wang Q, et al. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: a prospective study. BMC Infect Dis. 2021;21:1282. doi: 10.1186/s12879-021-07002-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Toomre D, Kandula S, Shaman J. Longitudinal Association of COVID-19 Hospitalization and Death with Online Search for Loss of Smell or Taste. Emerg Infect Dis. 2023;29:1711. doi: 10.3201/eid2908.230071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J Neurol Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 130.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain, Behav, Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329:1934–46. doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beretta S, Cristillo V, Camera G, Colleoni CM, Pellitteri G, Viti B, et al. Incidence and Long-term Functional Outcome of Neurologic Disorders in Hospitalized Patients With COVID-19 Infected With Pre-Omicron Variants. Neurology. 2023;101:e892–e903. doi: 10.1212/WNL.0000000000207534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Troxel AB, Frontera JA, Mendoza-Puccini C. The National Institutes of Health COVID-19 NeuroDatabank and NeuroBiobank: a national resource for learning, discovery, and progress. Front Neurol. 2021;11:615061. doi: 10.3389/fneur.2020.615061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen W-B, Logue J, Yang P, Baracco L, Elahi M, Reece EA, et al. SARS-CoV-2 invades cognitive centers of the brain and induces Alzheimer’s-like neuropathology. bioRxiv; (2022).
- 135.Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee M-H, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–68.e16. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fekete R, Simats A, Biro E, Cserep C, Schwarcz AD, Posfai B, et al. Infection-induced vascular inflammation in COVID-19 links focal microglial dysfunction with neuropathologies through IL-1/IL-6-related systemic inflammatory states. bioRxiv. (2023):2023.06. 23.546214.
- 137.Grant RA, Poor TA, Sichizya L, Diaz E, Bailey JI, Soni S, et al. Prolonged exposure to lung-derived cytokines is associated with inflammatory activation of microglia in patients with COVID-19. bioRxiv. (2023):2023.07. 28.550765. [DOI] [PMC free article] [PubMed]
- 138.Yeh C-F, Huang W-H, Lan M-Y, Hung W Lipopolysaccharide-Initiated Rhinosinusitis Causes Neuroinflammation and Olfactory Dysfunction in Mice. American Journal of Rhinology & Allergy. (2022):19458924221140965. [DOI] [PubMed]
- 139.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem senses. 2014;39:185–94. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 140.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J Virol. 2008;82:7264–75. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–24. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by Human Respiratory Coronaviruses. J Virol. 2000;74:8913–21. doi: 10.1128/JVI.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J Virol. 2018;92:e00404–18. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jacomy H, Talbot PJ. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 2003;315:20–33. doi: 10.1016/S0042-6822(03)00323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–6. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, et al. Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. J Infect Dis. 2016;213:712–22. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi J, Li Z, Zhang J, Xu R, Lan Y, Guan J, et al. PHEV infection: A promising model of betacoronavirus-associated neurological and olfactory dysfunction. PLOS Pathog. 2022;18:e1010667. doi: 10.1371/journal.ppat.1010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barnett E, Cassell M, Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–25. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barnett EM, Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 1993;194:185–91. doi: 10.1006/viro.1993.1248. [DOI] [PubMed] [Google Scholar]
- 151.Barthold S. Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol. 1988;76:502–6. doi: 10.1007/BF00686390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schwob JE, Saha S, Youngentob SL, Jubelt B. Intranasal Inoculation with the Olfactory Bulb Line Variant of Mouse Hepatitis Virus Causes Extensive Destruction of the Olfactory Bulb and Accelerated Turnover of Neurons in the Olfactory Epithelium of Mice. Chem Senses. 2001;26:937–52. doi: 10.1093/chemse/26.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Flanagan CE, Wise SK, DelGaudio JM, Patel ZM. Association of Decreased Rate of Influenza Vaccination With Increased Subjective Olfactory Dysfunction. JAMA Otolaryngol–Head Neck Surg. 2015;141:225–8. doi: 10.1001/jamaoto.2014.3399. [DOI] [PubMed] [Google Scholar]
- 154.Dumm RE, Wellford SA, Moseman EA, Heaton NS. Heterogeneity of Antiviral responses in the upper respiratory tract mediates differential non-lytic clearance of influenza viruses. Cell Rep. 2020;32:108103. doi: 10.1016/j.celrep.2020.108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yoshida T, Nishiyama Y, Kimura Y, Yokochi T, Kohsaka S, Goshima F, et al. Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J Gen Virol. 2002;83:2109–16. doi: 10.1099/0022-1317-83-9-2109. [DOI] [PubMed] [Google Scholar]
- 156.Zielinski MR, Souza G, Taishi P, Bohnet SG, Krueger JM. Olfactory bulb and hypothalamic acute-phase responses to influenza virus: effects of immunization. NIM. 2013;20:323–33. doi: 10.1159/000351716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leyva-Grado VH, Churchill L, Wu M, Williams TJ, Taishi P, Majde JA, et al. Influenza Virus- and Cytokine-Immunoreactive Cells in the Murine Olfactory and Central Autonomic Nervous Systems before and after Illness Onset. J Neuroimmunol. 2009;211:73–83. doi: 10.1016/j.jneuroim.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schlesinger RW. Incomplete Growth Cycle of Influenza Virus in Mouse Brain. Proc Soc Exp Biol Med. 1950;74:541–8. doi: 10.3181/00379727-74-17966. [DOI] [PubMed] [Google Scholar]
- 159.Bouvier NM, Lowen AC Animal models for influenza virus pathogenesis and transmission. (2010). 1530-63. [DOI] [PMC free article] [PubMed]
- 160.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol. 2017;2:eaam6970-eaam. doi: 10.1126/sciimmunol.aam6970. [DOI] [PubMed] [Google Scholar]
- 161.Klinkhammer J, Schnepf D, Ye L, Schwaderlapp M, Gad HH, Hartmann R, et al. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife.7:e33354. [DOI] [PMC free article] [PubMed]
- 162.van den Brand JMA, Stittelaar KJ, van Amerongen G, Reperant L, de Waal L, Osterhaus ADME, et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PloS One. 2012;7:e42343. doi: 10.1371/journal.pone.0042343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Plourde JR, Pyles JA, Layton RC, Vaughan SE, Tipper JL, Harrod KS. Neurovirulence of H5N1 infection in ferrets is mediated by multifocal replication in distinct permissive neuronal cell regions. PloS One. 2012;7:e46605. doi: 10.1371/journal.pone.0046605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richard M, van den Brand JMA, Bestebroer TM, Lexmond P, de Meulder D, Fouchier RAM, et al. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat Commun. 2020;11:766. doi: 10.1038/s41467-020-14626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mizuguchi M. Influenza encephalopathy and related neuropsychiatric syndromes. Influenza Other Respiratory Viruses. 2013;7:67–71. doi: 10.1111/irv.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Studahl M. Influenza virus and CNS manifestations. J Clin Virol. 2003;28:225–32. doi: 10.1016/S1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 167.Bauer L, Benavides FF, Kroeze EJV, de Wit E, van Riel D The neuropathogenesis of highly pathogenic avian influenza H5Nx viruses in mammalian species including humans. Trends Neurosci. (2023). [DOI] [PMC free article] [PubMed]
- 168.Lee N, Wong CK, Chan P. ults. Emerg Infect Dis. 2010;16:139–42. doi: 10.3201/eid1601.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Popescu CP, Florescu SA, Lupulescu E, Zaharia M, Tardei G, Lazar M, et al. Neurologic Complications of Influenza B Virus Infection in Adults, Romania. Emerg Infect Dis. 2017;23:574–81. doi: 10.3201/eid2304.161317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McCullers JA, Facchini S, Chesney PJ, Webster RG Influenza B virus encephalitis - PubMed. (1999). [DOI] [PubMed]
- 171.Newland JG, Romero JR, Varman M, Drake C, Holst A, Safranek T, et al. Encephalitis Associated with Influenza B Virus Infection in 2 Children and a Review of the Literature. Clin Infect Dis. 2003;36:e87–e95. doi: 10.1086/368184. [DOI] [PubMed] [Google Scholar]
- 172.Reyes C, Miranda S, Fica A, Navarrete M. Encephalitis caused by type B influenza virus in an adult. Report of one case. Rev Med Chil. 2019;147:922–7. [DOI] [PubMed]
- 173.Britton PN, Blyth CC, Macartney K, Dale RC, Li-Kim-Moy J, Khandaker G, et al. The Spectrum and Burden of Influenza-Associated Neurological Disease in Children: Combined Encephalitis and Influenza Sentinel Site Surveillance From Australia, 2013–2015. Clin Infect Dis. 2017;65:653–60. doi: 10.1093/cid/cix412. [DOI] [PubMed] [Google Scholar]
- 174.van Riel D, Leijten LM, Verdijk RM, GeurtsvanKessel C, van der Vries E, van Rossum AMC, et al. Evidence for influenza virus CNS invasion along the olfactory route in an immunocompromised infant. J Infect Dis. 2014;210:419–23. doi: 10.1093/infdis/jiu097. [DOI] [PubMed] [Google Scholar]
- 175.Aronsson F, Robertson B, Ljunggren H-G, Kristensson K. Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system. Viral Immunol. 2003;16:415–23. doi: 10.1089/088282403322396208. [DOI] [PubMed] [Google Scholar]
- 176.Schrauwen EJA, Herfst S, Leijten LM, van Run P, Bestebroer TM, Linster M, et al. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J Virol. 2012;86:3975–84. doi: 10.1128/JVI.06828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci USA. 2009;106:14063–8. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun H, Li H, Tong Q, Han Q, Liu J, Yu H, et al. Airborne transmission of human-isolated avian H3N8 influenza virus between ferrets. Cell. (2023). [DOI] [PubMed]
- 179.Helman SN, Adler J, Jafari A, Bennett S, Vuncannon JR, Cozart AC, et al., editors. Treatment strategies for postviral olfactory dysfunction: a systematic review. Allergy and asthma proceedings; (2022): OceanSide Publications, Inc. [DOI] [PMC free article] [PubMed]
- 180.Kurtenbach S, Goss GM, Goncalves S, Choi R, Hare JM, Chaudhari N, et al. Cell-based therapy restores olfactory function in an inducible model of hyposmia. Stem Cell Rep. 2019;12:1354–65. doi: 10.1016/j.stemcr.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lechner M, Liu J, Counsell N, Yan CH, Paun S, Eynon-Lewis N, et al., editors. High prevalence of persistent smell loss and qualitative smell dysfunction during the coronavirus disease 2019 (COVID-19) pandemic in the United States: Urgent need for clinical trials. Int Forum Allergy Rhinol. 2023. [DOI] [PMC free article] [PubMed]