Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disease occurring in the gut causing chronic diarrhea and abdominal pain with severe complications. Sesame cake is a by-product of sesame oil production, possessing various beneficial properties; however, little is known about the effect of sesame cake extract (SCE) against IBD. The aim of this study was to investigate the protective effect of SCE against dextran sulfate sodium (DSS)-induced colitis in mice. Administration of SCE was first performed at 7 days before treating mice with 2.5% DSS to induce colitis for 7 days. SCE pretreatment improved symptoms of DSS-induced colitis. In addition, SCE ameliorated histopathological damages of the mucus layer in colon tissues and decreased pro-inflammatory cytokines in colitis-induced mice. SCE also suppressed apoptosis and oxidative stress in colitis-induced colon tissues. Together, these findings suggest that SCE could be potential nutraceuticals for treating colitis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01367-1.
Keywords: Colitis, Sesame cake extract, Pro-inflammatory cytokines, Apoptosis, Oxidative stress
Introduction
Chronic inflammatory bowel disorder (IBD) such as Crohn’s disease and ulcerative colitis is a condition that causes severe inflammation in the intestine, leading to abdominal pain, diarrhea, and loss of weight (Griffiths, 1998). A variety of factors such as environmental, immune, and microbial factors contributing with the incidence of IBD have been elucidated (Kaser et al., 2010). These factors can exert aberrant barrier function and immune reaction, consequently provoking inflammatory response in the gut mucosa (Khor et al., 2011). The pathological inflammatory response is caused by numerous inflammatory mediators, such as pro-inflammatory cytokines and chemokines, causing serious damage to the intestinal epithelium (Neurath, 2014). Indeed, pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α have been found to be increased in the gut mucosa of IBD patients (Sanchez-Munoz et al., 2008), indicating that they have a key role in the pathogenesis of IBD.
Therapeutic approaches for anti-inflammation, immunosuppression, anti-adhesion, cell signaling kinase inhibitors, microbiota transplantation, and stem cell therapy have been introduced or currently being evaluated in clinical trials for IBD (Verstockt, 2018). Natural compounds and their derivatives have been spotlighted as alternative substitutes for treating IBD due to their safeness and effectivity (Duan et al., 2021). Various natural products derived from plants, such as catechins, anthocyanins, and terpenes, exhibit anti-inflammatory effects and have been successfully employed to treat IBD (Gupta et al., 2022).
Sesame (Sesamum indicum L.) seed oil has long been used as a health food and an herbal remedy worldwide (Morris et al., 2021). It contains many bioactive substances such as tocopherol, several lignans (sesamin, sesamolin, and sesaminol), and several phenolic compounds (ferulic, vanillic, and cinnamic acids) known to possess potential biological properties (Ben Othman et al., 2015). It exhibits preventive effects against a variety of diseases, such as hypercholesterolemic (Chen et al., 2005), inflammatory (Hsu and Parthasarathy, 2017), atherosclerotic (Wang et al., 2021), neuronal (Chung et al., 2010), and cardiovascular diseases (Dalibalta et al., 2020).
Sesame cake is a by-product of oil extraction in sesame oil industry and has been utilized as animal feeds or discarded (Elleuch et al., 2007). However, previous studies have revealed that sesame cake extract (SCE) contains numerous active compounds such as antioxidants, phytochemicals, phenolic acids, and lignans with beneficial functions (Esmaeilzadeh Kenari et al., 2014; Mekky et al., 2019). Especially, previous studies have demonstrated that SCE can improve brain functions including cognition and memory. A study using a mouse model with accelerated aging has demonstrated that sesaminol glucosides from SCE can attenuate cognitive deficits caused by aging due to their antioxidant activities (Um et al., 2009). Another recent clinical study on older adults with memory impairment has reported that supplement of SCE for 12 weeks can enhance verbal memory abilities and diminish plasma β-amyloid levels (Jung et al., 2021). However, advantageous effects of SCE on other diseases remain unclear.
Oxidative stress can be a major factor in the pathogenesis of IBD (Bourgonje et al., 2020). Overproduction of reactive oxygen species (ROS) further gives rise to imbalance between production of ROS and antioxidant activity, which in turn can lead to oxidative stress (Schieber and Chandel, 2014). Oxidative stress is known to cause mucosal layer damage in the intestine and lead to bacterial infection, which can stimulate immune responses, cause inflammation, and initiate colitis (Tian et al., 2017). Indeed, a clinical study has shown that colitis patients exhibit increased levels of ROS but decreased levels of antioxidants in the colonic mucosa (Sturniolo et al., 1998).
Nuclear factor E2-related factor2 (Nrf2) is a sensor protein that has an important role in protecting cells against oxidative stress. Upon oxidative stress, Nrf2 translocates to the nucleus from the cytosol to increase the expression of several antioxidative enzymes, such as NADPH: quinone oxidoreductase 1 (Nqo1), heme oxygenase1 (Hmox1), and glutamate-cysteine ligase catalytic subunit (Gclc), which confers protection of cells against oxidative stress, inflammation, and apoptosis (Jaramillo and Zhang, 2013). Much evidence has been accumulated that Nrf2 has a preventive effect of colitis through regulation of inflammatory cytokines and oxidative stress (Zhang et al., 2008).
DSS is a water-soluble, negative charged sulfated polysaccharide. It induces intestinal inflammation resulting from the damage to the epithelial monolayer lining the large intestine (Chassaing et al, 2014). The DSS-induced colitis model is very popular due to the advantages, such as rapidity, simplicity, and reproducibility. The objective of the present study was to determine whether SCE could prevent DSS-induced ulcerative colitis in mice and whether inhibition of oxidative stress was involved in such preventative effect.
Materials and methods
Reagents
DSS (colitis grade; molecular weight, 36,000–50,000) was purchased from MP bio-medicals (Santa Ana, CA, USA). Enzyme-Linked Immunosorbent Assay (ELISA) kits for TNFα, IL-1β, and IL-6 were obtained from Cusabio (Houston, TX, USA). Hematoxylin & Eosin (H & E) staining kit was purchased from Sigma (Burlington, MA, USA). Periodic Acid-Schiff (PAS)-Alcian blue staining kit was purchased from Abcam (Cambridge, UK). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining kit was purchased from Roche Diagnostics (Manheim, Germany). Dichloro-dihydro-fluorescein diacetate (DCFH-DA) dye was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Western blotting-related reagents were purchased from LPS Solution Co. (Daejeon, Korea), Thermo Fisher Scientific, or Roche Diagnostics. Primary and secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA), Santa Cruz Biotechnology (Santa Cruz, CA, USA), or Jackson Immuno Research Labs (West Grove, PA, USA). Detailed information of antibodies is listed in Table S1. Quantitative real-time (qRT)-PCR-related kits were purchased from New England Biolabs (NEB, Ipswich, MA, USA), Promega (Madison, WI, USA), or Kapa Biosystems (Boston, MA, USA).
Extraction of sesame cake
The sesame cakes used in this study were obtained from Queensbucket Co. (Iksan, Korea) and powdered with a mixer from Osaka Chemical Co. (Osaka, Japan). Sesame cake was extracted with ultrasound extraction method as previously described (Eom et al., 2021). Briefly, 500 mL water and 100 g sesame cake powder were mixed in a container and stirred. An ultrasound apparatus (AMMM-1000 W, MPI-ultrasonic Co., Ltd., Le Locle, Switzerland) powered by a constant frequency of 20 ± 0.05 kHz with a maximum power of 1000 W was used for extraction at room temperature for 16 h. Extracts were filtered to produce filtrates which were then concentrated and freeze-dried. The extraction yield was 2.97%. Dried extracts were stored at − 20 °C.
Animals
Male C57BL/6 mice aged 6 weeks (20–22 g) were purchased from Saeronbio Co. (Uiwang, Korea) and maintained under controlled environmental conditions (12 h light & dark cycle, 23 ± 2 °C, and humidity 60 ± 10%). The mice were fed with normal diet from Purina (Sungnam, Korea). All animals were allowed to access food and water freely. Protocols of animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Jeonbuk National University (Ethics No. JBNU 2022–084). For euthanasia, the mice were anesthetized with CO2 inhalation to minimize suffering.
Experimental design
After mice were acclimated for 7 days, they were randomly divided into seven groups: (1) control group, (2) SCE alone-treated group (300 mg/kg/day), (3) DSS alone-treated group, (4) DSS + 50 mg/kg/day SCE treated group, (5) DSS + 100 mg/kg/day SCE treated group, (6) DSS + 300 mg/kg/day SCE treated group, and (7) DSS + 200 mg/kg/day chlorogenic acid (CA)-treated group. Colitis was induced by 2.5% DSS for 7 days. For combined administration of SCE and DSS, SCE was first orally administrated by gavage for 14 days and 2.5% DSS was simultaneously given for 7 days by drinking water at day 8 after starting SCE administration (Fig. 1). CA as a positive drug was orally administrated at 200 mg/kg/day by gavage for 14 days.
Fig. 1.
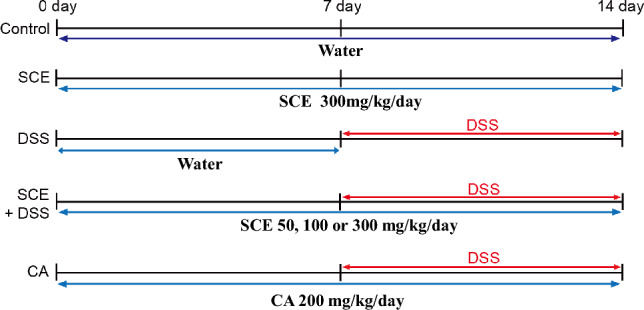
Experimental design for treatment with SCE or DSS for induction of colitis. SCE sesame cake extract; DSS dextran sulfate sodium; CA chlorogenic acid
Determination of disease activity index (DAI)
The colon length was measured when the mice were sacrificed at 7 days after starting DSS treatment. The mice were observed for loss of body weight stool consistency and fecal bleeding to access the severity of colitis. DAI was further calculated by scoring the degree of these three major clinical signs. The formula of DAI scoring is as follows: DAI = (combined score of weight loss, stool consistency and fecal bleeding)/3. The criteria of DAI score are shown as Table S2.
Histological analyses
The histological change of colon tissue was evaluated by H & E and PAS-Alcian blue staining. The colon tissues were embedded with paraffin and cut into 7 µm thickness after fixing with 4% paraformaldehyde. After that, they were stained with H & E staining. Histological injury score was calculated by the degree of pathological changes of crypts and inflammatory cell infiltration as shown in Table S3. The value was calculated the average value for each parameter. PAS-Alcian blue staining was conducted by the manufacturer’s protocols. Briefly, paraffin sectioned samples were immersed in 3% acetic acid solution for 2 min followed by incubation of Alcian blue (pH 2.5) for 20 min. They were then incubated with 1% periodic acid for 5 min and Schiff’s solution for 20 min. Finally, the tissue sections were immersed in hematoxylin for nuclear staining. The goblet cells were counted and normalized to crypt numbers.
ELISA assay of serum inflammatory cytokines
The blood samples from the caudal vena cava were collected in lithium heparin-containing tubes. Serum was collected by centrifugation of whole blood samples at 3000 rpm for 10 min and kept at − 80 °C. Serum levels of TNFα, IL-1β, and IL-6 were measured with ELISA kits according to the manufacturer’s instructions. Blood was obtained from the caudal vena cava and then centrifuged at 1,000 xg for 10 min to collect serum. Serum samples were incubated with biotin antibody followed by incubation with horseradish peroxidase-avidin for 1 h at 37 ℃. Finally, the enzymatic reaction was developed by incubating with 3,3′,5,5′-tetramethylbenzidine substrate for 30 min at 37 ℃. The optical density was measured at 450 nm with an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
TUNEL and DCFH-DA staining of the colon tissue
Colon tissues were cryosectioned to 5 µm in thickness after cryopreserving with optimal cutting temperature compound. They were then fixed with 4% paraformaldehyde for 10 min at room temperature. A TUNEL staining kit was then used to detect apoptotic cells with DNA fragmentation. Briefly, tissue sections were labelled with TUNEL reaction mixture for 1 h at 37 ℃ after permeabilizing with 0.1% Triton-X 100 for 2 min on ice. They were then incubated with 3 µM DCFH-DA dye for 20 min at room temperature to measure ROS production. Cells were analyzed with a fluorescence microscope (Olympus, Tokyo, Japan). Percentages of apoptotic cells were calculated as the number of TUNEL-positive cells over the total number of cells. Fluorescence intensity of DCFH-DA was measured at 485 nm excitation and 535 nm emission with a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
qRT-PCR analysis
Total RNAs were extracted from colon tissues using the kits (NEB) and reverse transcribed to cDNAs with a GoScript RT kit (Promega). These cDNAs were then used for qRT-PCR with a Thermal cycler (Takara) and a qPCR kit (Kapa Biosystems). Intensities of target genes were normalized against the intensity of 18S rRNA. Primer sequences are shown in Table S4.
Western blotting
Colon tissues were homogenized with radio-immunoprecipitation assay (RIPA) buffer contained protease and phosphatase inhibitors (Roche). Proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Membranes were then blocked with 5% BSA in Tris buffered saline with Tween 20 (TBST) solution for 1 h at room temperature. They were then incubated with appropriate primary antibodies overnight at 4 ℃ followed by incubation with secondary antibodies at room temperature for 2 h. Finally, proteins were visualized with a commercially available enhanced chemiluminescence (ECL) kit (Millipore Corp., Billerica, MA, USA). Primary antibodies used in this study are listed in Table S1. β-actin was used as an internal control.
Compositional analysis of SCE
Sesamin and sesamolin (Sigma) were purchased, dissolved in methanol, and filtered through 0.2 μm filters to prepare reference standard solutions. For construction of calibration curves, seven different concentrations (0.01875, 0.0325, 0.075, 0.15, 0.3, 0.6, and 1.2 mg/mL) of standard solutions were prepared by diluting stock solutions with high-performance liquid chromatography (HPLC) grade methanol. These solutions were then injected in triplicates. HPLC was used to quantitatively analyze the amount of each compound in the extract by comparison with commercially available standard compounds. After obtaining calibration curves of sesamin and sesamolin, reliable coefficients of determination (R2 > 0.999) were obtained. HPLC was performed on Alliance 2690 HPLC system equipped with Waters 2487 UV/Vis detector with a Waters Sunfire C18 column (5 µm, 4.6 × 250 mm; Milford, MA, USA). SCE was dissolved in HPLC grade methanol, sonicated for 30 min, and filtered through a 0.2 μm polytetrafluoroethylene membrane filter (Millipore, USA). The mobile phase A was water and mobile phase B was methanol. The flow rate was adjusted to 0.7 mL/min. The detection wavelength was set at 285 nm. The temperature was held constant at 25 ℃. The injection volume was 10 μl. The analysis was carried out thrice using three independent samples.
Statistical analysis
Multiple comparisons were performed with one-way analysis of variance and Bonferroni post hoc test using a Prism software (GraphPad Software Inc., San Diego, CA, USA). Values are shown as mean ± standard error of the mean. P < 0.05 was considered statistically significant.
Results and discussion
SCE ameliorates symptoms of colitis in DSS-induced colitis
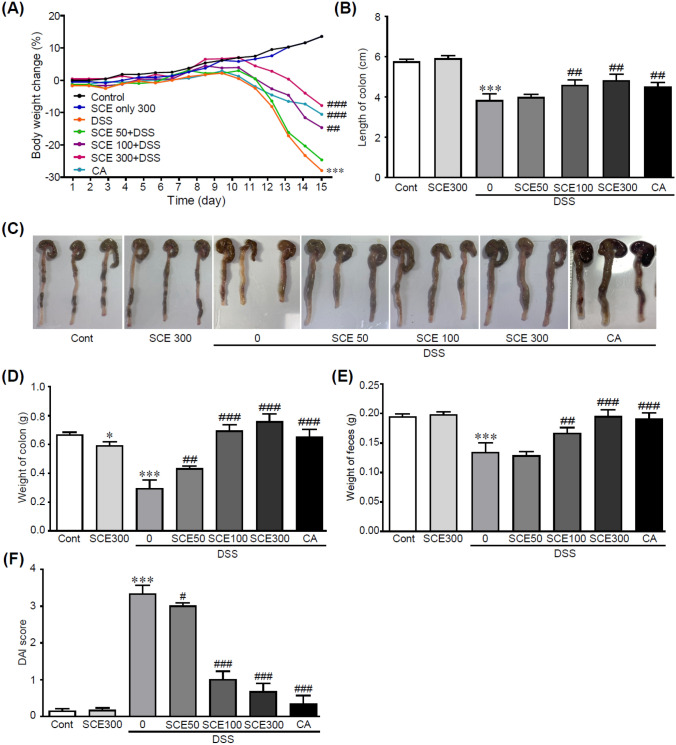
To induce colitis in this study, the colitis mouse model induced by DSS was utilized, which is the most widely used due to its high similarity to human colitis (Chassaing et al, 2014). To determine the protective effect of SCE on DSS-induced colitis, parameters related to symptoms of colitis, including weight, colon length, and rectal bleeding after treating with 50 mg/kg/day SCE, 100 mg/kg/day SCE, or 300 mg/kg/day SCE followed by 2.5% DSS treatment of mice were evaluated. The DAI score was also calculated by comprehensively evaluating parameters including weight loss, stool consistency, and fecal bleeding followed by criteria as shown in Table S2. DSS alone-treated mice showed significantly decreased body weights, whereas the loss of weight was dramatically inhibited by administration of SCE (Fig. 2A). Additionally, DSS treatment decreased fecal weight. It also induced diarrhea as a main symptom of colitis. Notably, administration of SCE suppressed the decrease of fecal weight caused by DSS treatment (Fig. 2B). The length and weight of colon were also significantly preserved when SCE was administrated to DSS-treated mice, which exhibited decreases of colon length and weight (Fig. 2C, D and E). DSS-alone-treated mice displayed high DAI score with a mean value of 3.3. Administration with 100 or 300 mg/kg/day SCE lowered the DAI score (1.0 and 0.67, respectively) than DSS alone-treatment (Fig. 2F). CA has proven to possess various pharmacological activities, especially anti-inflammation and anti-oxidation effects in colitis (Gao et al., 2019; Wan et al., 2021). CA treatment, as a positive drug, also inhibited the symptoms of colitis. Interestingly, 100 and 300 mg/kg/day SCE-treated groups were more protective than those in CA-treated group. These results indicate that SCE can effectively protect mice against DSS-induced colitis.
Fig. 2.
SCE attenuates symptoms of DSS-induced colitis. (A) Changes of body weights of mice with DSS-induced colitis with or without SCE treatment (n = 10). (B) Colon lengths, (C) Representative photographs of colons, (D) Colon weights, (E) Weight of feces, (F) Disease activity index (DAI) for mice with DSS-induced colitis with or without SCE treatment. *p < 0.05 and ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. DSS alone-treated group. Cont control; SCE sesame cake extract; DSS dextran sulfate sodium; CA chlorogenic acid
SCE attenuates injury of colon tissue in DSS-induced colitis
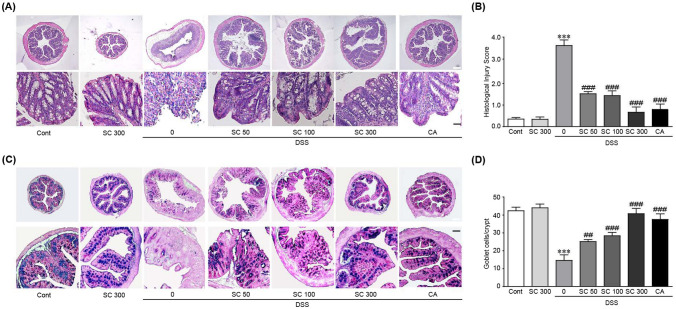
Histological examination of colon tissues revealed that severe inflammation was induced in DSS alone-treated mice. That is, infiltration of inflammatory cells into the submucosal layer and submucosal edema were observed. In addition, the morphology of the mucosal layer was not maintained due to severe damage of crypt (Fig. 3A). On the other hand, pretreatment with SCE dramatically prevented damage to colon tissues caused by DSS treatment as indicated by decreased infiltration of inflammatory cells, edema, and damage of crypt (Fig. 3A). These results were also shown in histological injury score, which displays high value in DSS alone treated group and decreased values in SCE pretreatments (Fig. 3B).
Fig. 3.
SCE ameliorates histopathological damages in colitis-induced colon tissues. (A) Hematoxylin and eosin (H&E) staining and (B) histological injury score. (C) periodic acid-Schiff (PAS)-Alcian blue staining and (D) number of goblet cells of colon tissues of mice with DSS-induced colitis with or without SCE treatment (n = 10). Cont control; SCE sesame cake extract; DSS dextran sulfate sodium; CA chlorogenic acid. White and black scale bars, 50 and 200 µm, respectively
Since mucins secreted by goblet cells in the colon play an essential role in gut defense mechanisms (Bankole et al., 2021), PAS-Alcian blue staining was next performed to detect neutral and acidic mucins in SCE with or without DSS-treated mice. PAS-Alcian blue staining showed that mucins (blue color) were significantly depleted in DSS alone-treated mice. SCE administration dramatically inhibited the depletion of mucins caused by DSS treatment (Fig. 3C and D). Especially, 300 mg/kg/day SCE treatment preserved the secretion of mucins to a level similar to that in the control group. CA treatment also ameliorated the pathological changes in colon tissues from DSS-treated mice. Collectively, these results suggest that SCE can effectively prevent pathological changes and defects in secretion of mucins induced by DSS treatment.
SCE attenuates the production of proinflammatory cytokines in DSS-induced colitis.
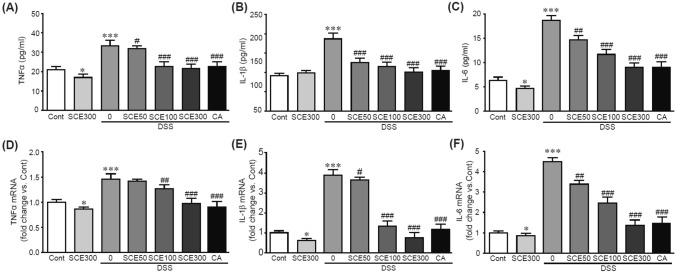
One of the classic symptoms of IBD disease is an increase in proinflammatory cytokines (Strober and Fuss, 2011). To evaluate inhibitory effects of SCE on the production of proinflammatory cytokines involved in colitis induced by DSS, serum levels and gene expression of typical proinflammatory cytokines such as TNFα, IL-1β, and IL-6 were determined. ELISA was performed to determine serum levels of these cytokines. Results showed increased levels of these cytokines in DSS alone-treated mice in comparison with those in control mice (Fig. 4A, B and C). Notably, these increased levels were significantly attenuated by SCE pretreatment. Similarly, gene expression levels of these cytokines in colon tissues were significantly increased by DSS treatment. However, those increased levels were attenuated by SCE pretreatment (Fig. 4D, E and F). These preventive effects of SCE were similar to those of CA. These results demonstrated protective effects of SCE on the production of proinflammatory cytokines in DSS-induced colitis.
Fig. 4.
SCE inhibits production of pro-inflammatory cytokines in colitis-induced mice. (A) TNFα, (B) IL-1β, and (C) IL-6 serum protein levels were determined by ELISA. qRT-PCR analysis was per-formed for mRNA expression levels of TNFα (D), IL-1β (E), and IL-6 (F) in colon tissues. All analyses were conducted thrice using 3–5 samples. *p < 0.05 and ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. DSS alone-treated group. Cont control; SCE sesame cake extract; DSS dextran sulfate sodium; CA chlorogenic acid
SCE inhibits apoptosis of colonic cells in DSS-induced colitis
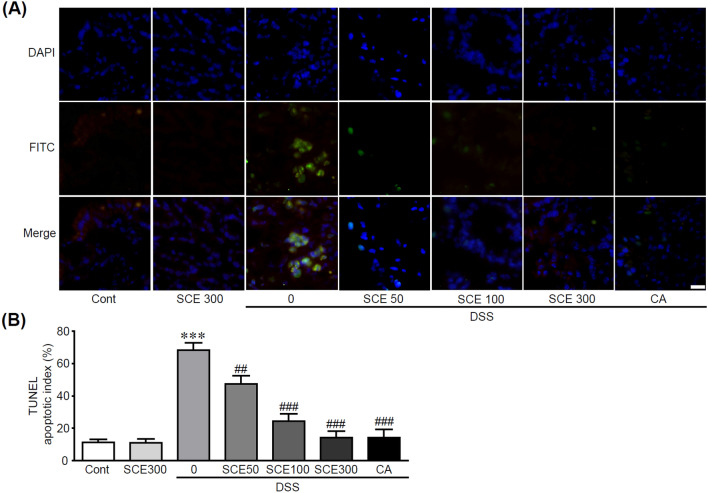
Since previous studies have suggested that apoptotic cell death in intestinal cells contributes to the progression of IBD (Liu et al., 2022), we further investigated whether SCE could inhibit apoptotic cell death in colonic cells when colitis was induced by DSS. For this purpose, TUNEL staining was performed to detect apoptotic cells using colon tissues from SCE with or without DSS-treated mice. Apoptotic cells detected by green fluorescence were dramatically increased in colon tissues from DSS alone-treated mice. On the other hand, these apoptotic cells gradually decreased as the concentration of SCE became higher (Fig. 5A). Moreover, apoptotic index calculated by percentage of the number of apoptotic cells over total number of cells was shown to be 68% for DSS alone-treated group. In the SCE group, the percentage of apoptotic cells was 47% at 50 kg/mg/day, 24% at 100 kg/mg/day, and 14.2% at 300 kg/mg/day. In the CA and DSS cotreated group, the percentage was 14% (Fig. 5B), indicating that SCE could effectively inhibit apoptotic cell death induced by DSS treatment. In patients with IBD, an increase in apoptosis-induced colon cells has been observed (Ramachandran et al., 2000). This apoptotic process could cause a breakdown of balance between cellular proliferation and cell death for maintaining the intestinal barrier (Edelblum et al., 2006). Subsequently, it can result in a barrier defect, which can further cause microbial invasion and inflammation. Therefore, inhibiting apoptotic cell death in colon cells undergoing colitis has an important role for maintaining the function of colon. Here, we demonstrated that SCE could effectively ameliorate apoptotic responses in colon cells with colitis induced by DSS.
Fig. 5.
SCE attenuates apoptotic cell death in colitis-induced colon tissues. (A) TUNEL staining was performed using colon tissues from colitis-induced mice with or without SCE treatment (n = 10). Cells showing green fluorescence were apoptotic cells. (B) Percentage of apoptotic cells versus total cells is shown as an apoptotic index. ***p < 0.001 vs. control group. ##p < 0.01 and ###p < 0.001 vs. DSS alone-treated group. Cont control; SCE sesame cake extract; DSS dextran sulfate sodium; CA chlorogenic acid. Scale bar, 50 µm
SCE inhibits oxidative stress and activates Nrf2 signaling pathway in DSS-induced colitis
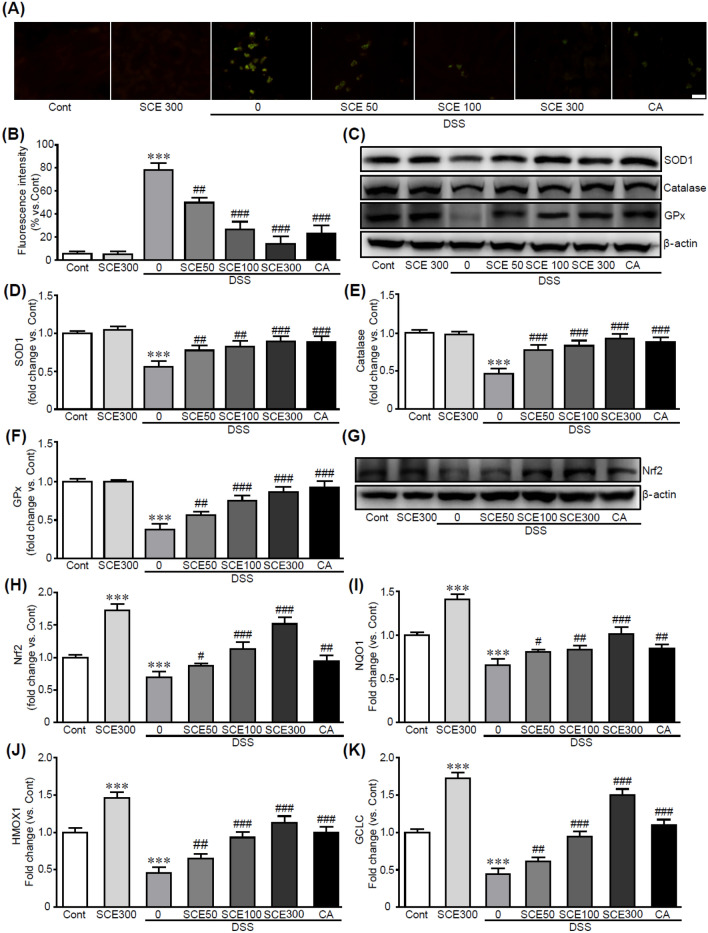
Antioxidant therapy targeting oxidative stress can be a good strategy for treating colitis. Thus, we further investigated the anti-oxidative effect of SCE on DSS-induced colitis to explore the molecular mechanism involved in the inhibitory effect of SCE on the DSS-induced colitis. Immunostaining using DCFH-DA dye as an indicator of ROS was performed for colon tissue treated with SCE with/without DSS. As shown in Fig. 6A, cells with green fluorescence in which ROS production was observed were significantly increased in DSS alone-treated colon tissues than in the control group. However, DCFH-DA-positive cells were markedly decreased when SCE was administrated followed by DSS treatment, indicating that SCE inhibited ROS production induced by DSS (Fig. 6B). Moreover, western blot analysis using antioxidants including superoxide dismutase 1, catalase, and glutathione peroxidase demonstrated that expression levels of these proteins were significantly increased by SCE administration, whereas DSS treatment decreased their expression compared to the control (Fig. 6C, D, E and F). CA treatment also inhibited these oxidative stress responses.
Fig. 6.
SCE attenuates oxidative stress in colitis-induced colon tissues. Colon tissues from co-litis-induced mice with or without SCE treatment were stained with DCFH-DA for detecting ROS overproduced cells with green fluorescence (A). (B) Measured fluorescence intensity. (C, D, E, and F) Protein expression levels of antioxidants were analyzed by western blotting (n = 5). (G and H) Western blot analysis and band density for Nrf2 protein. (I, J, and K) The mRNA expression of Nrf2 target genes including Nqo1, Hmox1, and Gclc in colon tissues was performed by qRT-PCR analysis in triplicate. ***p < 0.001 vs. control group. ##p < 0.01 and ###p < 0.001 vs. DSS alone-treated group. Cont control; SCE sesame cake extract; DSS dextran sulfate sodium; CA chlorogenic acid. Scale bar, 50 µm
Nrf2 has been shown to mediate various cell biological outcomes through suppression of oxidation and cellular stress (Chang et al., 2021; Mitsuishi et al., 2012). Previous studies have proven that Nrf2 deficiency exacerbate colonic injury in a colitis mouse model (Khor et al., 2008; Osburn et al., 2007), whereas activation of Nrf2 protect colon tissue against colitis (Wang et al., 2016). These protective actions of Nrf2 are mediated by upregulation of antioxidative enzymes and inhibition of inflammation and apoptotic responses (Kim et al., 2010; Xu et al., 2017). Since Nrf2 has been proven to protect the colon tissue from colitis (Liu et al., 2021), we focused on the effects of SCE on Nrf2 signaling pathway. Toward this, we determined the expression level of Nrf2 protein. The western blot analysis showed that Nrf2 protein were dramatically preserved by pretreatment of SCE, otherwise, it was significantly decreased in colitis-induced colon tissues by DSS (Fig. 6G and H). In addition, Nrf2 target genes with antioxidant properties, including Nqo1, Hmox1, and Gclc, were also increased when SCE was pretreated in colitis-induced colon tissues (Fig. 6I, J, and K). Similar to the effects of SCE, CA treatment activated the Nrf2-related signaling pathway. Therefore, SCE has potential effects against oxidative stress induced by DSS treatment by preserving the expression of antioxidants mediated by activation of Nrf2-related signaling pathway.
Identification of sesamin and sesamolin as active components in SCE by HPLC analysis
Accumulating evidence has shown that SCE contains numerous bioactive compounds. Among these bioactive compounds, lignans are polyphenolic substances produced by plant cells (Durazzo et al., 2018). They possess diverse biological or pharmacological activities, including anti-inflammatory, antitumor, anti-cardiovascular, and anti-neuronal activities (Adlercreutz, 2007; Hu et al., 2021; Rodriguez-Garcia et al., 2019). Since SCE has been shown to exert biological activities including anti-oxidation and anti-inflammation effects through its bioactive lignans including sesamin and sesamolin (Mekky et al., 2019), these lignans in SCE were analyzed and quantified using commercially available lignans as standards. HPLC analysis showed that extracts contained 2.54 mg/g of sesamin and 1.76 mg/g of sesamolin (Fig. S1 and Table S5). Thus, contents of both lignans in SCE could be used for standardization, further suggesting that their beneficial effects against DSS-induced colitis are possibly mediated by sesamin and sesamolin, at least in part.
Natural compounds and their derivatives have been spotlighted as alternative substitutes for treating IBD due to their safeness and effectivity (Duan et al., 2021). Indeed, many natural compounds that are effective in IBD treatment have been identified (Duan et al., 2021). Various natural products derived from plants, such as catechins, anthocyanins, and terpenes, exhibit anti-inflammatory effects, and have been successfully employed to treat IBD (Gupta et al., 2022).
In this study, we demonstrated that SCE can ameliorated symptoms of colitis induced by DSS by inhibiting pathological alterations and inflammatory responses in colon tissues of mice. Furthermore, the preventive effect of SCE is medicated by suppressing oxidative stress through activation of Nrf2-related signaling pathway. Therefore, SCE might be utilized to establish a preventive or therapeutic strategy for treating colitis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
D.K.Y. was funded by the Ministry of Science and Technology, Republic of Korea (NRF- 2021R1I1A1A01054531). K.W.P. was funded by the Ministry of Science and Technology, Republic of Korea (NRF-2020R1A2B5B02001592).
Declarations
Conflict of interest
J.Y.P. has ownership interest in Queensbucket company. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kye Won Park, Email: kwpark@skku.edu.
Dong Kwon Yang, Email: dkyang0502@jbnu.ac.kr.
References
- Adlercreutz H. Lignans and human health. Critical Reviews in Clinical Laboratory Sciences. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- Bankole E, Read E, Curtis MA, Neves JF, Garnett JA. The relationship between mucins and ulcerative colitis: A Systematic Review. Journal of Clinical Medicine. 2021;10:1935. doi: 10.3390/jcm10091935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Othman S, Katsuno N, Kanamaru Y, Yabe T. Water-soluble extracts from defatted sesame seed flour show antioxidant activity in vitro. Food Chemistry. 2015;175:306–314. doi: 10.1016/j.foodchem.2014.11.155. [DOI] [PubMed] [Google Scholar]
- Bourgonje AR, Feelisch M, Faber KN, Pasch A, Dijkstra G, van Goor H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends in Molecular Medicine. 2020;26:1034–1046. doi: 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- Chang SH, Jang J, Oh S, Yoon JH, Jo DG, Yun UJ, Park KW. Nrf2 induces Ucp1 expression in adipocytes in response to beta3-AR stimulation and enhances oxygen consumption in high-fat diet-fed obese mice. BMB Reports. 2021;54:419–424. doi: 10.5483/BMBRep.2021.54.8.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice.Vol. 104 In:Current protocols in immunology. John Wiley & Sons, Inc., Danvers, MA, USA pp. 15.25.1–15.25.14. (2014) [DOI] [PMC free article] [PubMed]
- Chen PR, Chien KL, Su TC, Chang CJ, Liu TL, Cheng HC, Tsai CM. Dietary sesame reduces serum cholesterol and enhances antioxidant capacity in hypercholesterolemia. Nutrition Research. 2005;25:559–567. doi: 10.1016/j.nutres.2005.05.007. [DOI] [Google Scholar]
- Chung BH, Lee JJ, Kim JD, Jeong D, Lee H, Choe J, Ha KS, Kwon YG, Kim YM. Angiogenic activity of sesamin through the activation of multiple signal pathways. Biochemical and Biophysical Research Communications. 2010;391:254–260. doi: 10.1016/j.bbrc.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Dalibalta S, Majdalawieh AF, Manjikian H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharmaceutical Journal. 2020;28:1276–1289. doi: 10.1016/j.jsps.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Cheng S, Li L, Liu Y, Wang D, Liu G. Natural anti-inflammatory compounds as drug candidates for Inflammatory bowel disease. Frontiers in Pharmacology. 2021;12:684486. doi: 10.3389/fphar.2021.684486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A, Lucarini M, Camilli E, Marconi S, Gabrielli P, Lisciani S, Gambelli L, Aguzzi A, Novellino E, Santini A, Turrini A, Marletta L. Dietary lignans: definition, description and research trends in databases development. Molecules. 2018;23:2251. doi: 10.3390/molecules23123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflammatory Bowel Diseases. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- Elleuch M, Besbes S, Roiseux O, Blecker C, Attia H. Quality characteristics of sesame seeds and by-products. Food Chemistry. 2007;103:641–650. doi: 10.1016/j.foodchem.2006.09.008. [DOI] [Google Scholar]
- Eom SJ, Zu HD, Lee J, Kang MC, Park J, Song KM, Lee NH. Development of an ultrasonic system for industrial extraction of unheated sesame oil cake. Food Chemistry. 2021;354:129582. doi: 10.1016/j.foodchem.2021.129582. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh Kenari R, Mohsenzadeh F, Amiri ZR. Antioxidant activity and total phenolic compounds of Dezful sesame cake extracts obtained by classical and ultrasound-assisted extraction methods. Food Science & Nutrition. 2014;2:426–435. doi: 10.1002/fsn3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Wang C, Yu L, Sheng T, Wu Z, Lin Y, Gong Y. Chlorogenic acid attenuates dextran dodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. BioMed Research International. 2019;2019:679789. doi: 10.1155/2019/6769789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths AM. Inflammatory bowel disease. Nutrition. 1998;14:788–791. doi: 10.1016/S0899-9007(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Gupta M, Mishra V, Gulati M, Kapoor B, Kaur A, Gupta R, Tambuwala MM. Natural compounds as safe therapeutic options for ulcerative colitis. Inflammopharmacology. 2022;30:397–434. doi: 10.1007/s10787-022-00931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E, Parthasarathy S. Anti-inflammatory and antioxidant effects of sesame oil on atherosclerosis: A descriptive literature review. Cureus. 2017;9:e1438. doi: 10.7759/cureus.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li Y, Sampson L, Wang M, Manson JE, Rimm E, Sun Q. Lignan intake and risk of coronary heart disease. Journal of the American College of Cardiology. 2021;78:666–678. doi: 10.1016/j.jacc.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes & Development. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SJ, Jung ES, Ha KC, Baek HI, Park YK, Han SK, Chae SW, Lee SO, Chung YC. Efficacy and safety of sesame oil cake extract on memory function improvement: A 12-Week, randomized, double-blind, placebo-controlled pilot study. Nutrients. 2021;13:2606. doi: 10.3390/nu13082606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annual Review of Immunology. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, Kong AN. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prevention Research. 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation Research. 2010;1–2:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Liu H, Johnston LJ, Wang F, Ma X. Triggers for the Nrf2/ARE signaling pathway and its nutritional regulation: Potential therapeutic applications of ulcerative colitis. International Journal of Molecular Sciences. 2021;22:11411. doi: 10.3390/ijms222111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zeng Y, Wen Y, Huang X, Liu Y. Natural products modulate cell apoptosis: A promising way for the treatment of ulcerative colitis. Frontiers in Pharmacology. 2022;13:806148. doi: 10.3389/fphar.2022.806148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekky RH, Abdel-Sattar E, Segura-Carretero A, Contreras MDM. Phenolic compounds from sesame cake and antioxidant activity: A new insight for agri-food residues significance for sustainable development. Foods. 2019;8:432. doi: 10.3390/foods8100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi Y, Motohashi H, Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Frontiers in Oncology. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JB, Wang ML, Tonnis BD. Variability for oil, protein, lignan, tocopherol, and fatty acid concentrations in eight sesame (Sesamum indicum L.) genotypes. Industrial Crops and Products. 2021;164:113355. doi: 10.1016/j.indcrop.2021.113355. [DOI] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nature Reviews Immunology. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, Kensler TW. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. International Journal of Cancer. 2007;121:1883–1891. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. Journal of Gastroenterology and Hepatology. 2000;15:109–120. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia C, Sanchez-Quesada C, Toledo E, Delgado-Rodriguez M, Gaforio JJ. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules. 2019;24:917. doi: 10.3390/molecules24050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World Journal of Gastroenterology. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturniolo GC, Mestriner C, Lecis PE, D'Odorico A, Venturi C, Irato P, Cecchetto A, Tropea A, Longo G, D'Inca R. Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scandinavian Journal of Gastroenterology. 1998;33:644–649. doi: 10.1080/00365529850171936. [DOI] [PubMed] [Google Scholar]
- Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Medicine and Cellular Longevity. 2017;2017:4535194. doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um MY, Choi WH, Ahn JY, Kim S, Kim MK, Ha TY. Sesaminol glucosides improve cognitive deficits and oxidative stress in SAMP8 mice. Food Science and Biotechnology. 2009;18:1311–1315. [Google Scholar]
- Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. Journal of Gastroenterology. 2018;53:585–590. doi: 10.1007/s00535-018-1449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F, Cai XY, Wang MY, Chen L, Zhong RQ, Liu L, Yi B, Hou FJ, Zhang HF. Chlorogenic acid supplementation alleviates dextran sulfate sodium (DSS)-induced colitis via inhibiting inflammatory responses and oxidative stress, improving gut barrier integrity and Nrf-2/HO-1 pathway. Journal of Functional Foods. 2021;87:104808. doi: 10.1016/j.jff.2021.104808. [DOI] [Google Scholar]
- Wang Y, Wang H, Qian C, Tang J, Zhou W, Liu X, You Q, Hu R. 3-(2-Oxo-2-phenylethylidene)-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one (compound 1), a novel potent Nrf2/ARE inducer, protects against DSS-induced colitis via inhibiting NLRP3 inflammasome. Biochemical Pharmacology. 2016;101:71–86. doi: 10.1016/j.bcp.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Wang JS, Tsai PH, Tseng KF, Chen FY, Yang WC, Shen MY. Sesamol ameliorates renal injury-mediated atherosclerosis via inhibition of oxidative stress/IKKalpha/p53. Antioxidants. 2021;10:1519. doi: 10.3390/antiox10101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Chen L, Chen X, Wen Y, Yu C, Yao J, Wu H, Wang X, Xia Q, Kong X. The triterpenoid CDDO-imidazolide ameliorates mouse liver ischemia-reperfusion injury through activating the Nrf2/HO-1 pathway enhanced autophagy. Cell Death & Disease. 2017;8:e2983. doi: 10.1038/cddis.2017.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guan L, Wang X, Wen T, Xing J, Zhao J. Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radical Research. 2008;42:362–371. doi: 10.1080/10715760801993076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.