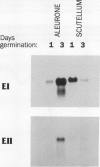
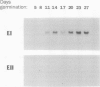
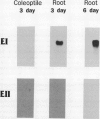
Abstract
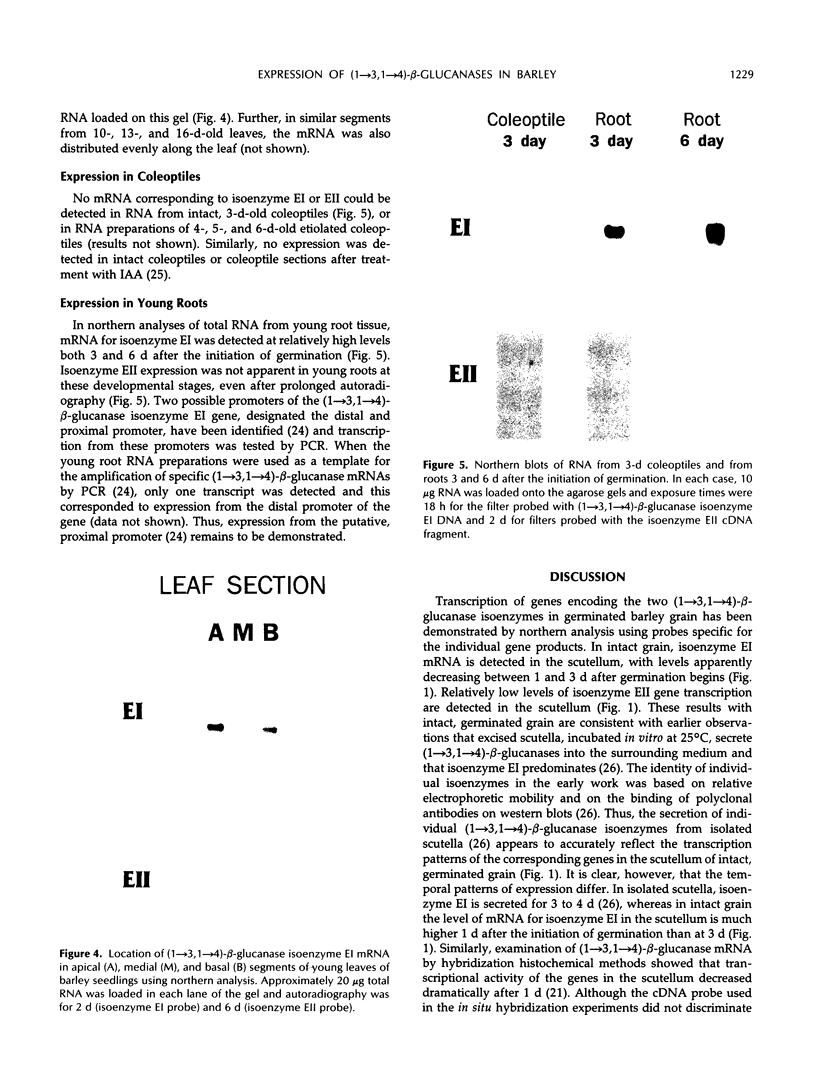
Two genes encode (1→3,1→4)-β-d-glucan 4-glucanohydrolase (EC 3.2.1.73) isoenzymes EI and EII in barley (Hordeum vulgare L.). Specific DNA probes have been used in Northern analyses to examine the developmental regulation of individual (1→3,1→4)-β-glucanase genes in the aleurone and scutellum of germinated grain and in young leaves and young roots. In aleurone and scutella excised from germinated grain, mRNAs encoding both isoenzymes are present but developmental patterns differ between the two tissues. Thus, levels of both isoenzyme EI and EII mRNA increase significantly in the aleurone between 1 and 3 days after the initiation of germination. In the scutellum, isoenzyme EI mRNA predominates and decreases as germination proceeds. Isoenzyme EI mRNA appears in young leaves approximately 8 days after the initiation of germination and levels rise until about 20 days. Enzyme activity in leaf extracts parallels the development of isoenzyme EI mRNA. No isoenzyme EII mRNA is detected in the leaves in this period. Analysis of RNA from different leaf segments indicates that the isoenzyme EI mRNA is distributed relatively evenly along the length of the leaf. In young roots, mRNA encoding (1→3,1→4)-β-glucanase isoenzyme EI is detected at high levels 3 to 6 days after the initiation of germination; again, little or no isoenzyme EII mRNA is found. Overall, transcription of the (1→3,1→4)-β-glucanase isoenzyme EII gene appears to be restricted to the germinating grain, whereas isoenzyme EI is expressed in a wider range of tissues during seedling development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. W., Feix G. A functional splice site in the 5' untranslated region of a zein gene. Nucleic Acids Res. 1990 Jan 11;18(1):111–117. doi: 10.1093/nar/18.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fincher G. B., Lock P. A., Morgan M. M., Lingelbach K., Wettenhall R. E., Mercer J. F., Brandt A., Thomsen K. K. Primary structure of the (1-->3,1-->4)-beta-D-glucan 4-glucohydrolase from barley aleurone. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2081–2085. doi: 10.1073/pnas.83.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield R. D., Nevins D. J. Hydrolytic Activity and Substrate Specificity of an Endoglucanase from Zea mays Seedling Cell Walls. Plant Physiol. 1987 Jan;83(1):203–207. doi: 10.1104/pp.83.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M., Hong Y. N., Schopfer P. Acid- and Enzyme-Mediated Solubilization of Cell-Wall beta-1.3,beta-1.4-d-Glucan in Maize Coleoptiles : Implications for Auxin-Mediated Growth. Plant Physiol. 1991 Apr;95(4):1012–1018. doi: 10.1104/pp.95.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoson T., Nevins D. J. beta-d-Glucan Antibodies Inhibit Auxin-Induced Cell Elongation and Changes in the Cell Wall of Zea Coleoptile Segments. Plant Physiol. 1989 Aug;90(4):1353–1358. doi: 10.1104/pp.90.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouhe M., Nevins D. J. Inhibition of auxin-induced cell elongation of maize coleoptiles by antibodies specific for cell wall glucanases. Plant Physiol. 1991 Jun;96(2):426–431. doi: 10.1104/pp.96.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Modulation of gene expression by auxin. Bioessays. 1989 Aug-Sep;11(2-3):52–58. doi: 10.1002/bies.950110204. [DOI] [PubMed] [Google Scholar]
- Litts J. C., Simmons C. R., Karrer E. E., Huang N., Rodriguez R. L. The isolation and characterization of a barley 1,3-1,4-beta-glucanase gene. Eur J Biochem. 1990 Dec 27;194(3):831–838. doi: 10.1111/j.1432-1033.1990.tb19476.x. [DOI] [PubMed] [Google Scholar]
- Loi L., Ahluwalia B., Fincher G. B. Chromosomal Location of Genes Encoding Barley (1-->3, 1-->4)-beta-Glucan 4-Glucanohydrolases. Plant Physiol. 1988 Jun;87(2):300–302. doi: 10.1104/pp.87.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttenegger D. G., Nevins D. J. Transient Nature of a (1 --> 3), (1 --> 4)-beta-d-Glucan in Zea mays Coleoptile Cell Walls. Plant Physiol. 1985 Jan;77(1):175–178. doi: 10.1104/pp.77.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slakeski N., Baulcombe D. C., Devos K. M., Ahluwalia B., Doan D. N., Fincher G. B. Structure and tissue-specific regulation of genes encoding barley (1----3, 1----4)-beta-glucan endohydrolases. Mol Gen Genet. 1990 Dec;224(3):437–449. doi: 10.1007/BF00262439. [DOI] [PubMed] [Google Scholar]
- Stuart I. M., Loi L., Fincher G. B. Development of (1-->3,1-->4)-beta-d-Glucan Endohydrolase Isoenzymes in Isolated Scutella and Aleurone Layers of Barley (Hordeum vulgare). Plant Physiol. 1986 Feb;80(2):310–314. doi: 10.1104/pp.80.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N. Complete Nucleotide Sequence of a Hordeum vulgare Gene Encoding (1-->3, 1-->4)-beta-Glucanase Isoenzyme II. Plant Physiol. 1991 Aug;96(4):1382–1384. doi: 10.1104/pp.96.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Fincher G. B. Purification and chemical properties of two 1,3;1,4-beta-glucan endohydrolases from germinating barley. Eur J Biochem. 1982 Jan;121(3):663–669. doi: 10.1111/j.1432-1033.1982.tb05837.x. [DOI] [PubMed] [Google Scholar]