Abstract
The binary actin–ADP-ribosylating Clostridium botulinum C2 toxin consists of the enzyme component C2I and the binding component C2II, which are separate proteins. The active component C2I enters cells through C2II by receptor-mediated endocytosis and membrane translocation. The N-terminal part of C2I (C2IN), which consists of 225 amino acid residues but lacks ADP-ribosyltransferase activity, was identified as the C2II contact site. A fusion protein (C2IN-C3) of C2IN and the full-length C3-like ADP-ribosyltransferase from Clostridium limosum was constructed. The fusion protein C2IN-C3 ADP-ribosylated Rho but not actin in CHO cell lysates. Together with C2II, C2IN-C3 induced complete rounding up of CHO and HeLa cells after incubation for 3 h. No cell rounding was observed without C2II or with the original C3-like transferase from C. limosum. The data indicate that the N-terminal 225 amino acid residues of C2I are sufficient to cause the cellular uptake of C. limosum transferase via the binding component of C2II, thereby increasing the cytotoxicity of the C3-like exoenzyme several hundred-fold.
Various bacterial exotoxins attack eukaryotic cells by covalent modification of intracellular targets. It is believed that at least four steps are essential for the action of these protein toxins (19). At first, toxins bind through a binding domain or a specific binding component to a surface receptor of the target cells. This is followed by receptor-mediated endocytosis. After refolding, the protein toxins translocate into the cytosol, where the enzyme component modifies a specific target, causing functional alterations of the target cell. Recent structure-function analysis of diphtheria toxin, which can be taken as a prototype for protein toxins, revealed three functional domains responsible for receptor binding, membrane translocation, and enzyme activity (8). In most toxins, these functional domains are located on a single toxin chain (e.g., Pseudomonas exotoxin A [37]), are positioned on different chains which are linked by disulfide bonds (e.g., diphtheria toxins and botulinum neurotoxins) (8, 9, 20), or are located on specific components which are noncovalently associated (e.g., cholera toxin and pertussis toxin (13, 18). In contrast, the enzyme and the binding/translocation components of binary bacterial protein toxins such as Clostridium botulinum C2 toxin (4, 10) or anthrax toxin (17) are separate proteins that meet at the surface of the target cell.
The binary actin–ADP-ribosylating C2 toxin consists of components C2I (Mr, 49,394) and C2II (22). The binding component C2II is an ∼100-kDa protein (after trypsin activation, ∼80 kDa [21]) which assembles with an unknown receptor of the target cell, thereby inducing a binding site for the enzymatic component C2I (23). After internalization and translocation into the cytosol, C2I ADP-ribosylates monomeric G-actin at Arg-177 (1, 29, 34), thereby inhibiting actin polymerization (1, 36).
C2 toxin is related to C. perfringens iota toxin, another member of the family of binary actin–ADP-ribosylating toxins (4, 26, 30, 31). Studies on NAD photoaffinity labeling (33) and site-directed mutagenesis of iota toxin (25) showed that the catalytic site of the enzyme component of iota toxin is located in its C-terminal part. Recent studies in our laboratory identified a similar location of the catalytic site of C2 toxin at the C-terminal part of the enzyme component C2I (26a). From these data, we proposed that the 225-amino-acid N-terminal part of C2I (C2IN) is responsible for contact of C2I with a docking site on C2II which is formed after C2II binding to the target cell receptor. Here we studied the interaction of C2IN with its binding component C2II. Moreover, we used this toxin fragment to construct C2IN-C3, a chimeric protein of C2IN with C. limosum transferase.
The C. limosum C3-like exoenzyme (Mr, ∼23,000) inactivates the small GTP-binding protein Rho by ADP-ribosylation at Asn-41 (3, 14, 28). During last years, C3-like toxins have been valuable tools for the elucidation of the function of Rho GTPases. However, the use of C3 and related C3-like transferases is hampered by the fact that these enzymes do not enter cells readily. Here we report on a fusion toxin consisting of the N-terminal part of C2IN and the C3-like transferase from C. limosum that enters the cells via the binding component C2II of C. botulinum, thereby increasing the sensitivity of target cells for C3-like transferase at least several hundred-fold.
MATERIALS AND METHODS
Materials.
Cell culture medium was obtained from Biochrom (Berlin, Germany), fetal calf serum was obtained from PAN Systems (Aidenbach, Germany), and cell culture materials were obtained from Falcon (Heidelberg, Germany). C. botulinum C2 toxin was purified and activated with trypsin as described previously (21, 22). C3-like exoenzyme from C. limosum was purified as described previously (14). Antibodies against C2I and C3 were raised in rabbits against the respective whole proteins. Donkey anti-rabbit antibody coupled to peroxidase and the enhanced chemiluminescence detection kit were purchased from Amersham (Braunschweig, Germany). The nitrocellulose blotting membrane was from Schleicher & Schuell (Dassel, Germany). Low-molecular-weight protein marker was obtained from Bio-Rad (Hercules, Calif.). Oligonucleotides were obtained from BIG (Denzlingen, Germany), the pGEX2T vector (included in the glutathione S-transferase [GST] gene fusion system) and glutathione-Sepharose 4B were from Pharmacia Biotech (Uppsala, Sweden), DNA molecular weight marker (lambda HindIII) and restriction enzymes were from Boehringer Mannheim (Mannheim, Germany), and T4 ligase and competent Escherichia coli cells were from Stratagene (Heidelberg, Germany). PCR was performed with the Gene Amp PCR System 2400 from Perkin-Elmer (Langen, Germany), and DNA sequencing was done with a Cycle Sequencing Ready Reaction kit (ABI PRISM) from Perkin-Elmer. Thrombin and phalloidin-rhodamine were from Sigma (Deisenhofen, Germany). [32P]NAD (30 Ci/mmol) was from DuPont NEN (Bad Homburg, Germany).
Construction of C2IN and C2IN-C3.
The C2I gene (1,293 bp) from C. botulinum KZZ 1577(92-13) (a gift from S. Nakamura, Kanazawa, Japan) was amplified by PCR with 300 ng of chromosomal DNA in a total volume of 100 μl with 2 U of Taq DNA polymerase in a reaction mixture (10 mM Tris, 1.5 mM MgCl2, 50 mM KCl [pH 8.3]) including deoxynucleoside triphosphates (200 μM each) and 15 pmol of the primers C2IC (5′-AGATCTATGCCAATAATAAAAGAACCC-3′), containing a BglII site, and C2IN (5′-GGATCCCTAAATCTCTTTATTTTGTATAAC-3′), containing a BamHI site. Amplification was done by 30 cycles of denaturing at 94°C for 10 s, primer annealing at 50°C for 30 s, and extension at 68°C for 2 min. The resulting PCR product (1 μl) was cloned into pCR2.1 vector (Invitrogen, NV Leek, The Netherlands) according to the manufacturer’s instructions (Fig. 1A). For expression experiments, the C2I gene was excised with BglII/BsaBI and cloned into BamHI/SmaI-digested pGEX2T, resulting in plasmid pGEX2T-C2I. pGEX2T-C2IN was constructed by BamHI digestion of pGEX2T-C2I. This was possible because C2I from strain KZZ 1577(92-13) contains a BamHI site at position 673 (first position in the recognition sequence; the complete sequence of C. botulinum C2I from strain KZZ 1577 has been submitted to the EMBL database) which is not present in the published sequence (12). Religation of the ∼6,000-bp pGEX2T-C2IN fragment followed, and the vector was transformed into competent E. coli cells (Fig. 1B). For construction of pGEX-C2IN-C3, the C. limosum C3 gene (7) was amplified by PCR using primers 5′-C3oS (5′-GTAGATCTCCTTATGCGGATTCTTTTAAGG-3′) and 3′C3oS (5′-TGTCGTAATAATTTTTCTATTCCTAGGAC-3′), which contain additional BglII and BamHI sites, respectively, and cloned into pCR2.1 vector. C3 was excised from this vector by restriction with BglII and BamHI, ligated with BamHI-digested pGEX-C2IN, and transformed into competent E. coli cells (Fig. 1C and D). C2IN and C2IN-C3 were sequenced by using the sequencing primers 5′ pGEX2T-58 and 3′ pGEX2T-43. For sequencing of the C2IN-C3 boundary, the primer C2IN-C3′ (5′-GCTATTATAACTACTATAAAGGG-3′) was used. The cycle sequencing reaction was performed according to the manufacturer’s instructions.
FIG. 1.
Construction of plasmid pGEX-C2IN. The C2I gene (1,293 bp) from C. botulinum KZZ 1577 was amplified from chromosomal DNA by PCR using primers C2IC, containing a BglII site, and C2IN, containing a BamHI site, and cloned into pCR2.1 vector (A). For expression experiments the C2I gene was excised with BglII/BsaBI and cloned into BamHI/SmaI-digested pGEX2T, resulting in plasmid pGEX2T-C2I (B). pGEX2T-C2IN was constructed by BamHI digestion of pGEX2T-C2I and religation of the pGEX2T-C2IN fragment. The construct was identified by DNA sequencing. To construct the fusion toxin C2IN-C3 (D), the C. limosum C3 gene was excised from the pCR2.1 vector harboring C3 (C) by restriction with BglII and BamHI and ligated with BamHI-digested pGEX-C2IN. The construct was confirmed by DNA sequencing.
Expression and purification of recombinant proteins.
Recombinant GST fusion proteins were produced in E. coli transformed with the respective DNA fragment in the pGEX2T vector and purified according to the manufacturer’s instructions. In brief, E. coli harboring plasmid pGEX2T-C2I, pGEX2T-C2IN, or pGEX2T-C2IN-C3 was grown at 37°C in Luria-Bertani (LB) medium containing ampicillin (100 μg/ml) to an optical density at 600 nm of 0.8, and isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM. The cultures were incubated at 29°C for an additional 20 h, and the cells were sedimented for 10 min at 4°C at 7,700 × g, resuspended in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, and disrupted by sonication. Cellular debris was sedimented for 10 min at 4°C at 12,000 × g, and the resulting supernatant was incubated for 30 min at room temperature with a 50% slurry of glutathione-Sepharose 4B in PBS (2 ml/100 ml). The suspension was centrifuged at 500 × g for 5 min; the pellet was washed five times with 10 bed volumes of PBS and finally incubated with thrombin (3.25 NIH units/ml of bead suspension) to cleave the fusion proteins from GST. After thrombin cleavage, the suspension was centrifuged for 10 min at 500 × g, and the resulting supernatant was analyzed for the relevant protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis with antiserum against C2I or C3.
SDS-PAGE and Western blotting.
All buffers used for SDS-PAGE (11% gel) were formulated by the methods of Laemmli (16). For immunoblot analysis, the proteins were electroblotted from the gel onto a nitrocellulose membrane, using a semidry system. The membrane was blocked for 30 min with 5% nonfat dry milk in PBS containing 0.05% Tween 20 (PBS-T) followed by 1-h incubation with either anti-C2I antibody (rabbit, 1:2,000 in PBS-T) or anti-C3 antibody (rabbit, 1:10,000 in PBS-T). After washing with PBS-T, the blots were probed for 1 h with donkey anti-rabbit antibody coupled to horseradish peroxidase (1:2,000 in PBS-T) and washed, and proteins were detected with the Amersham enhanced chemiluminescence system as instructed by the manufacturer.
ADP-ribosylation assay.
The in vitro ADP-ribosylation assay for G-actin and for Rho was performed with rat brain lysate as described previously (1, 2, 15). For analysis of the ADP-ribosylation of cellular Rho after treatment of cells with fusion toxin, cells were scraped into cold PBS and sonicated, and 200 μg of protein was incubated with 18 ng of C. limosum C3 ADP-ribosyltransferase and [32P]NAD for 30 min at 37°C. The reaction was stopped by addition of Laemmli buffer, and labeled proteins were analyzed by SDS-PAGE and phosphorimaging.
Cytotoxicity assay with culture cells.
CHO-K1, the C2-resistant mutant cell line CHO-C2RK14 (11), and HeLa cells were cultivated in tissue culture flasks at 37°C and 95% CO2 in Ham’s F-12–Dulbecco’s minimal essential medium and Dulbecco’s modified Eagle medium, (1:1) respectively. Both media contained 5% heat-inactivated (30 min, 56°C) fetal calf serum, 2 mM l-glutamate, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Cells were routinely trypsinized and reseeded twice a week. For cytotoxicity assays, subconfluent monolayer cells (about 105 cells/cm2) in 24-well plates containing coverslips were treated for different times with 200 ng of activated C2II per ml, 100 ng of C2I per ml, and variable amounts of either C2IN or C2IN-C3. Cells growing on coverslips were washed with PBS and fixed in 4% paraformaldehyde (PFA) in PBS for 30 min. After washing in PBS, the coverslips were embedded in Kaiser’s gelatin on glass slights, and the rounded cells were counted. For actin staining, cells were fixed in 4% PFA in PBS containing 0.1% Triton X-100 for 30 min. Cells were washed and incubated for 30 min with phalloidin-rhodamine (600 ng/ml), and after extensive washing, the coverslips were embedded in gelatin and subjected to fluorescence microscopy. All experiments were performed at least three times.
RESULTS
Cloning, expression, and characterization of C2IN.
To analyze C2IN (amino acids 1 to 225) for ADP-ribosyltransferase activity and for its ability to bind to C2II, C2IN was expressed as a GST-C2IN fusion protein in E. coli. The C2IN protein was purified as described in Materials and Methods and analyzed by SDS-PAGE (Fig. 2A) and by Western blotting with anti-C2I antiserum (Fig. 2B). No ADP-ribosylation of actin was observed in the presence of C2IN, suggesting that the active site of the transferase is not located at the N-terminal part of C2I. Accordingly, C2IN did not induce any cytotoxic effect in the presence of C2II when applied to HeLa cells (not shown).
FIG. 2.
Analysis of C2I and C2IN proteins. C2I and C2IN proteins were expressed as GST fusion proteins in E. coli and cleaved with thrombin from glutathione-Sepharose beads. One microgram of each protein was subjected to SDS-PAGE and either stained with Coomassie blue (A) or detected by a Western blot analysis with an antiserum raised against C2I (B). Lane 1, C2I; lane 2, C2IN.
C2IN is responsible for binding to C2II.
Next, we tested whether C2IN interferes with the interaction of full-length C2I and C2II. To this end, CHO cells were incubated with trypsin-activated C2II (200 ng/ml) and C2I (100 ng/ml) in the presence of increasing concentrations (100, 300, 600, and 1,000 ng/ml) of C2IN at 37°C. After 3 h, cells were fixed on coverslips and the number of rounded cells per field was determined. Figure 3 shows that C2IN effectively competed for C2I-induced cell rounding, corroborating the view that the C2IN harbors the toxin interaction site.
FIG. 3.
Inhibition of the cytotoxic effects of C2I by C2IN. CHO cells were incubated with C2II (200 ng/ml) plus C2I (100 ng/ml) in the presence of increasing concentrations (100, 300, 600, and 1,000 ng/ml) of C2IN at 37°C. After 3 h, the number of rounded cells was determined.
Cloning and expression of C2IN-C3 fusion protein.
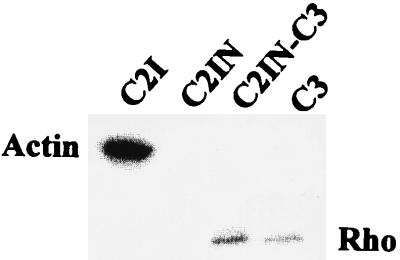
Based on the findings described above, we constructed the C2IN-C3 fusion protein. The fusion protein was expressed in E. coli and identified with anti-C2I antibody as well as with anti-C3 antibody as an ∼50-kDa protein (C2IN [∼25 kDa] plus C3 [∼23 kDa]). The antiserum against C2I showed no cross-reactivity with C. limosum transferase (Fig. 4A, lane 4) but recognized C2I (lane 1), C2IN (lane 2), and the C2IN-C3 fusion protein (lane 3). On the other hand, C3 (Fig. 4B, lane 4) and C2IN-C3 protein (lane 3) but not C2I or C2IN cross-reacted with anti-C3-antiserum.
FIG. 4.
Immunoblot analysis of wild-type C2I, C2IN, C2IN-C3, and C3 proteins. Proteins were expressed as GST fusion proteins in E. coli and cleaved with thrombin from glutathione-Sepharose beads. Proteins (200 ng of each) were subjected to SDS-PAGE and analyzed by Western blotting with anti-C2I antiserum (A) and anti-C3 antiserum (B). Lanes: 1, wild-type C2I; 2, C2IN; 3, C2IN-C3; 4, C3.
ADP-ribosylation of Rho by the C2IN-C3 fusion protein.
To compare C2I, C2IN, C2IN-C3, and C3 with respect to their substrate specificities in vitro, we performed an ADP-ribosylation assay with rat brain lysate. The autoradiography showed ADP-ribosylation of actin by C2I (Fig. 5, lane 1), no signal with C2IN (lane 2), and ADP-ribosylation of Rho by C3 toxin (lane 4). The C2IN-C3 fusion toxin ADP-ribosylated Rho but not actin, indicating the substrate specificity typical for C3 (Fig. 5, lane 3).
FIG. 5.
ADP-ribosylation of actin and Rho in rat brain lysate by C2I, C2IN, C2IN-C3, and C3. Rat brain lysates were ADP-ribosylated by C2I (50 ng), C2IN (50 ng), C2IN-C3 (50 ng), and C3 (15 ng) in the presence of [32P]NAD as described in Materials and Methods. Labeled proteins were analyzed by SDS-PAGE and phosphorimaging (shown).
Cytotoxic effects of C2IN-C3 on cultured cells.
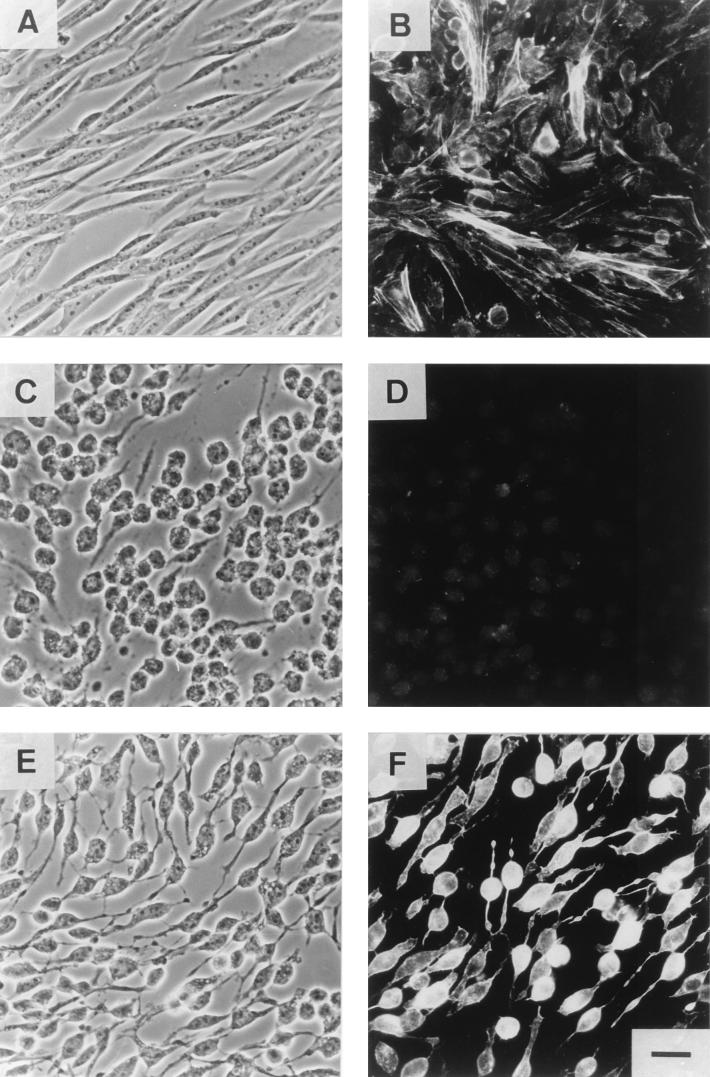
Next, we studied whether C2II was able to catalyze the transfer of the fusion protein C2IN-C3 into eukaryotic cells. For the cytotoxicity assay, subconfluent CHO monolayer cells were incubated with 200 ng of activated C2II per ml plus 100 ng of C2I (Fig. 6 C and D) or 200 ng of C2IN-C3 (Fig. 6 E and F) per ml. After 3 h of incubation, C2I/C2II and C2IN-C3/C2II-induced rounding up of cells and redistribution of the actin cytoskeleton were detected. Thus, destruction of the actin cytoskeleton was induced by ADP-ribosylation of G-actin in the case of C2I and by ADP-ribosylation of Rho in the case of C2IN-C3. Staining of F-actin with phalloidin-rhodamine showed the difference between the cytoskeleton destruction induced by C2 or by C2IN-C3 toxin. As a consequence of total disassembly of the actin filaments, only a very weak rhodamine fluorescence was observed after treatment of cells with the complete C2 toxin. In contrast, incubation of the cells with C2II and the fusion protein C2IN-C3 resulted in amorphous staining of residual F-actin characteristic for C3 toxin. The same cytotoxic effects of the various toxin combinations were detected in HeLa cells (data not shown). To test possible cytotoxic activity of the individual toxin proteins, cells were incubated with either 200 ng of C2II, 200 ng of C2IN-C3, or 1 μg of C. limosum C3 exoenzyme per ml. Incubation of the cells with the individual toxin components for 3 h did not cause any changes in cell morphology or in F-actin assembly of the cells (not shown). To test the specificity of the C2II-mediated uptake of the fusion toxin, CHO-C2RK14 cells, which most likely lack a functional C2II receptor, were used. No effect of C2II/C2IN-C3 treatment was observed when these cells were incubated for 3 h with the toxin. This finding corroborates the view that the delivery of C2IN-C3 fusion toxin into the cell occurred by a C2II receptor-mediated pathway.
FIG. 6.
Cytotoxic effects of the chimeric toxin C2IN-C3 on CHO cells. (A and B) Control cells; (C and D) cells treated with C2II (200 ng/ml) and C2I (100 ng/ml); (E and F) cells treated with C2II (200 ng/ml) and C2IN-C3 (200 ng/ml). After 3 h, the cells were washed and fixed for microscopy (A, C, and E) and for actin staining with phalloidin-rhodamine (B, D, and F). Bar = 25 μm.
To analyze the time dependence of the effects induced by the fusion toxin, the proteins were added to cells growing on coverslips, and every 30 min a sample was fixed with PFA. As shown in Fig. 7A, no morphological changes were observed when cells were treated with up to 30 μg of C. limosum C3 toxin per ml. In the presence of the chimeric toxin C2IN-C3/C2II (200 ng of each per ml), CHO cells started to round up after 60 min, and 120 min after addition of the toxin, more than 50% of the cells were round. HeLa cells were less sensitive, but more than 80% of cells were round after 3 h of incubation. To check C2IN-C3-catalyzed ADP-ribosylation of Rho in intact cells, the cells were lysed and Rho was ADP-ribosylated in lysates from treated cells in the presence of radiolabeled NAD and C3 toxin. As shown in Fig. 7B and C, a time-dependent decrease of radiolabeled Rho protein was observed. After 2.5 h of incubation with C2IN-C3/C2II, the cellular Rho was ADP-ribosylated by about 80%, and after 3 h (Fig. 7C), no radiolabeling was detected in the cell lysates, indicating the modification of Rho by C2IN-C3 in intact cells.
FIG. 7.
Time course of C2IN-C3/C2II action on cells. CHO and HeLa cells were treated either with C2IN-C3 (200 ng/ml) and C2II (200 ng/ml) or with C. limosum C3 toxin (30 μg/ml) and incubated at 37°C. Every 30 min, cells were fixed for determination of the number of rounded cells (A) and in parallel CHO cells were lysed for ADP-ribosylation of Rho (B and C). Lanes show [32P]ADP-ribosylation of Rho after incubation of CHO cells with C2II/C2IN-C3 for 0 (lane 1, B), 30 (lane 2, B), 60 (lane 3, B), 90 (lane 4, B), 120 (lane 5, B), 150 (lane 6, B), and 180 (lane 2, C) min and the 180-min control (lane 1, C).
DISCUSSION
We report on a C3-like Rho-ADP-ribosylating chimeric toxin that is delivered into eukaryotic cells by use of the binary C2 toxin from C. botulinum as a carrier system. C2 toxin is composed of two individual and separate protein subunits, C2I and C2II. Both components of this system are without overt toxicity individually but exhibit their potent cytotoxic effects when applied together. C2I possesses ADP-ribosyltransferase activity and modifies selectively G-actin, eventually leading to destruction of the cytoskeleton. C2II binds to the surface of the target cell, thereby inducing a binding site for C2I and subsequently mediating the internalization and membrane translocation of the enzyme component C2I.
An essential prerequisite for the construction of a fusion toxin on the basis of the binary C2 toxin was the identification of the C2I-C2II interaction site. Several findings indicated that the C-terminal part of C2I harbors the active site of the transferase. First we identified the conserved catalytic glutamic acid residue of C2I as Glu-388 (26a) in the C-terminal part of C2I. The C-terminal location of the catalytic domain of C2I is in agreement with the structure of the related C. perfringens iota toxin (26). In contrast to a recent study (12) that could not identify significant sequence similarity between C2I and iota toxin, application of the PC Gene program showed about 32% amino acid sequence identity of C2I with the enzyme component of iota toxin. In iota toxin, the active site has been identified by NAD photoaffinity labeling (33), by site-directed mutagenesis (25), and by sequence comparison with other transferases (33). Thus, we suggest that the N-terminal part of C2I is involved in the interaction with C2II. This hypothesis was corroborated by the finding that C2IN, which consists of the N-terminal 225 amino acid residues of C2I, competed effectively with full-length C2I for toxin uptake and intoxication of culture cells.
We constructed a fusion protein consisting of the N-terminal 225 amino acid residues of C. botulinum C2I and the full-length C. limosum ADP-ribosyltransferase C3. C3 is known to inactivate the small GTPase Rho by ADP-ribosylation at Asn-41. When the chimeric protein was applied together with C2II to monolayer HeLa or CHO wild-type cells, rounding up of cells and destruction of the actin cytoskeleton were induced. These effects are most likely the consequence of ADP-ribosylation of Rho by the chimeric toxin. Accordingly, no radiolabeling of Rho proteins was detected in the presence of [32P]NAD and C3 in the lysates of treated cells. Therefore, we conclude that the N-terminal 225 amino acid residues of the enzyme component of C2 toxin are sufficient for the interaction with C2II and subsequent delivery of this polypeptide into the cytosol.
Because C3 or C3-like toxins contain no binding or transport components, it is believed that these exoenzymes enter cells nonspecifically, e.g., via pinocytosis. Because of the poor cell accessibility of C3, the exoenzyme must be applied at high concentrations (usually >10 μg/ml) for cell culture experiments, and the incubation time is usually extended to 24 h. We observed morphological changes as early as 2 h after addition of the chimeric toxin C2IN-C3. A similar kinetic is observed with the native C2 toxin. Thus, it appears that the chimeric toxin has an uptake mechanism similar to that of C2 toxin. This inference is supported by the findings that the C2-resistant mutant cell line CHO-C2RK14, which is suggested to be defective in the C2II receptor, showed no morphological changes after treatment with the fusion toxin component C2IN-C3 and C2II. At present, however, we do not know the precise cellular entry mechanism of C2 toxin or of the chimeric toxin. It was reported that C2 toxin is taken up by receptor-mediated endocytosis (23, 29) and that both components (C2I and C2II) reach the same endosome compartment (23). Like other bacterial toxins (e.g., diphtheria toxin and anthrax toxin) that are transported into cells, the binding component C2II is capable of inducing formation of cation-selective channels (27). However, channel formation was studied only with artificial membranes, and the role of small membrane channels in the uptake of various bacterial toxins is still unclear. Moreover, recent studies with a diphtheria toxin-C3 fusion toxin showed that the uptake path of the chimeric toxin differed somehow from that of the native diphtheria toxin (6). Studies to check this possibility in the case of the C2-C3 chimeric fusion toxin are under way.
Various bacterial toxins are used as tools to transport toxic components or proteins of interest into eukaryotic cells. Well-known examples are the immunotoxins which often consist of a cell binding ligand coupled to toxins or their active components (24, 32, 35, 38). Diphtheria toxin and Pseudomonas exotoxin A are frequently used as the toxin parts. In these cases, the binding, translocation, and toxic components are part of one chimeric construct. Anthrax toxin was the first binary toxin (actually a tripartite protein toxin [17]) used as a cellular delivery system (5). Polypeptide fused to the N-terminal fragments of the lethal factor of anthrax toxin are effectively transported into the eukaryotic cells by means of the protective antigen that is the binding and transport component of anthrax toxin (17). The advantage of the usage of binary toxins is the nontoxicity of the individual components. Moreover, the fusion proteins of the toxin fragment and the polypeptide of interest are rather small, a fact that largely reduces technical problems with the expression of the chimeric proteins.
In summary, the contact site for binding of C2I to C2II is located in the N-terminal fragment of C2I. This fragment of the N-terminal 225 amino acid residues is sufficient to allow the transfer of polypeptides into the cytosol, indicating that the binary system of C. botulinum C2 toxin can be used as a protein carrier system to deliver fusion proteins into eukaryotic cells.
ACKNOWLEDGMENTS
The excellent technical assistance of Ulrike Müller and Brigitte Neufang is gratefully acknowledged.
This study was financially supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 388).
REFERENCES
- 1.Aktories K, Bärmann M, Ohishi I, Tsuyama S, Jakobs K H, Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- 2.Aktories K, Just I. In vitro ADP-ribosylation of Rho by bacterial ADP-ribosyltransferases. Methods Enzymol. 1995;256:184–195. doi: 10.1016/0076-6879(95)56023-8. [DOI] [PubMed] [Google Scholar]
- 3.Aktories K, Mohr C, Koch G. Clostridium botulinum C3 ADP-ribosyltransferase. Curr Top Microbiol Immunol. 1992;175:115–131. doi: 10.1007/978-3-642-76966-5_6. [DOI] [PubMed] [Google Scholar]
- 4.Aktories K, Wille M, Just I. Clostridial actin-ADP-ribosylating toxins. Curr Top Microbiol Immunol. 1992;175:97–113. doi: 10.1007/978-3-642-76966-5_5. [DOI] [PubMed] [Google Scholar]
- 5.Arora N, Leppla S H. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 6.Aullo P, Giry M, Olsnes S, Popoff M R, Kocks C, Boquet P. A chimeric toxin to study the role of the 21 kDa GTP binding protein rho in the control of actin microfilament assembly. EMBO J. 1993;12:921–931. doi: 10.1002/j.1460-2075.1993.tb05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böhmer J, Jung M, Sehr P, Fritz G, Popoff M, Just I, Aktories K. Active site mutation of the C3-like ADP-ribosyltransferase from Clostridium limosum—analysis of glutamic acid 174. Biochemistry. 1996;35:282–289. doi: 10.1021/bi951784+. [DOI] [PubMed] [Google Scholar]
- 8.Choe S, Bennett M J, Fujii G, Curmi P M G, Kantardjieff K A, Collier R J, Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992;357:216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- 9.Collier R J. Three-dimensional structure of diphtheria toxin. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker; 1995. pp. 81–93. [Google Scholar]
- 10.Considine R V, Simpson L L. Cellular and molecular actions of binary toxins possessing ADP-ribosyltransferase activity. Toxicon. 1991;29:913–936. doi: 10.1016/0041-0101(91)90076-4. [DOI] [PubMed] [Google Scholar]
- 11.Fritz G, Schroeder P, Aktories K. Isolation and characterization of a Clostridium botulinum C2 toxin-resistant cell line: evidence for possible involvement of the cellular C2II receptor in growth regulation. Infect Immun. 1995;63:2334–2340. doi: 10.1128/iai.63.6.2334-2340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii N, Kubota T, Shirakawa S, Kimura K, Ohishi I, Moriishi K, Isogai E, Isogai H. Characterization of component-I gene of botulinum C2 toxin and PCR detection of its gene in clostridial species. Biochem Biophys Res Commun. 1996;220:353–359. doi: 10.1006/bbrc.1996.0409. [DOI] [PubMed] [Google Scholar]
- 13.Hol W G J, Sixma T K, Merritt E A. Structure and function of E. coli heat-labile enterotoxin and cholera toxin B pentamer. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker; 1995. pp. 185–223. [Google Scholar]
- 14.Jung M, Just I, van Damme J, Vandekerckhove J, Aktories K. NAD-binding site of the C3-like ADP-ribosyltransferase from Clostridium limosum. J Biol Chem. 1993;268:23215–23218. [PubMed] [Google Scholar]
- 15.Just I, Mohr C, Schallehn G, Menard L, Didsbury J R, Vandekerckhove J, van Damme J, Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J Biol Chem. 1992;267:10274–10280. [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Leppla S H. Anthrax toxins. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker; 1995. pp. 543–572. [Google Scholar]
- 18.Locht C, Antoine R. Pertussis toxin. In: Aktories K, editor. Bacterial toxins tools in cell biology and pharmacology. Weinheim, Germany: Chapman & Hall; 1997. pp. 33–45. [Google Scholar]
- 19.Montecucco C, Papini E, Schiavo G. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett. 1994;346:92–98. doi: 10.1016/0014-5793(94)00449-8. [DOI] [PubMed] [Google Scholar]
- 20.Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys. 1995;28:423–472. doi: 10.1017/s0033583500003292. [DOI] [PubMed] [Google Scholar]
- 21.Ohishi I. Activation of botulinum C2 toxin by trypsin. Infect Immun. 1987;55:1461–1465. doi: 10.1128/iai.55.6.1461-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohishi I, Iwasaki M, Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980;30:668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohishi I, Yanagimoto A. Visualizations of binding and internalization of two nonlinked protein components of botulinum C2 toxin in tissue culture cells. Infect Immun. 1992;60:4648–4655. doi: 10.1128/iai.60.11.4648-4655.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastan I, FitzGerald D. Pseudomonas exotoxin: chimeric toxins. J Biol Chem. 1989;264:15157–15160. [PubMed] [Google Scholar]
- 25.Perelle S, Domenighini M, Popoff M R. Evidence that Arg-295, Glu-378, and Glu-380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 1996;395:191–194. doi: 10.1016/0014-5793(96)01035-6. [DOI] [PubMed] [Google Scholar]
- 26.Perelle S, Gibert M, Boquet P, Popoff M R. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect Immun. 1993;61:5147–5156. doi: 10.1128/iai.61.12.5147-5156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Preiss, J., et al. Unpublished data.
- 27.Schmid A, Benz R, Just I, Aktories K. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes: formation of cation-selective channels and inhibition of channel function by chloroquine and peptides. J Biol Chem. 1994;269:16706–16711. [PubMed] [Google Scholar]
- 28.Sekine A, Fujiwara M, Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- 29.Simpson L L. The binary toxin produced by Clostridium botulinum enters cells by receptor-mediated endocytosis to exert its pharmacologic effects. J Pharmacol Exp Ther. 1989;251:1223–1228. [PubMed] [Google Scholar]
- 30.Simpson L L, Stiles B G, Zapeda H H, Wilkins T D. Molecular basis for the pathological actions of Clostridium perfringens iota toxin. Infect Immun. 1987;55:118–122. doi: 10.1128/iai.55.1.118-122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiles B G, Wilkens T D. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect Immun. 1986;54:683–688. doi: 10.1128/iai.54.3.683-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strom T B, Anderson P L, Rubin-Kelley V E, Williams D P, Kiyokawa T, Murphy J R. Immunotoxins and cytokine toxin fusion proteins. Ann N Y Acad Sci. 1991;636:233–250. doi: 10.1111/j.1749-6632.1991.tb33455.x. [DOI] [PubMed] [Google Scholar]
- 33.van Damme J, Jung M, Hofmann F, Just I, Vandekerckhove J, Aktories K. Analysis of the catalytic site of the actin ADP-ribosylating Clostridium perfringens iota toxin. FEBS Lett. 1996;380:291–295. doi: 10.1016/0014-5793(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 34.Vandekerckhove J, Schering B, Bärmann M, Aktories K. Botulinum C2 toxin ADP-ribosylates cytoplasmic β/γ-actin in arginine 177. J Biol Chem. 1988;263:696–700. [PubMed] [Google Scholar]
- 35.Vitetta E S, Thorpe P E, Uhr J W. Immunotoxins: magic bullets or misguided missiles? Trends Pharmacol Sci. 1993;14:148–154. doi: 10.1016/0165-6147(93)90199-t. [DOI] [PubMed] [Google Scholar]
- 36.Wegner A, Aktories K. ADP-ribosylated actin caps the barbed ends of actin filaments. J Biol Chem. 1988;263:13739–13742. [PubMed] [Google Scholar]
- 37.Wick M J, Iglewski B H. Pseudomonas aeruginosa exotoxin A. In: Moss J, Vaughan M, editors. ADP-ribosylating toxins and G proteins. Washington, D.C: American Society for Microbiology; 1990. pp. 31–43. [Google Scholar]
- 38.Williams D P, Snidert C E, Strom T B, Murphy J R. Structure/function analysis of interleukin-2-toxin (DAB486-IL-2) J Biol Chem. 1990;265:11885–11889. [PubMed] [Google Scholar]