Abstract
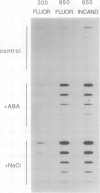
In Mesembryanthemum crystallinum, phosphoenolpyruvate carboxylase is synthesized de novo in response to osmotic stress, as part of the switch from C3-photosynthesis to Crassulacean acid metabolism. To better understand the environmental signals involved in this pathway, we have investigated the effects of light on the induced expression of phosphoenolpyruvate carboxylase mRNA and protein in response to stress by 400 millimolar NaCl or 10 micromolar abscisic acid in hydroponically grown plants. When plants were grown in high-intensity fluorescent or incandescent light (850 microeinsteins per square meter per second), NaCl and abscisic acid induced approximately an eightfold accumulation of phosphoenolpyruvate carboxylase mRNA when compared to untreated controls. Levels of phosphoenolpyruvate carboxylase protein were high in these abscisic acid- and NaCl-treated plants, and detectable in the unstressed control. Growth in high-intensity incandescent (red) light resulted in approximately twofold higher levels of phosphoenolpyruvate carboxylase mRNA in the untreated plants when compared to control plants grown in high-intensity fluorescent light. In low light (300 microeinsteins per square meter per second fluorescent), only NaCl induced mRNA levels significantly above the untreated controls. Low light grown abscisic acid- and NaCl-treated plants contained a small amount of phosphoenolpyruvate carboxylase protein, whereas the (untreated) control plants did not contain detectable amounts of phosphoenolpyruvate carboxylase. Environmental stimuli, such as light and osmotic stress, exert a combined effect on gene expression in this facultative halophyte.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang Y. C., Walling L. L. Abscisic Acid Negatively Regulates Expression of Chlorophyll a/b Binding Protein Genes during Soybean Embryogeny. Plant Physiol. 1991 Nov;97(3):1260–1264. doi: 10.1104/pp.97.3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Dai Z., Ku M. S., Edwards G. E. Induction of Crassulacean Acid Metabolism in the Facultative Halophyte Mesembryanthemum crystallinum by Abscisic Acid. Plant Physiol. 1990 Jul;93(3):1253–1260. doi: 10.1104/pp.93.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman J. C., Meyer G., Michalowski C. B., Schmitt J. M., Bohnert H. J. Salt stress leads to differential expression of two isogenes of phosphoenolpyruvate carboxylase during Crassulacean acid metabolism induction in the common ice plant. Plant Cell. 1989 Jul;1(7):715–725. doi: 10.1105/tpc.1.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin P. M., Sarokin L., Memelink J., Chua N. H. Molecular light switches for plant genes. Plant Cell. 1990 May;2(5):369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliber T. E. Endogenous Abscisic Acid Content Correlates with Photon Fluence Rate and Induced Leaf Morphology in Hippuris vulgaris. Plant Physiol. 1989 Mar;89(3):732–734. doi: 10.1104/pp.89.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiltinan M. J., Marcotte W. R., Jr, Quatrano R. S. A plant leucine zipper protein that recognizes an abscisic acid response element. Science. 1990 Oct 12;250(4978):267–271. doi: 10.1126/science.2145628. [DOI] [PubMed] [Google Scholar]
- Hartung W., Radin J. W., Hendrix D. L. Abscisic Acid Movement into the Apoplastic solution of Water-Stressed Cotton Leaves: Role of Apoplastic pH. Plant Physiol. 1988 Mar;86(3):908–913. doi: 10.1104/pp.86.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Benedyk M., Chua N. H. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol Cell Biol. 1989 Nov;9(11):4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., LaFavre A. K. Interactions between red light, abscisic acid, and calcium in gravitropism. Plant Physiol. 1989;89:875–878. doi: 10.1104/pp.89.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem J. A., Olson S. W., Schmitt J. M., Bohnert H. J. Salt Stress Increases the Level of Translatable mRNA for Phosphoenolpyruvate Carboxylase in Mesembryanthemum crystallinum. Plant Physiol. 1987 Aug;84(4):1270–1275. doi: 10.1104/pp.84.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant A. L., Cohen A., Moses M. S., Bray E. A. Nucleotide sequence and spatial expression pattern of a drought- and abscisic Acid-induced gene of tomato. Plant Physiol. 1991 Nov;97(3):900–906. doi: 10.1104/pp.97.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Thuleau P. Ca2+ Channels in Higher Plant Cells. Plant Cell. 1991 Jun;3(6):555–559. doi: 10.1105/tpc.3.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. C., De Armond R. L., Bohnert H. J. Influence of NaCl on Growth, Proline, and Phosphoenolpyruvate Carboxylase Levels in Mesembryanthemum crystallinum Suspension Cultures. Plant Physiol. 1992 Feb;98(2):626–631. doi: 10.1104/pp.98.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]