Abstract
Chlamydia pneumoniae is a respiratory pathogen that has been associated with chronic inflammatory diseases such as asthma and atherosclerosis. Recent isolation of C. pneumoniae from human carotid and coronary atheromas provides additional support for a role of this organism in atherogenesis. We characterized the coronary strain C. pneumoniae A-03 by sequence analysis of the major outer membrane protein gene (omp1). In addition, the in vitro activities of A-03 and three respiratory strains of C. pneumoniae (BAL-16, TW-183, and T-2634) were examined in infected human umbilical vein endothelial cells (HUVEC) by analysis of the production of interleukin-8 (IL-8), monocyte chemotactic protein 1 (MCP-1), and soluble intercellular cell adhesion molecule 1 (sICAM-1). Sequence analysis of omp1 of C. pneumoniae A-03, compared to prototype strains TW-183 and AR-39, revealed five nucleotide changes resulting in nonsynonymous codons. Of interest was a nonconservative amino acid substitution (Ser to Pro) in position 61 of variable segment 1. In vitro, the extent of MCP-1, IL-8, and sICAM-1 production was dependent on the C. pneumoniae strain examined at low multiplicities of infection following 24 h of incubation. Strain A-03 displayed the lowest stimulatory activity in infected HUVEC, while T-2634 induced the highest levels of MCP-1, IL-8, and sICAM-1 among all strains examined. Heat-inactivated C. pneumoniae failed to stimulate production of these proteins by all strains tested. In contrast, only partial inhibition was observed by UV-inactivated organisms. Results from this study demonstrate that unlike prototype respiratory strains of C. pneumoniae, the coronary strain A-03 displays divergence in the omp1 gene. In addition, the stimulation of chemokines and adhesion molecules involved in the recruitment of leukocytes to sites of inflammation by C. pneumoniae may be important in the pathogenesis of diseases associated with this organism, including atherosclerosis.
Chlamydia pneumoniae is a respiratory pathogen that causes sinusitis, bronchitis, pneumonia, and other acute respiratory infections (12, 13, 23). Infection with this organism has also been associated with chronic inflammatory diseases such as asthma (14) and atherosclerosis (24, 26, 29, 32). The role of C. pneumoniae in atherosclerosis remains unclear despite the detection of this bacterium in atheromas by numerous studies (21, 22, 33), including a report from our laboratory documenting the isolation of C. pneumoniae from the coronary artery of a patient with severe coronary atherosclerosis (30). Isolation of this organism from a carotid artery atheroma has been reported as well (17).
The pathologic significance of C. pneumoniae in chronic inflammatory diseases is not well understood. This organism is capable of remaining viable in the host despite antibiotic treatment of chronic respiratory infections following acute illness (15). One of the hallmarks of diseases such as asthma and atherosclerosis includes the accumulation of blood-borne leukocytes into the inflamed tissues in response to antigenic stimuli. This process is initiated with the binding of leukocytes to activated endothelium via induced expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and leukocyte function antigen 1 (27). Leukocyte chemotaxis and migration across the endothelium are modulated by numerous chemokines (i.e., chemotactic cytokines), including interleukin-8 (IL-8) and monocyte chemotactic protein 1 (MCP-1), which have specificities for neutrophils and monocytes, respectively (2). The fact that these chemokines have been detected in human atheromas suggests that they may play important pathophysiologic roles in atherogenesis (28, 36).
The in vivo effects of C. pneumoniae on the production of inflammatory mediators have not been studied in detail. Lung pathology in mice models is characterized by patchy interstitial pneumonitis, with polymorphonuclear leukocyte infiltration in the early stage and mononuclear cell infiltration in the late stage (39). In previous in vitro studies, we have shown inhibition of C. pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha (TNF-α) (34). Induction of proinflammatory cytokines such as TNF-α, IL-1, and IL-6 by C. pneumoniae has been studied in human monocytic cells (16). The capability of C. pneumoniae to replicate in vitro in human endothelial cells, aortic smooth muscle cells, and macrophages has been shown previously (8, 10, 11, 19). Infection of endothelial cells results in the stimulation of adhesion molecules such as endothelial-leukocyte adhesion molecule 1, ICAM-1, and vascular cell adhesion molecule 1 (20). Data concerning the production of chemokines such as IL-8 and MCP-1 by endothelial cells in response to C. pneumoniae infection have not been reported.
Although isolation of C. pneumoniae from coronary and carotid atheromas strengthens the role of this organism in atherosclerosis, a cause-and-effect relation between C. pneumoniae and the development of atherosclerotic lesions has not been established. Previous molecular characterization of a carotid isolate has been performed by using Southern hybridization analysis of genomic digests and sequence analysis of the major outer membrane protein (MOMP) gene (omp1) (17). Similar studies have not been documented with the coronary isolate, designated C. pneumoniae A-03.
Prior evidence has shown conservation in the omp1 genes of several C. pneumoniae strains (9, 18, 25), while considerable sequence variation is present among various C. trachomatis serovars. Unlike C. pneumoniae, specific antigenic determinants that distinguish different species, subspecies, and serovars of C. trachomatis are located in the MOMP (1, 40). Different serovars of C. pneumoniae have not yet been identified; however, antigenic variation among strains has been observed by immunoblot studies (3, 35), and serovar-specific antigenic determinants may reside in a 65-kDa protein, as reported by Jantos et al. (18).
The present study was aimed at characterizing C. pneumoniae A-03 at the molecular level and to examine its biological activity in vitro. Our first goal was to determine whether diversity existed in the omp1 gene of this strain compared to prototype strains of C. pneumoniae. Subsequent studies focused on the ability of A-03 and three respiratory strains of C. pneumoniae to stimulate the production of MCP-1, IL-8, and soluble ICAM-1 (sICAM-1) in human endothelial cells in vitro.
MATERIALS AND METHODS
Cell lines.
Human umbilical vein endothelial cells (HUVEC; ATCC 1730-CRL) were maintained in Ham’s F12K medium (Sigma, St. Louis, Mo.) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin-amphotericin B (Fungizone) mix (BioWhittaker, Walkersville, Md.), 30 μg of endothelial cell growth supplement per ml, and 100 μg of heparin (Sigma) per ml. Cells were grown in 75-cm2 culture flasks and transferred into 24-well plates containing gelatin-coated glass coverslips. HUVEC were seeded at 2 × 105 cells/well and allowed to adhere overnight in media without endothelial cell growth supplement prior to infection.
HEp-2 cells (ATCC CCL-23) were maintained in Iscove’s minimal essential medium (Cellgro, Washington, D.C.) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1% (vol/vol) nonessential amino acids, 10 mM HEPES, 10 μg of gentamicin per ml, and 25 μg of vancomycin per ml. Cells were maintained in 75-cm2 flasks and passed into 1-dram vials for use in propagation of C. pneumoniae strains.
C. pneumoniae strains.
C. pneumoniae A-03 (ATCC VR-1452) was previously isolated in our laboratory from an atheroma of a patient with coronary artery disease (30). C. pneumoniae TW-183 and AR-39 were obtained from the Washington Research Foundation, Seattle. C. pneumoniae BAL-16 and T-2634 were kindly provided by Margaret Hammerschlag, State University of New York, Brooklyn. Two additional clinical isolates from the University of Louisville, UL-029 and UL-083, were also selected for comparative sequence analysis of omp1. Bacterial strains were propagated in HEp-2 cell monolayers by a modification of the procedures described by Wong et al. (38). Briefly, confluent monolayers of HEp-2 cells in 1-dram vials were infected separately with each strain. C. pneumoniae were previously suspended in inoculation medium (supplemented Iscove’s minimal essential medium plus 4 mg of glucose per ml [pH 7.5]) before addition to the HEp-2 monolayers. Infection was done by centrifugation at 800 × g for 1 h at 4°C. Infected cultures were then incubated at 37°C in 5% CO2 for 30 min. The medium was replaced with growth medium (inoculation medium plus 1 μg of cycloheximide per ml), and infected cultures were incubated for 48 to 72 h. Sequential passages in 1-dram vials were done before inoculation into 75-cm2 HEp-2 monolayers. C. pneumoniae was harvested by disruption of the monolayers with sterile glass beads, sonication, and low-speed centrifugation at 200 × g. Aliquots of C. pneumoniae were titrated and stored at −70°C in inoculation medium.
omp1 gene amplification and sequencing.
DNA from C. pneumoniae A-03, TW-183, AR-39, BAL-16, T-2634, UL-029, and UL-083 was extracted from elementary body suspensions by lysis with 10 μg of proteinase K per ml in TE buffer (10 mM Tris-HCl [pH 8.3], 1 mM EDTA, 0.45% [vol/vol] Tween 20–Nonidet P-40) incubation at 55°C, phenol-chloroform extraction, precipitation with 95% ethanol, and resuspension in TE as previously described (7). One microliter of DNA was amplified by using primers that flank the omp1 gene of C. pneumoniae (CS-F and CF-B) (7). Automated sequencing of the amplified product was performed with a model 377 automated sequencer (Perkin Elmer-ABI, Foster City, Calif.) as instructed by the manufacturer.
Infection of HUVEC.
HUVEC monolayers in 24-well plates were infected separately with C. pneumoniae A-03, TW-183, BAL-16, and T-2634 suspended in inoculation medium. Cells were inoculated with 2 × 105 inclusion-forming units (IFU) of each strain per well, resulting in a multiplicity of infection (MOI) of 1:1. In other experiments, a higher inoculum concentration of 2 × 106 IFU/well (MOI of 10:1) was used for strains A-03 and BAL-16 to study a dose-dependent activation of HUVEC. In addition to infection with viable C. pneumoniae, HUVEC were infected with organisms that had been previously inactivated by heat (90°C for 30 min) or UV light (12-h exposure to a model UVSL-25 Mineralight UV lamp at a distance of 2 cm). When such preparations were examined for viable organisms in HEp-2 cell cultures, none were detected. Inoculation of HUVEC with viable or inactivated C. pneumoniae was followed by centrifugation at 800 × g for 1 h at 4°C. After 30 min of incubation at 37°C with 5% CO2, cell monolayers were washed with Hanks’ balanced salt solution and the medium was replaced with growth medium lacking cycloheximide. Since the bacterial inoculum may contain remnants of HEp-2 cells, mock-infected controls were included. These consisted of HUVEC treated with crude lysates of HEp-2 cells and were processed in the same manner as infected cells. Uninfected, mock-infected, and infected cells were incubated at 37°C in 5% CO2, and culture supernatants were collected after 6, 24, and 48 h. Supernatants were stored at −20°C until chemokine and sICAM-1 measurements were performed. The response of HUVEC to stimulation with 500 U of human recombinant TNF-α (Promega, Madison, Wis.) per ml was used as a positive control.
To examine the growth of C. pneumoniae in HUVEC, infected cells were scraped at 48 h postinfection (p.i.), resuspended in inoculation medium, titrated, and inoculated in fresh HEp-2 monolayers. Growth titers were obtained from replicate wells and expressed as IFU/milliliter. In addition, separate HUVEC monolayers on glass coverslips were fixed with methanol, and chlamydial inclusions were stained with the Pathfinder Chlamydia culture confirmation system (Kallestad, Chaska, Minn.) according to manufacturer’s instructions. Infectivity of C. pneumoniae was assessed by counting the numbers of inclusions per high-power field (HPF), using epifluorescence microscopy at a magnification of ×400. Viability of HUVEC during infection was determined by trypan blue dye exclusion analysis.
Chemokine and adhesion molecule measurements.
Levels of MCP-1, IL-8, and sICAM-1 were measured from the supernatants of uninfected, mock-infected, infected, and TNF-α-treated cells by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, Minn.). The ELISAs were performed according to the manufacturer’s instructions.
Data analysis.
Raw data of experimental groups from the ELISAs were subjected to analysis of variance with the Tukey-HSD multiple-comparison test. A P of <0.05 was used as the alpha value to determine statistical significance for all analyses.
RESULTS
omp1 sequence analysis of C. pneumoniae strains.
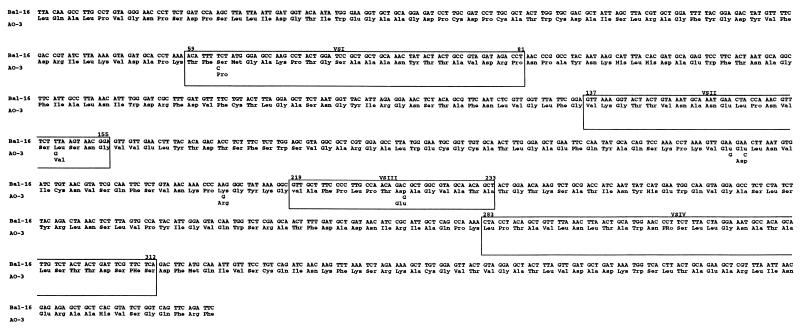
The sequence of the omp1 gene of the coronary atheroma C. pneumoniae A-03 strain was determined and compared to the gene sequences of six respiratory strains of C. pneumoniae. The omp1 sequences of C. pneumoniae BAL-16, T-2634, and two clinical isolates from the University of Louisville (UL-029 and UL-083) were identical to those of the prototype strains TW-183 and AR-39. Figure 1 shows comparison of the omp1 gene sequences of C. pneumoniae A-03 and BAL-16. Six nucleotide changes were noted in the omp1 gene sequence of C. pneumoniae A-03, with five of these resulting in nonsynonymous codons. One substitution was found in each of the variable segments 1, 2, and 3 (VS1, -2, and -3), while the remaining two localized at conserved regions of omp1. VS4 sequences were identical among all strains examined. Significantly, the amino acid change in VS1 of C. pneumoniae A-03 (Ser to Pro at position 61) was nonconservative. The remaining amino acid changes were synonymous.
FIG. 1.
omp1 gene sequence of C. pneumoniae BAL-16, which is identical to that of the prototype strain TW-183. The corresponding C. pneumoniae A-03 sequence is shown below that of BAL-16. Positions of VS 1 to -4 are (VSI to -IV) shown. Deduced amino acid sequences are shown below the codons.
Secretion of MCP-1 and IL-8 by HUVEC in response to C. pneumoniae infection.
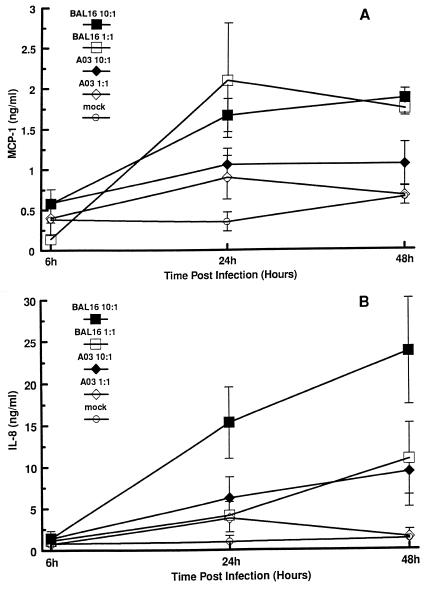
The in vitro activities of C. pneumoniae A-03 and the respiratory strains BAL-16, T-2634, and TW-183 were examined in infected endothelial cells by analysis of the production of the chemokines MCP-1 and IL-8. The kinetics of MCP-1 and IL-8 secretion were determined from HUVEC infected with C. pneumoniae A-03 and BAL-16 at MOIs of 1:1 and 10:1 after 6, 24, and 48 h of incubation. As shown in Fig. 2A, secretion of MCP-1 was induced in a time-dependent fashion in response to C. pneumoniae A-03 and BAL-16. At 6 h p.i., levels of MCP-1 were not higher than those in mock-infected controls, even at an MOI of 10:1. Following 24 and 48 h of infection, significant increases in MCP-1 production were observed in response to C. pneumoniae BAL-16 at both MOIs compared to mock-infected controls. These increases were approximately fivefold at 24 h (P < 0.01) and threefold at 48 h (P < 0.002). In contrast, induction of MCP-1 by C. pneumoniae A-03 did not reach statistical significance compared to mock-infected controls, even at an MOI of 10:1. After 24 h of infection, levels of MCP-1 increased approximately threefold in response to A-03 at both MOIs and remained elevated after 48 h only with an MOI of 10:1. Concentrations of MCP-1 in uninfected cells ranged from 0.2 to 0.5 ng/ml, while treatment of cells with 500 U of human recombinant TNF-α per ml, used as a positive control, increased MCP-1 production from 0.8 to 12.5 ng/ml during the 48-h length of the assays.
FIG. 2.
Time course secretion of MCP-1 and IL-8 by HUVEC in response to infection with C. pneumoniae. HUVEC monolayers were inoculated with C. pneumoniae A-03 or BAL-16 at MOIs of 1:1 and 10:1. Mock-infected cells were treated with a suspension of lysed HEp-2 cells (see Materials and Methods). Levels of MCP-1 (A) and IL-8 (B) from culture supernatants were measured by ELISA. Data points represent the means ± standard errors of the means of three separate experiments. In each experiment, duplicate wells were assayed separately for each condition for MCP-1 or IL-8 secretion.
The stimulation of IL-8 in response to C. pneumoniae A-03 and BAL-16 was also time dependent (Fig. 2B). In addition, raising the MOI from 1:1 to 10:1 had a greater effect on the secretion of IL-8 compared to MCP-1 during the 48-h period of infection. When an MOI of 1:1 was used, neither strain A-03 nor strain BAL-16 induced significant production of IL-8 compared to mock-infected controls after 24 or 48 h of infection. At 24 h p.i., levels of IL-8 increased approximately fourfold in response to either strain at an MOI of 1:1 and remained elevated after 48 h only with strain BAL-16. As opposed to strain A-03, C. pneumoniae BAL-16 stimulated a significant increase in IL-8 secretion following 24 and 48 h of infection when the MOI was raised to 10:1. Compared to mock-infected controls, this increase was approximately 16-fold at 24 h (P < 0.001) and 19-fold at 48 h (P < 0.006). At the same MOI, levels of IL-8 in response to C. pneumoniae A-03 increased approximately sixfold at 24 h and sevenfold at 48 h. Supernatants of uninfected cells contained concentrations of IL-8 ranging from 0.1 to 0.7 ng/ml, while TNF-α-treated cells secreted 1.1 to 18.2 ng of IL-8 per ml during the experiments.
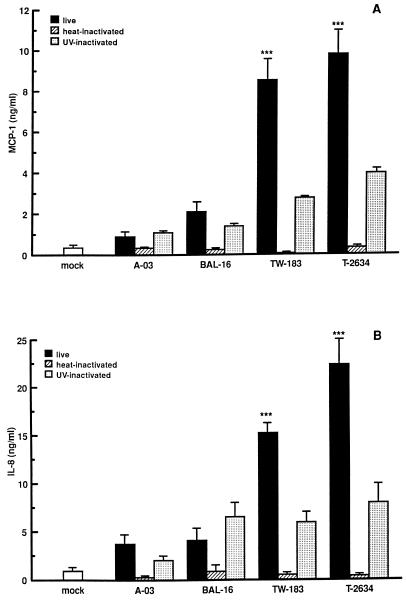
In addition to C. pneumoniae A-03 and BAL-16, strains TW-183 and T-2634 were examined for the ability to induce MCP-1 and IL-8 production in HUVEC. As seen in Fig. 3, the extent of MCP-1 and IL-8 secretion by HUVEC infected at an MOI of 1:1 depended on the C. pneumoniae strain examined. There was a significant difference in the relative amounts of MCP-1 and IL-8 stimulated by strains TW-183 and T-2634 as measured at 24 h p.i. compared to strains A-03 and BAL-16 (P < 0.001 in Fig. 3A and B, respectively). Differences in the induction of these proteins between strains displaying the lowest and highest stimulatory activities (A-03 and T-2634) were approximately 11-fold for MCP-1 and 6-fold for IL-8.
FIG. 3.
Extent of MCP-1 and IL-8 secretion by HUVEC infected with different strains of C. pneumoniae. HUVEC monolayers were inoculated with C. pneumoniae A-03, BAL-16, TW-183, or T-2634 at an MOI of 1:1. Equivalent inoculum concentrations of each strain were inactivated by heat or UV light before infection (see Materials and Methods). Mock-infected cells were treated with a suspension of lysed HEp-2 cells. Levels of MCP-1 (A) and IL-8 (B) from culture supernatants were measured following 24 h of incubation by ELISA. Bars indicate the means ± standard errors of the means of four separate experiments. In each experiment, duplicate wells were assayed separately for each condition for MCP-1 or IL-8 secretion. ∗∗∗, P < 0.001.
The ability of nonviable C. pneumoniae to stimulate the production of MCP-1 and IL-8 was also examined. Heat treatment of bacteria completely inhibited the stimulation of MCP-1 and IL-8 by all C. pneumoniae strains tested. In contrast, only partial inhibition was observed by UV-inactivated organisms. UV light treatment of strain A-03 had no effect on the MCP-1 response, while the response to strains BAL-16, TW-183 and T-2634 decreased compared to viable organisms by approximately 35, 65, and 60%, respectively (Fig. 3A). Similar results were obtained for IL-8 secretion in response to UV-inactivated strains TW-183 and T-2634, while a 45% reduction was observed in response to UV-inactivated strain A-03 and no effect was observed for strain BAL-16 (Fig. 3B).
Production of sICAM-1 by HUVEC in response to C. pneumoniae infection.
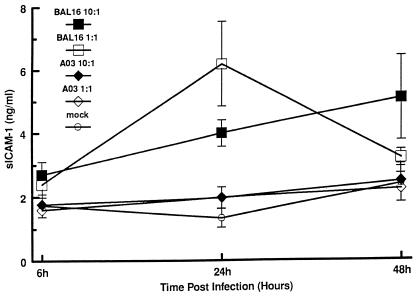
The in vitro activities of C. pneumoniae A-03, BAL-16, TW-183, and T-2634 were also examined by analysis of sICAM-1 production by infected HUVEC. Figure 4 depicts the kinetics of sICAM-1 production in response to infection with C. pneumoniae A-03 and BAL-16. At 24 h p.i., strain BAL-16 stimulated significant increases in sICAM-1 production compared to mock-infected controls at both MOIs. These increases were approximately fivefold at an MOI of 1:1 (P < 0.03) and threefold at an MOI of 10:1 (P < 0.004). Contrary to this, infection with C. pneumoniae A-03 caused little if any increase in sICAM-1 production after 24 and 48 h, even at an MOI of 10:1. Elevated levels of sICAM-1 were maintained at 48 h p.i. in cells infected with strain BAL-16 at both MOIs (P < 0.02 for an MOI of 10:1). In comparison to the MCP-1 experiments, raising the MOI from 1:1 to 10:1 did not increase levels of sICAM-1 stimulation by both strains at 24 h. Concentrations of sICAM-1 from uninfected HUVEC were comparable to those for mock-infected controls, whereas production of this protein from cells treated with 500 U of TNF-α per ml fluctuated from 2.1 to 22 ng/ml throughout the experiments.
FIG. 4.
Time course production of sICAM-1 by HUVEC in response to infection with C. pneumoniae. HUVEC monolayers were inoculated with C. pneumoniae A-03 or BAL-16 at MOIs of 1:1 and 10:1. Mock-infected cells were treated with a suspension of lysed HEp-2 cells (see Materials and Methods). Levels of sICAM-1 from culture supernatants were measured by ELISA. Data points represent the means ± standard errors of the means of three separate experiments. In each experiment, duplicate wells were assayed separately for each condition for sICAM-1 production.
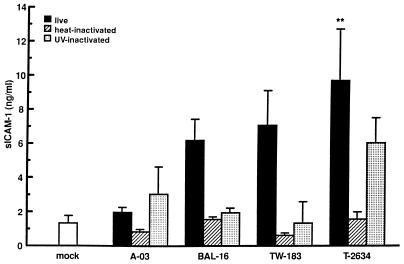
The production of sICAM-1 in response to C. pneumoniae A-03, BAL-16, TW-183, and T-2634 is shown in Fig. 5. The extent of sICAM-1 production by HUVEC infected at an MOI of 1:1 depended on the C. pneumoniae strain examined, similar to that seen in the MCP-1 and IL-8 experiments. The fold increase in sICAM-1 levels compared to mock-infected controls after 24 h ranged from 1.5 for strain A-03 to 7.2 for T-2634 (P < 0.01 for strain T-2634). Heat inactivation of the four C. pneumoniae strains tested diminished sICAM-1 stimulation entirely. Induction of this protein by UV-inactivated C. pneumoniae compared to viable bacteria had no effect for strain A-03, was completely inhibited for TW-183, and decreased approximately 70 and 40% for strains BAL-16 and T-2634, respectively.
FIG. 5.
Extent of sICAM-1 production by HUVEC infected with different strains of C. pneumoniae. HUVEC monolayers were inoculated with C. pneumoniae A-03, BAL-16, TW-183, or T-2634 at an MOI of 1:1. Equivalent inoculum concentrations of each strain were inactivated by heat or UV light before infection (see Materials and Methods). Mock-infected cells were treated with a suspension of lysed HEp-2 cells. Levels of sICAM-1 from culture supernatants were measured following 24 h of incubation by ELISA. Bars indicate the means ± standard errors of the means of four separate experiments. In each experiment, duplicate wells were assayed separately for each condition for sICAM-1 production. ∗∗, P < 0.01.
Growth of C. pneumoniae in HUVEC.
HUVEC supported similar replication of all four strains of C. pneumoniae examined, with growth titers ranging from 6.5 × 103 to 9.8 × 103 IFU/ml when an MOI of 1:1 was used. At the same MOI, numbers of chlamydial inclusion bodies per HPF, as well as the percentages of HUVEC with one or more inclusion body per HPF, were similar among all strains after 48 h of infection (data not shown). Inactivation of C. pneumoniae by heat or UV light resulted in nonproductive infection. All C. pneumoniae strains had similar effects on viability of infected HUVEC at 24 (>80%) or 48 h p.i. (>70%), as determined by trypan blue dye exclusion analysis.
DISCUSSION
Infections caused by C. pneumoniae are generally localized to the respiratory tract, are frequently asymptomatic, and may become chronic following acute illness despite appropriate antibiotic therapy (15). A role for C. pneumoniae in chronic inflammatory diseases has been suggested by data showing a propensity for patients with previous respiratory infection to develop asthmatic bronchitis (14) and by the physical and serological evidence implicating an association of this organism with atherosclerosis (21, 22, 24, 26, 29, 32, 33).
Isolation of C. pneumoniae from human carotid (17) and coronary (30) atheromas provides further support for an association of this bacterium with atherosclerosis by demonstrating the presence of viable organisms within lesions. Previous analysis of the MOMP gene sequence (omp1) of a carotid isolate indicated no differences with prototype C. pneumoniae respiratory strains (17). The present study demonstrates that C. pneumoniae coronary atheroma strain A-03 displays omp1 divergence from prototype respiratory strains, as well as from four additional respiratory isolates. In agreement with previous reports showing conservation of omp1 sequences among different respiratory strains of C. pneumoniae (9, 18, 25), we found the omp1 gene sequences of respiratory strains BAL-16, T-2634, UL-029, and UL-083 to be identical to those of the prototype strains TW-183 and AR-39. Among the nucleotide changes observed in the omp1 gene sequence of C. pneumoniae A-03, three were located in the variable segments with one resulting in a nonconservative amino acid substitution in the VS1 region.
For C. trachomatis serovars, the diversity of nucleotide sequences within the variable segments of omp1 genes accounts for the antigenic variation of the MOMP (37). Epitope mapping of the C. trachomatis MOMP has shown that three of the four variable segments (VS1, VS2, and VS4) contain serovar-, subspecies-, and species-specific antigenic determinants (1, 40). In contrast, the genetic homogeneity between different respiratory strains of C. pneumoniae in the omp1 gene may correlate with the inability to identify specific antigenic determinants in the MOMP. Previous immunoblot analyses of C. pneumoniae respiratory strains with anti-C. pneumoniae rabbit sera or human sera from patients with C. pneumoniae infection have shown that the recognition of the MOMP is genus reactive (5, 6). Even though evidence suggests that the C. pneumoniae MOMP is not immunodominant (6), further characterization of C. pneumoniae A-03 by serological studies is required to determine whether the omp1 substitutions correlate with distinct antigenic reactivities in the MOMP of this strain.
The results from our in vitro studies demonstrate that infection of endothelial cells with different strains of C. pneumoniae elicits the production of MCP-1, IL-8, and sICAM. Induction of these proteins in HUVEC was time dependent, with no increases detected early after infection (6 h) in response to C. pneumoniae A-03 and BAL-16. The extent of endothelial cell stimulation by C. pneumoniae A-03, BAL-16, TW-183, and T-2634 was strain dependent at low MOIs following 24 h of incubation. Strain A-03 exhibited the lowest stimulatory activity, while T-2634 induced the highest levels of MCP-1, IL-8, and sICAM-1 among all strains examined. The heterogeneity observed among these strains in the activation of HUVEC could not be explained by differences in the extent of chlamydial replication, since similar growth titers, as well as numbers of inclusion-containing cells, were observed for all four strains. These findings may reflect intrinsic differences among strains of C. pneumoniae in the activation of endothelial cells in vitro.
A variety of mechanisms may be involved in the stimulation of chemokines and adhesion molecules by C. pneumoniae in endothelial cells. Activation of HUVEC by C. pneumoniae may involve an autocrine pathway via IL-1α or TNF-α production in a fashion similar to that described for C. trachomatis and C. psittaci in epithelial cells (31). The results obtained with heat-inactivated and UV-inactivated organisms suggest that a heat-labile component may be required for the activation of HUVEC by C. pneumoniae. Analogous to what has been described for C. trachomatis and C. psittaci entry of HeLa cells and L cells (4), UV-inactivated C. pneumoniae may still possess the ability to invade endothelial cells, thereby activating a cascade of signaling mechanisms associated with phagocytosis. The complete inhibition of MCP-1, IL-8, and sICAM-1 stimulation by heat treatment of C. pneumoniae suggests that chlamydial lipopolysaccharide may not be important in eliciting the production of these inflammatory mediators from endothelial cells.
The increased production of chemokines and adhesion molecules by endothelial cells in response to C. pneumoniae infection may be important for recruiting leukocytes to the site of infection. This event presumably would result in both protective and deleterious consequences since the inflammatory response can lead to clearance of the infection as well as contribute to tissue damage. Although a casual relationship between C. pneumoniae and the development of atherosclerosis awaits further investigations, the ability of this organism to trigger an inflammatory response from endothelial cells suggests a potential role for C. pneumoniae in the pathology associated with this disease.
ACKNOWLEDGMENTS
This study was supported by a grant from the Heart and Lung Institute, Jewish Hospital Foundation, Louisville, Ky.
We thank Linda Jane Goldsmith, University of Louisville Biostatistics Center, for assistance in statistical analysis of data.
REFERENCES
- 1.Baehr W, Zhang Y-X, Joseph T, Su H, Nano F E, Everett K D E, Caldwell H D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci USA. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemotactic and inflammatory cytokines—CXC and CC proteins. Adv Exp Med Biol. 1993;353:1–10. doi: 10.1007/978-1-4615-2952-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Black C M, Johnson J E, Farshy C E, Brown T M, Berdal B P. Antigenic variation among strains of Chlamydia pneumoniae. J Clin Microbiol. 1991;29:1312–1316. doi: 10.1128/jcm.29.7.1312-1316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne G I, Moulder J W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978;19:598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell L A, Kuo C-C, Grayston J T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect Immun. 1990;58:93–97. doi: 10.1128/iai.58.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell L A, Kuo C-C, Wang S-P, Grayston J T. Serological response to Chlamydia pneumoniae infections. J Clin Microbiol. 1990;28:1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean D, Shama A, Schachter J, Dawson C R. Molecular identification of an avian strain of Chlamydia psittaci causing severe keratoconjuctivitis in a bird fancier. Clin Infect Dis. 1995;20:1179–1185. doi: 10.1093/clinids/20.5.1179. [DOI] [PubMed] [Google Scholar]
- 8.Fryer R H, Woods M L, Rodgers G M. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Invest Med. 1997;45:168–174. [PubMed] [Google Scholar]
- 9.Gaydos C A, Quinn T C, Bobo L D, Eiden J J. Similarity of Chlamydia pneumoniae strains in the variable domain IV region of the major outer membrane protein gene. Infect Immun. 1992;60:5319–5323. doi: 10.1128/iai.60.12.5319-5323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godzik K L, O’Brien E R, Wang S K, Kuo C-C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayston J T, Wang S P, Kuo C C, Campbell L A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989;8:191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- 13.Grayston J T. Chlamydia pneumoniae strain TWAR pneumonia. Annu Rev Med. 1992;43:317–323. doi: 10.1146/annurev.me.43.020192.001533. [DOI] [PubMed] [Google Scholar]
- 14.Hahn D L, Dodge R W, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult onset asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 15.Hammerschlag M R, Chirgwin K, Roblin P M, Gelling M, Dumornay W, Mandel L, Smith P, Schachter J. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis. 1992;14:178–182. doi: 10.1093/clinids/14.1.178. [DOI] [PubMed] [Google Scholar]
- 16.Heinemann M, Susa M, Simnacher V, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L A, Campbell L A, Kuo C-C, Rodriguez D I, Lee A, Grayston J T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 18.Jantos C A, Heck S, Roggendorf R, Sen-Gupta M, Hegemann J H. Antigenic and molecular analyses of different Chlamydia pneumoniae strains. J Clin Microbiol. 1997;35:620–623. doi: 10.1128/jcm.35.3.620-623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaukoranta-Tolvanen S S, Laitinen K, Saikku P, Leinonen M. Chlamydia pneumoniae multiplies in human endothelial cells in vitro. Microb Pathog. 1994;16:313–319. doi: 10.1006/mpat.1994.1032. [DOI] [PubMed] [Google Scholar]
- 20.Kaukoranta-Tolvanen S S E, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 21.Kuo C-C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 22.Kuo C-C, Gown A M, Benditt E P, Grayston J T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 23.Kuo C C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linnanmäki E, Leinonen M, Mattila K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae-specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 25.Melgosa M P, Kuo C-C, Campbell L A. Sequence Analysis of the major outer membrane gene of Chlamydia pneumoniae. Infect Immun. 1991;59:2195–2199. doi: 10.1128/iai.59.6.2195-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melnick S L, Shahar E, Folsom A R, Grayston J T, Sorlie P D, Wang S-P, Szklo M. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 27.Munro J M. Endothelial-leukocyte adhesive interactions in inflammatory diseases. Eur Heart J. 1993;14:K72–K77. [PubMed] [Google Scholar]
- 28.Nelken N A, Coughlin S R, Gordon D, Wilcox J N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puolakkainen M, Kuo C-C, Shor A, Wang S-P, Grayston J T, Campbell L A. Serological response to Chlamydia pneumoniae in adults with coronary arterial fatty streaks and fibrolipid plaques. J Clin Microbiol. 1993;31:2212–2214. doi: 10.1128/jcm.31.8.2212-2214.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez J A, Ahkee S, Summersgill J T, Ganzel B L, Ogden L L, Quinn T C, Gaydos C A, Bobo L L, Hammerschlag M R, Roblin P M, LeBar W, Grayston J T, Kuo C-C, Campbell L A, Patton D L, Dean D, Schachter J. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y-X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saikku P, Mattila K, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 33.Shor A, Kuo C-C, Patton D L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. South Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 34.Summersgill J T, Sahney N N, Gaydos C A, Quinn T C, Ramirez J A. Inhibition of Chlamydia pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect Immun. 1995;63:2801–2803. doi: 10.1128/iai.63.7.2801-2803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagels G, Rasmussen S, Timms P. Comparison of Chlamydia pneumoniae isolates by Western blot (immunoblot) analysis and DNA sequencing of the omp2 gene. J Clin Microbiol. 1994;32:2820–2823. doi: 10.1128/jcm.32.11.2820-2823.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang N, Tabas I, Winchester R, Ravalli S, Rabani L E, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem. 1996;271:8837–8842. doi: 10.1074/jbc.271.15.8837. [DOI] [PubMed] [Google Scholar]
- 37.Ward M E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 38.Wong K H, Skelton S K, Chan Y K. Efficient culture of Chlamydia pneumoniae with cell lines derived from the human respiratory tract. J Clin Microbiol. 1992;30:1625–1630. doi: 10.1128/jcm.30.7.1625-1630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z-P, Kuo C-C, Grayston J T. A mouse model of Chlamydia pneumoniae strain TWAR pneumonitis. Infect Immun. 1993;61:2037–2040. doi: 10.1128/iai.61.5.2037-2040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Y, Zhang Y-X, Watkins N G, Caldwell H D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the fifteen Chlamydia trachomatis serovars. Infect Immun. 1989;57:1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]