Abstract
Preventing local tumor recurrence while promoting bone tissue regeneration is an urgent need for osteosarcoma treatment. However, the therapeutic efficacy of traditional photosensitizers is limited, and they lack the ability to regenerate bone. Here, a piezo-photo nanoheterostructure is developed based on ultrasmall bismuth/strontium titanate nanocubes (denoted as Bi/SrTiO3), which achieve piezoelectric field-driven fast charge separation coupling with surface plasmon resonance to efficiently generate reactive oxygen species. These hybrid nanotherapeutics are integrated into injectable biopolymer hydrogels, which exhibit outstanding anticancer effects under the combined irradiation of NIR and ultrasound. In vivo studies using patient-derived xenograft models and tibial osteosarcoma models demonstrate that the hydrogels achieve tumor suppression with efficacy rates of 98.6 % and 67.6 % in the respective models. Furthermore, the hydrogel had good filling and retention capabilities in the bone defect region, which exerted bone repair therapeutic efficacy by polarizing and conveying electrical stimuli to the cells under mild ultrasound radiation. This study provides a comprehensive and clinically feasible strategy for the overall treatment and tissue regeneration of osteosarcoma.
Keywords: Surface plasmon resonance, Piezo-enhanced photodynamic, Piezoelectric stimulation, Anti-osteosarcoma, Osteogenesis
Graphical abstract
Highlights
-
•
Piezoelectric-photosensitizers demonstrates narrow band gaps and high-efficiency ROS production.
-
•
The nanoheterojunction shows exceptional piezo-enhanced NIR photodynamic therapy.
-
•
The mechanism of the piezoelectrical/photocatalytic coupling process is elucidated.
-
•
A tumor microenvironment-responsive hydrogel delivery platform is designed.
-
•
Piezoelectric stimulation accelerates bone defect healing via the PI3K/AKT pathway.
1. Introduction
Osteosarcoma is the most common primary malignant bone tumor in adolescents under the age of 20 [1,2]. It is characterized by the production of tumor-like bone matrix, strong local tissue invasion, and stubborn postoperative characteristics that pose a serious threat to human health [3]. Approximately 10–15 % of newly diagnosed osteosarcoma patients present with metastatic disease, predominantly in the lungs, resulting in a 5-year survival rate of only 20 % [4]. The standardized clinical treatment for osteosarcoma involves surgery in combination with neoadjuvant chemotherapy [[5], [6], [7]]. However, traditional intravenous chemotherapy has limited therapeutic efficacy due to inadequate local drug concentration within the tumor and the potential for systemic side effects [6]. Moreover, osteosarcoma exhibits strong bone tissue invasion, which may lead to large bone defects that cannot heal itself, resulting in lifelong disability and pain [8]. Therefore, there is an urgent need to develop innovative therapeutic strategies as alternatives, aiming to prevent the recurrence of bone tumors and facilitate the healing of bone defects.
Over the last decade, near-infrared (NIR) light-triggered photodynamic therapy (PDT) has been extensively studied as a treatment for solid tumors through the generation of excessive toxic reactive oxygen species (ROS) by photocatalysis, owing to its non-invasiveness, precise spatial and temporal precision, and ability to penetrate thick tissue [9,10]. However, most inorganic photocatalysis under NIR light often has low ROS generation efficiency, owing to the inefficient separation and fast recombination of photo-generated electrons and holes [[11], [12], [13], [14]]. High concentrations of photosensitizers and high irradiation intensity are required for adequate therapeutic effect, which may cause significant side effects on normal tissues [15].
Recently, introducing piezoelectric stimuli to photocatalysis has proven to be an effective strategy for enhancing the efficiency of photocatalytic ROS generation [[16], [17], [18]]. In detail, piezoelectric materials can be polarized, rapidly creating a built-in electric field that expedites the separation and transfer of photo-induced carriers when subjected to ultrasonics. Benefitting from the piezoelectric effect, some piezo-photo heterojunctions, such as ZnO/CuS [19], BiFeO3/TiO2 [20,21], and BiFeO3/COF [22], have been employed as effective piezo-photocatalysts. These materials demonstrate augmented ultraviolet and visible light photocatalytic activity, particularly under sunlight conditions, for applications in water splitting and pollutant degradation. Nevertheless, few piezo-photo heterostructure materials have been explored for the treatment of solid tumors or other related diseases primarily due to their limited piezo-photocatalytic performance in the NIR light range [23,24]. The current energy band gap of the piezoelectric heterojunction exceeds 2.9 eV, significantly surpassing the photon energy of 808 nm NIR light, which renders it incapable of absorbing NIR light and low ROS production [[25], [26], [27], [28]]. Therefore, constructing a novel piezo-photosensitizer with narrow band gap, rapid carrier separation and excellent piezoelectricity is expected to facilitate highly effective osteosarcoma elimination via piezo-enhanced PDT under 808 nm near-infrared light.
The effectiveness of bone defect therapy following osteosarcoma is currently hindered by insufficient medication concentration in the lesion region and the inadequacy of existing implants to match its complex shape [29,30]. To overcome these challenges, it is imperative to develop a method for delivering nanomedicine to the lesion site and filling the bone deficiency. Hydrogel, as a novel type of implant, can serve as a promising delivery platform for a wide range of substances, including small-molecule drugs, biomacromolecules, and nanoparticles [31,32]. Even so, the hydrogel-based delivery systems for antitumor therapy are very limited, since the low sensitivity to the tumor microenvironment of the mostly reported hydrogels restricts the precise modulation of therapeutic drug release behavior [33,34]. Rationally designing the hydrogel network to achieve precise release of therapeutic agents in response to tumor microenvironmental stimuli (pH and ROS) can increase local drug concentrations and reduce systemic side effects [[35], [36], [37]]. Additionally, by endowing hydrogel implants with injectability and self-healing ability, they can fill and conform to complex-shaped bone defects and promote bone defect repair [38,39].
In this study, the Bi/SrTiO3 (BST) nanoheterostructure was synthesized and integrated into an in situ-forming injectable biopolymer hydrogel for anti-osteosarcoma and osteogenesis combination therapy (Scheme 1). The designed nanocomposite hydrogel is degraded by responding to the tumor microenvironment (pH < 6.7), enabling the sustainable release of BST NPs. Benefiting from the local surface plasmon resonance (LSPR) effect and low bandgap width, the BST nanoheterojunction can respond to a wider range of light wavelengths (ultraviolet to near-infrared). During the ultrasonic (US) and NIR turn-on stages, the piezoelectric field drives rapid charge separation coupled LSPR, resulting in the rapid production of a substantial quantity of ROS for the prompt eradication of osteosarcoma cells. The in vivo therapeutic effects of BST NPs are excellent in human malignant patient-derived xenografts (PDX) and tibial orthotopic osteosarcoma models through piezo-enhanced photodynamic therapy. Furthermore, in the NIR switch-off stage, endocytosed BST NPs coupled with ultrasound treatment generate intracellular electrical stimulation to promote osteogenic differentiation and accelerate bone healing in vitro. Overall, plasma metal/piezoelectric heterojunction-integrated biopolymer hydrogels achieve anti-osteosarcoma and accelerated bone healing through piezoelectric-enhanced photodynamic and electrically stimulated switchable states.
Scheme 1.
Piezoelectric field-driven fast charge separation coupling with surface plasmon resonance enhanced near-infrared photodynamic therapy for anti-osteosarcoma and osteogenesis (a) The amino group of BST and gelatin, as well as the aldehyde group of oxidized chondroitin sulfate, were utilized for cross-linking via Schiff base bonds to prepare a BST/Gelatin/OCS nanocomposite injectable hydrogel (BGO hydrogel). Importantly, the dynamically covalent (Schiff base bond) cross-linked BGO hydrogel continuously released BST NPs in response to the tumor microenvironment (pH < 6.7). (b) Benefiting from piezoelectric field-driven fast charge separation coupling with surface plasmon resonance, BST NPs demonstrate a remarkable capability to efficiently generate ROS in response to NIR, thereby effectively eliminating tumor cells. Furthermore, in the NIR switch-off stage, endocytosed BST NPs coupled with ultrasound treatment generate intracellular electrical stimulation to promote osteogenic differentiation and accelerate bone healing in vitro.
2. Methods
2.1. Preparation of ST nanoparticles
For the method of preparing ST nanoparticles, please refer to the published papers [40]. Briefly, a mixed solution of 0.1 mM tetrabutyl titanate solution (Aladdin, China) and 0.2 mM Sr(OH)2 (Aladdin, China) was prepared in a 1:1 vol ratio. KOH was then added so that its concentration was 1.25 Mm. The mixed solution underwent hydrothermal reaction at 200 °C for 48 h, and the product was obtained through centrifugation and washing, resulting in ST nanopowder.
To obtain BST nanopowder, first prepare an aqueous solution of 0.02 mmol/mL bismuth nitrate pentahydrate (Aladdin, China). Next, add 0.3 g of ST particles to the solution and ultrasonically disperse the mixture for 10 min to ensure even distribution of the particles. Then, prepare a 0.5 mmol/mL aqueous solution of sodium borohydride (Aladdin, China) and mix it with the bismuth nitrate pentahydrate solution in a 1:1.5 vol ratio. Allow the resulting mixture to react for 30 min before immediately centrifuging, washing, and drying the solution to obtain the BST nano powder.
2.2. Characterization of ST and BST nanoparticles
The morphological and structural properties, elemental composition, particle size, and ferroelectricity of ST and BST nanoparticles were characterized using a combination of techniques, including scanning electron microscopy (SEM; Merlin, Zeiss, Germany), transmission electron microscopy (TEM; JEM-2100F, JEOL, Japan), energy-dispersive X-ray spectroscopy (EDX), dynamic light scattering (DLS; Zetasizer Nano ZS, Malvern, UK), and atomic force microscopy (AFM; Multimode 8, Bruker, Germany). The electron-hole recombination rate of the sample was measured by a fluorescence spectrophotometer (FLS980, Edinburg, UK). Furthermore, ultraviolet–visible–near-infrared (UV–Vis–NIR) absorption spectroscopy was employed to evaluate the light absorption capabilities of BST heterojunctions. X-ray photoelectron spectroscopy (XPS; ESCALAB XI+, Thermo-Fisher, USA) studies the elemental composition of nanoparticles. The photocurrent was investigated using a light source with an 808 nm near-infrared wavelength. To detect reactive oxygen species produced by BST, a 0.2 mol/mL DMPO methanol solution containing 500 μg/mL material and a 5 mg/mL TEMP aqueous solution containing 500 μg/mL material were prepared. The signals of superoxide radicals and singlet oxygen were detected by electron spin resonance (EMXPlus-10/12, Bruker, Germany) respectively.
2.3. Electrical signal detection of BST NPs
A laboratory mechano-electrical coupling device was utilized to investigate the difference and stability of the mechano-electrical response of BST NPs. This device was comprised of a self-made mechanical compression cycle device, a high-precision general-purpose digital instrument (DMM7510, Keithley, USA), and a computer. To measure the electrical signal output of the BST, a small device was fabricated. Initially, a mixed solution of ethanol and perfluorosulfonic acid at a volume ratio of 4:1 was prepared. BST powder was then added to the solution, with a concentration of 10 M. The solution was air-dried on conductive glass for 4 h to form a uniform BST powder film. A conductive layer was then formed by spraying platinum onto the BST powder film, and a closed electrode was formed by attaching one electrode to the platinum film and the other electrode to a substrate on conductive glass without BST powder.
2.4. Dye degradation experiment
The piezoelectric-photocatalytic properties of the sample material were evaluated through a methylene blue (MB) degradation experiment. Initially, 10 mg of methylene blue dye (Aladdin, China) was weighed and dissolved in 1000 mL of deionized water, resulting in a final concentration of 10 mg/L. During the experiment, the sample was maintained at a concentration of 200 μg/mL and was stirred for 1 h in the dark to achieve adsorption-desorption equilibrium. The solution was then subjected to either ultrasound or near-infrared light irradiation, with samples collected at regular intervals. After high-speed centrifugation, 1.5 mL of the supernatant was taken and analyzed using ultraviolet–visible spectrum detection within a detection range of 200–800 nm.
2.5. Characterization of BGO hydrogels
The rheological properties of the hydrogel, including gel time, shear-thinning properties, and self-healing behavior, were evaluated by a rotational rheometer (MCR301, Anton Paar, Austria). Oscillatory time sweeps were performed at an oscillating frequency of 1 Hz and a strain amplitude of 5 % to record the storage modulus (G′) and loss modulus (G") for determining the gel time. A hydrogel cylinder (Φ 25 mm × 2 mm) was prepared, and then the frequency-modulus curves were recorded at a strain amplitude of 5 % for angular frequencies ranging from 0.1 to 100 rad/s. The change in viscosity of the hydrogel was recorded in frequency scan mode at shear rates ranging from 0 to 100 s-1 to assess the hydrogel' shear-thinning behavior. In addition, the rhodamine B (R104960, Aladdin, China) stained hydrogel was injected into the PBS solution at 37 °C with a syringe to observe its injectability. Two identical hydrogel disks (Φ 20 mm × 3 mm) were prepared, with one stained red with Rhodamine B dye. The excitation wavelength for the BGO hydrogel is 555 nm, and the emission wavelength is 580 nm. The steps involved in the self-healing experiment include preparing two identical hydrogel disks (Φ20mm × 3 mm), with one dyed red using rhodamine B dye. Subsequently, the two hydrogel disks are symmetrically cut in half and ligated with the exchanged fragments at 37 °C for 5 min. The reattached hydrogel sheet was taken up with tweezers and pulled to examine the macroscopic self-healing ability. The electron-hole recombination rate of the sample was measured by a fluorescence spectrophotometer (FLS980, Edinburg, UK), and the temperature change curve was obtained as a result. A reactive oxygen species (ROS) detection kit (KGT010-1, KeyGEN Bio TECH, China) was used to determine the ability of materials to produce ROS. In the presence of ROS, the fluorescent probe DCFA-DA was hydrolyzed and oxidized into fluorescent DCF, and the fluorescence intensity was measured by a microplate spectrophotometer (Epoch2, BioTek, USA) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm every 2 min for a total of 10 min.
To detect treatment-induced temperature changes in vitro, BGO-2 hydrogel samples were situated in a 48-well plate, and 800 μL of PBS was added, followed by the measurement of the initial temperature. Subsequently, ultrasound and near-infrared treatment were conducted, with ultrasonic treatment parameters set at (1.0 MHz, 2.0 W/cm2, 5 min, 50 % duty cycle) and near-infrared light treatment parameters at (808 nm, 1.0 W/cm2, 5 min). For monitoring treatment-induced changes in body temperature, 100 μL of BGO-2 hydrogel was injected into the tumor site, and near-infrared light and ultrasound treatments were administered. The solution temperature was recorded every 5 s. Thermal imaging cameras captured images to document the temperature and generate a temperature profile.
To detect treatment-induced temperature changes in vitro, BGO-2 hydrogel samples were situated in a 48-well plate, and 800 μL of PBS was added, followed by the measurement of the initial temperature. Subsequently, ultrasound and near-infrared treatment were conducted, with ultrasonic treatment parameters set at (1.0 MHz, 2.0 W/cm2, 50 % duty cycle) and near-infrared light treatment parameters at (808 nm, 1.0 W/cm2). For monitoring treatment-induced changes in body temperature, 100 μL of BGO-2 hydrogel was injected into the tumor site, and near-infrared light and ultrasound treatments were administered. Thermal imaging cameras captured images to document the temperature and generate a temperature profile.
2.6. Evaluation of in vivo degradation and cytotoxicity assay of BGO-2 hydrogels
UMR106, Saos-2, and 143B cells were cultivated in 25 cm2 cell culture flasks within a humidified environment at 37 °C with 5 % CO2. The cell culture was maintained in high-glucose DMEM medium supplemented with 1 % penicillin/streptomycin and 10 % FBS. Adherent osteosarcoma cells were detached using 2 mL of 0.25 % trypsin and subsequently subcultured for subsequent experiments.
The cytotoxicity of BGO-2 hydrogels towards UMR106, 143B, and Saos-2 osteosarcoma cells was evaluated using a Calcein-AM/PI Live/Dead assay kit (C2015S, Beyotime, China). The ultrasonic parameters used were as follows: 1.0 MHz, 2.0 W/cm2, 50 % duty cycle, and 5 min, while the near-infrared light parameters were 1.0 W/cm2 for 5 min. After a 48-h culture in complete medium, the cells were stained according to the provided instructions and observed under a fluorescent inverted microscope (Axio Observer, Zeiss, Germany) to evaluate the cellular response to different hydrogel surfaces. The proliferation of osteosarcoma cells co-cultured on various hydrogel surfaces for 24 h was assessed using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), and the optical density (OD) at 450 nm was measured with a microplate spectrophotometer (Epoch2, BioTek, USA). Material hemocompatibility is a critical factor to consider for the in vivo application of biomaterials. To evaluate the hemocompatibility of the hydrogels, Triton was used as the positive control and PBS was used as the negative control. The hydrogels were co-incubated with 1 ml of whole blood at 37 °C for 2 h, followed by centrifugation at 1000 rpm for 10 min at 4 °C. The OD value at 540 nm was then measured using a microplate spectrophotometer.
Nine male Prague Dawley (SD) rats, weighing 250 ± 50 g and aged 7–9 weeks, were randomly divided into three groups (n = 4). The subcutaneous implantation of BGO-2 nanocomposite hydrogel was performed on the back of the rats. The degradation of BGO-2 hydrogel in vivo was observed by excising the tissue at the implantation site after 3, 5, and 7 days. The local inflammatory response was assessed by performing hematoxylin and eosin (H&E) staining using the C0105S kit (Beyotime, China).
2.7. In vitro antitumor performance test of BGO-2 hydrogel
To assess intracellular ROS levels in 143B osteosarcoma cells, we utilized a reactive oxygen species (ROS) detection kit (KGT010-1, KeyGEN BioTECH, China). The kit utilizes 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) which is hydrolyzed by esterase to generate DCFH. DCFH is then oxidized into fluorescent DCF by ROS, and the fluorescence intensity of DCF is used to determine the level of intracellular ROS. The cells were cultured for 72 h and then harvested and incubated with DCFH-DA at 37 °C for 30 min. ROS fluorescence was observed by laser confocal microscopy (CLSM; TCS SP8, Leica, Germany). Initially, intracellular reactive oxygen species (ROS) content was assessed using a microplate reader. ROS fluorescence was detected with an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The percentage of ROS production is calculated using the formula ROS production% = (C/C0) %. In the measurement of ROS, C0 signifies the initial optical density (OD) value of ROS in the blank group, while C represents the OD value of intracellular ROS after treatment with an external field.
Detection of mitochondrial membrane potential: To further investigate the effect of hydrogels on apoptosis in 143B osteosarcoma cells, a mitochondrial membrane potential assay kit with JC-1 (C2006, Beyotime, China) was applied. The red aggregates and green monomers of the JC-1 probe represent integrated and disrupted mitochondrial membrane, respectively. 143B cells were incubated for 48 h and then stained with a JC-1 probe for 20 min at 37 °C. The stained cells were then observed under a CLSM. The fluorescence intensity was analyzed using ImageJ software.
Cell apoptosis analysis was performed using the Apoptosis and Necrosis Detection Kit (C1062S, Beyotime, China). Following the manufacturer's instructions, the cells were washed with PBS and then treated with Hoechst 33342 and propidium iodide dissolved in the provided buffer solution. After a 30-min incubation at 4 °C, the cells were washed again with PBS and observed under an inverted fluorescence microscope. Living cells were identified by blue fluorescence, while early apoptotic, late apoptotic, and necrotic cells were identified by red fluorescence. Images were captured at the same location using appropriate filter sets for each fluorescence signal.
2.8. Evaluation of the osteogenic assay of BST NPs in vitro
There are four experimental groups: Blank, US, BST, and BST+US. Bone marrow mesenchymal stem cells (BMSCs) were seeded in each well at a density of 0.5 × 104 and incubated for 12 h. Next, 100 μg/ml BST working solution was added, and the cells were further incubated in osteoblast induction medium, refreshed every other day. The ultrasound parameters used were: 1.0 MHz frequency, 1.0 W/cm2 power, 50 % duty cycle, and a 2-min duration.
ALP activity was assessed using an ALP kit (A059-3-1, Nanjing, China) according to the manufacturer's instructions. ALP expression was examined using a stereomicroscope (MZ10F, Leica, Germany). The effects of BST and ultrasound on the osteogenic differentiation-related proteins and genes of BMSCs were evaluated using real-time quantitative PCR (RT-qPCR) to measure the expression of osteogenesis-related genes, such as ALP, Runx2, OPN, OCN, and PI3K/AKT. The primer sequences used in RT-qPCR are listed in Table S1 [41]. In addition, immunofluorescence staining for Runx-2 and OCN was performed on day 7, and CLSM was used to visualize BMSCs after counterstaining with Actin Tracker Green and DAPI. Briefly, BMSCs were co-cultured with different samples for 24 h and then incubated in medium containing 2.5 mmol/L Fluo-4 AM for 1 h. Fluorescence images were captured using CLSM.
2.9. Animal experiments
Animal procedures involving tibia osteosarcoma model were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Beijing Jishuitan Hospital (Grant No.202106-01). To establish the tibial osteosarcoma model, a small burr hole was created in the center of the tibia using an 18-gauge needle. Subsequently, 2 × 106 143B cells suspended in approximately 50 μL of PBS were inoculated into the defected medullary cavity of each female mouse. Once the tumor volume reached approximately 100 mm3, the mice with successfully established tumor models were randomly divided into five groups, with each group consisting of five mice. Subsequently, 100 μL of the BGO-2 hydrogel was administered to the tumor site via injection. On days 1 and 3, the treatment consisted of near-infrared light irradiation (1.0 W/cm2, 5 min) followed by ultrasound radiation (1.0 MHz, 2.0 W/cm2, 5 min, 50 % duty cycle). Weigh the body weight of the nude mice every other day and measure the length (a) and width (b) of the tumor in the mice. Subsequently, calculate the tumor volume (V: mm^3) using the formula: V = (a * b^2)/2. After completion of the experiment, the remaining tumors and important organs such as the heart, liver, spleen, lung, and kidney were collected and preserved in 4 % paraformaldehyde for subsequent histological and micro-CT analyses [42].
Animal procedures involving human-derived samples were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Beijing Jishuitan Hospital and were approved by the Animal Ethics Committee of the same hospital (Grant No. K2022-117-00). Following surgical procedures, fresh tumor tissue was obtained from the patient and subsequently cut into small pieces (approximately 2 mm × 2 mm × 2 mm) within a sterile environment using a Petri dish. The prepared tissue was then transplanted onto the dorsal region of female nude mice using forceps, and the surgical incision was securely closed with absorbable sutures. Upon reaching a tumor volume of approximately 100 mm3, mice that successfully established the tumor model were randomly assigned to 5 groups, each comprising 5 mice. Subsequently, 100 μl of BGO-2 hydrogel was administered to the tumor site via injection. On days 1 and 3, the treatment consisted of near-infrared light irradiation (1.0 W/cm2, 5 min) followed by ultrasound radiation (1.0 MHz, 2.0 W/cm2, 5 min, 50 % duty cycle).
To assess the in vivo biocompatibility of dynamic electrical stimulation, we collected blood from anesthetized mice via the eye and processed it to obtain serum through centrifugation. Subsequently, we analyzed liver and kidney function-related indicators in these serum samples using a biochemical analyzer (BC-5000vet, Mindray, China). Additionally, we assessed the hemocompatibility of piezoelectrically enhanced near-infrared photodynamic therapy following methods outlined in the referenced literature [5]. As controls, we used PBS as the negative control and Triton X-100 solution as the positive control. In brief, we introduced 1 ml of rat whole blood to the samples and incubated them for 2 h. After centrifugation (1000 rpm) at 4 °C for 10 min, we measured absorbance at 540 nm using a microplate reader.
2.10. In vivo bone regeneration of BGO-2 hydrogel in the rat critical-sized calvarial defect model
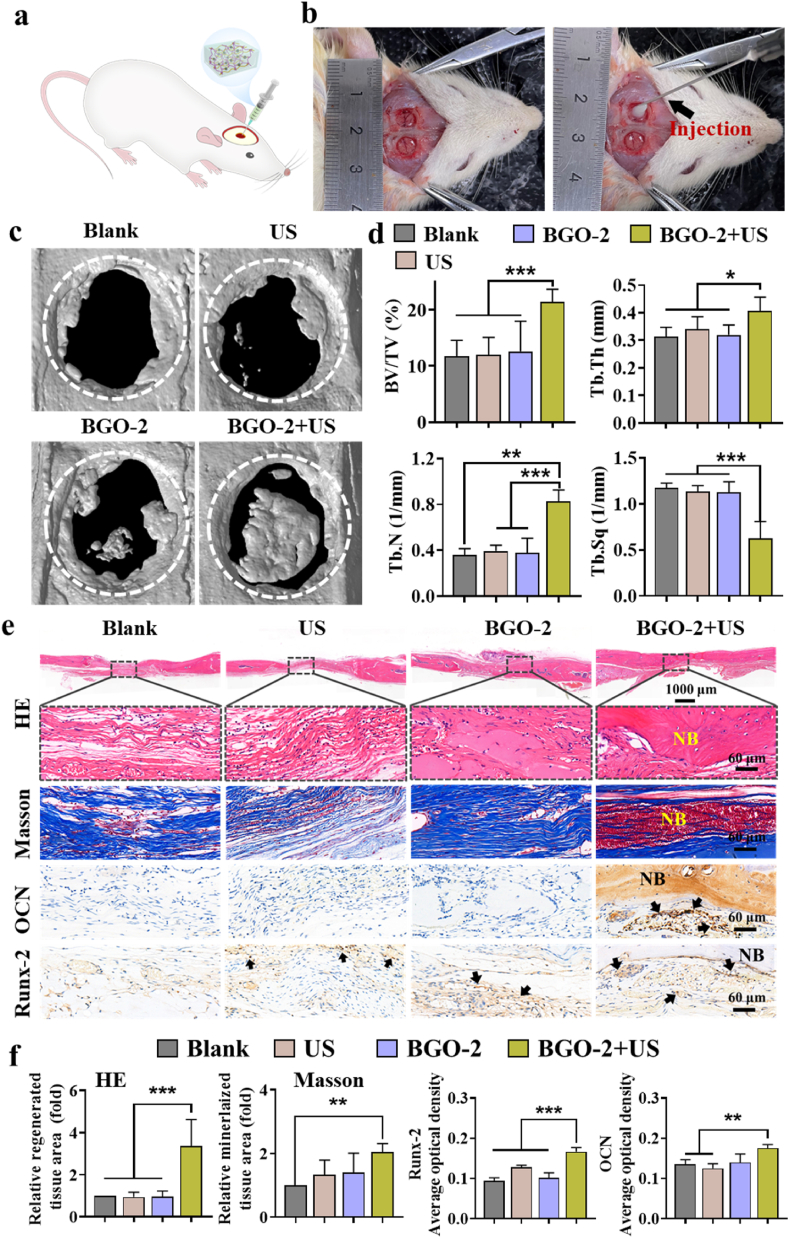
Sixteen Sprague-Dawley (SD) rats aged 6–8 weeks were randomly divided into four groups (n = 3), group Blank, US, BGO-2 and BGO-2+US. The ultrasonic parameters are: 1.0 MHz, 1.0 W/cm2, 5 min, 50 % duty cycle. Under general anesthesia, a critical bone defect area of 5 mm in diameter was drilled on each side of the rat's cranial mid-suture. Rats in Blank group were left untreated, while in the BGO-2 group were injected with BGO-2 hydrogel to fill the defect area, followed by careful suturing of the wound in layers. 8 weeks after implantation, serum was collected from rats for analysis of biochemical parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total protein (TP) to assess the systemic toxicity of the material. Then the rats were euthanized by carbon dioxide asphyxiation and cervical dislocation. The calvarials were completely separated and fixed in 4 % paraformaldehyde (PFA) for further micro-computed tomography (Micro-CT; μCT 100, Scanco Medicine AG, Switzerland) measurements of various bone parameters, including bone volume fraction (BV/TV), bone surfaces (BS), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) to evaluate the in vivo bone regeneration of BGO-2 hydrogel. Calvarial samples were decalcified, gradient dehydrated, and sectioned for H&E staining (C0105S, Beyotime, China), Masson trichrome (MT) staining (G1340, Solarbio, China), and immunohistochemical staining for OCN and Runx2. Major organs such as heart, liver, spleen, lung and kidney were also harvested and fixed in 4 % PFA to observe the accumulation of hydrogel degradation products and pathological changes in the tissues by H&E staining.
2.11. Statistics
SPSS 22.0 statistical software was carried out for statistical analysis, and one-way analysis of variance (ANOVA) followed by Tukey's post hoc test was performed to determine the statistically significant differences (P), with a P-value <0.05 deemed significant.
3. Results and discussion
3.1. Synthesis and characterizations of BST NPs
In order to construct heterojunction materials with coupled piezoelectric effect and SPR effect, Bi/SrTiO3 heterojunction nanoparticles (BST NPs) were synthesized using a two-step procedure (Fig. 1a). Briefly, SrTiO3 nanoparticles (ST NPs) were produced by a hydrothermal reaction. Next, NaBH4 were used as a strong reducing agent to reduce Bi3+ to Bi metal nanoparticles on the surface of ST NPs, resulting in the formation of BST NPs. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images revealed that the ST NPs had a cubic morphology and a smooth surface (Fig. 1b–d). The high-resolution TEM (HRTEM) image showed a lattice spacing of 0.276 nm, corresponding to the (110) plane of tetragonal SrTiO3 (Fig. 1e). Following the interaction with a powerful reducing agent, the surface of BST generated ultra-small particles and became rougher. (Fig. 1f–h). Moreover, the HRTEM image revealed that ultra-small nanoparticles with a size of 3.0 ± 1.2 nm were uniformly distributed on the surface of BST NPs. The measured lattice fringes were 0.319 nm, corresponding to the (012) plane of Bi, respectively (Fig. 1i). Furthermore, the element mapping analysis confirmed that the nanoparticles were mainly composed of Sr, Ti, O, and Bi elements, and the Bi elements were uniformly distributed on the surface of BST particles (Fig. 1j). Furthermore, the element mapping analysis confirmed that the nanoparticles were mainly composed of Sr, Ti, O, and Bi elements, and the Bi elements were uniformly distributed on the surface of BST particles (Fig. S1).
Fig. 1.
Fabrication process and characterizations of BST NPs. (a) The scheme of synthetic procedure of BST NPs. (b) SEM image of ST NPs. (c) and (d) TEM of ST NPs. (e) HRTEM of ST NPs. (f) SEM image of BST NPs. (g) and (h) TEM of BST NPs. (i) HRTEM of BST NPs. (j) XRD patterns of different NPs. (k) Survey XPS, and (l) high-resolution Bi 4f XPS spectra of BST NPs.
The average hydrodynamic diameters of BST and ST are consistent (120 and 123 nm, respectively). However, introduction of Bi increased the zeta potential of BST relative to that of ST (Figs. S2a and S2b). This is primarily because the positive surface of Bi promotes its interaction with ST on the negative surface. X-ray diffraction (XRD) were used to further analyze the crystal structures of the ST and BST NPs (Fig. 1k). The XRD pattern showed that BST is a typical perovskite crystal form, and the (012), (104) and (110) crystal planes of Bi are newly added. The results demonstrated that growing Bi on the surface of ST does not change the perovskite crystal structure of ST, which is consistent with the Raman spectra results (Fig. S2c). To further investigate the chemical composition and valence state of BST NPs, X-ray photoelectron spectroscopy (XPS) was performed. In addition to the four primary peaks of Sr 3d, O 1s, Ti 2p, and C 1s in both ST and BST NPs, the XPS result showed that the BST NPs sample additionally has a distinctive peak with Bi 4f at 161.4 eV (Fig. 1l). Moreover, the high-resolution Bi 4f spectrum of BST showed that the Bi 4f7/2 and Bi 4f5/2 peaks were located at 158.5 and 164.3 eV, respectively, indicating the existence of metallic Bi state. The above results prove that Bi was successfully loaded on the surface of BST, and a nano-ferroelectric heterojunction structure was constructed.
3.2. Piezo-electronic and photo-electronic properties of BST NPs
To investigate the piezoelectric-electronic properties of BST and ST NPs, atomic force microscopy (AFM) was used to examine their ferroelectricity. The butterfly-shaped amplitude curve of the BST nanoparticles demonstrated significant hysteresis and excellent local piezoelectric response (Fig. 2a). Additionally, the phase hysteresis loop indicated that the polarization direction of BST can be phase-switched by 180° (Fig. 2b). These phenomena are consistent with those of ST NPs (Fig. S3), indicating that the heterojunction BST composed of Bi and ST has excellent ferroelectric properties. Based on the tetragonal crystal structure of barium titanate (Fig. 2c), the crystal exhibited spontaneous polarization within a certain temperature range due to Ti ions deviating from the central position of the TiO6 octahedron [43]. When an external mechanical force is applied, the Ti ions move further away from the center of the TiO6 octahedron, resulting in the appearance of positive and negative charges on the two opposite surfaces, forming a piezoelectric potential (Fig. 2c) [44]. The piezoelectric potential changes of BST NPs before and after mechanical stimulation were calculated using finite element simulation in COMSOL software. As shown in Fig. 2f, the potential difference between the upper and lower surfaces of the BST NPs increases to 356 mV after applying 1 × 108 Pa mechanical force, indicating that the BST NPs have excellent mechanical-electrical response characteristics. Furthermore, to detect electrical signals produced by BST nanoparticles in response to ultrasound stimulation, a laboratory-made dynamic ultrasound device and a high-precision general-purpose digital meter (Keithley, DMM7510) were used (Fig. 2d) [45,46]. As shown in Fig. 2e, the electrical signal output of the BST NPs increased with increasing ultrasound intensity. BST can generate a maximum voltage of 25 mV at an ultrasonic power of 2 W/cm2 (Fig. 2e). This suggests that the introduction of Bi metal on the surface of ST to form a heterostructure does not change its piezoelectric-electronic effect. More importantly, this osteogenesis-friendly electrical stimulation is expected to promote bone defect regeneration after bone tumor surgery.
Fig. 2.
Piezoelectrical properties and local surface plasmon resonance (LSPR) of BST NPs. (a) Amplitude butterfly loop, and (b) phase hysteresis loop of BST NPs. (c) Crystal structure changes of BST NPs in response to mechanical stimuli. (d) Schematic diagram of testing mechanical-electrical response characteristics. (e) Open-circuit cyclic voltage output of BST NPs under different ultrasound intensities. (f) Calculation of the piezoelectric potential of BST NPs using finite element simulations under an applied force of 1 × 108 Pa. (g) UV–vis adsorption spectrum, and (h) Kubelka–Munk plots. (i) Current density of ST and BST NPs under NIR stimulation. (j) Current density of BST NPs under US and NIR stimulation. (k) Impedance curves of ST and BST NPs. (l) PL spectrum. (m) Simulated local electric field distribution maps of Bi NPs on the BST NPs.
Due to the half-metal-semiconductor transition effect of Bi NPs, they exhibit SPR properties which means they can have more applications in the near-infrared region [47]. The UV–vis diffuse reflectance spectrum (DRS) confirmed the SPR excitation of Bi NPs, resulting in a new near-infrared absorption band in the range of 808–1200 nm after the loading of Bi NPs onto BST nanocubes. The bandgaps (Eg) of BST NPs and ST NPs wes calculated to be 2.49 and 2.92 eV, respectively, using the Kubelka-Munk curve. The change in the energy band of BST NPs with heterojunction can directly influence the movement of electrons and holes at the interface between Bi metal and piezoelectric ST NPs [48,49].
Electrochemical amperometric measurements were used to investigate the carrier separation efficiency of BST NPs under US or NIR irradiation. Fig. 2i demonstrates that BST NPs generate higher photocurrent intensities in response to NIR irradiation relative to ST NPs. Notably, the current density of the BST electrode under simultaneous light irradiation and ultrasonic vibration was 3.5 A cm−2, which was much higher than the current densities under light and vibration alone (Fig. 2j). Moreover, Nyquist plot of the heterojunction BST NPs showed a smaller semicircle than ST NPs (Fig. 2k). This indicating that the piezoelectric field can enhance the interfacial charge transfer ability generated by the SPR effect. The results of photoluminescence (PL) spectrum are consistent with the above findings. The PL fluorescence intensity of heterojunction BST NPs was lower than that of ST NPs, indicating that electrons and holes at the heterojunction interface were less likely to recombine (Fig. 2l). In order to theoretically prove the existence of the LSPR effect in the BST heterojunction, further electromagnetic simulations were performed on the Bi NPs on the BST NPs surface. As shown in Fig. 2m, Bi NPs can generate a significantly enhanced electric field in response to 808 nm near-infrared light irradiation. The above results confirm that the piezoelectric field of BST NPs can regulate the generation, separation/recombination, and transport of charges and carriers in photoelectric processes.
3.3. Piezo-augmented photodynamic properties of BST NPs
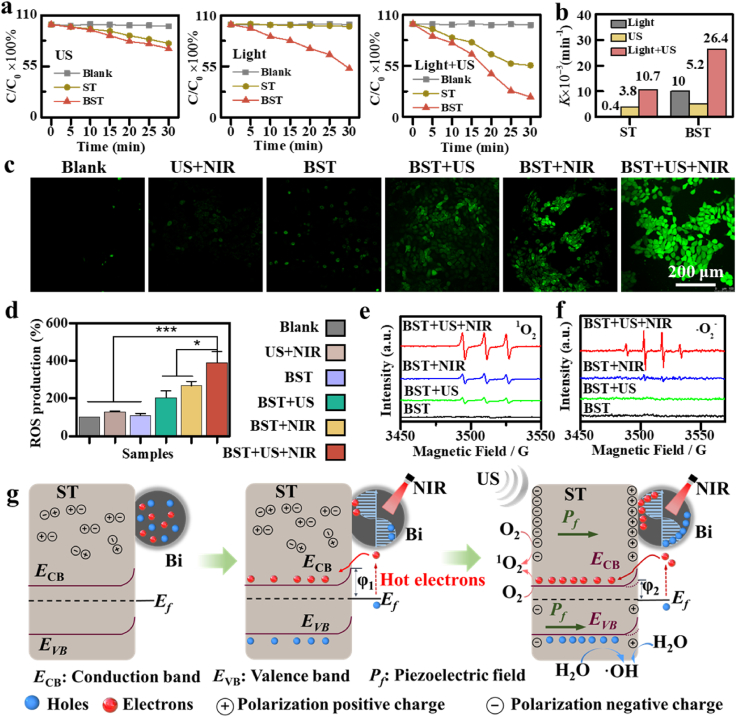
The coupling effect of BST's unique piezoelectric effect and SPR effect was further investigated in the context of the piezo-photocatalytic capacity of BST to degrade methylene blue (MB) dye. As shown in Fig. 3a, the MB degradation ability of BST NPs under NIR irradiation (2.0 W/cm2) is significantly higher than that of bare ST NPs, which is mainly due to the enhanced photocatalytic activity exhibited by the BST heterostructure associated with the SPR effect. Under US irradiation (1.0 MHz, 2.0 W/cm2, 50 % duty cycle) alone, the MB degradation rate of BST NPs was indistinguishable from that of ST NPs, indicating that the electric polarization charges generated in BST crystals under ultrasonic treatment were difficult to transfer to Bi NPs to drive the catalytic reaction. Remarkably, the BST nanoheterostructure exhibited a dye degradation capability of 78.4 % within 0.5 h when subjected to ultrasonic vibration and near-infrared radiation. Additionally, the corresponding first-order kinetic rate constants (k) results showed that, compared with pure ST NPs or a single external field response, BST NPs with heterojunction respond to ultrasound and near-infrared light irradiation at the same time, enhancing its ability to degrade dyes (Fig. 3b). The above results confirmed that the BST NPs with piezoelectric effect coupled with SPR effect exhibited efficient dye degradation function under ultrasonic and near-infrared irradiation.
Fig. 3.
Piezo-augmented photocatalytic properties of BST NPs. (a) Degradation curves of methylene blue. (b) First-order rate constant K for methylene blue dye degradation. (c) Intracellular ROS levels treated with BST NPs under different conditions. (d) Intracellular ROS level after different treatments. ESR spectra of 1O2 (e) and •OH (f). (g) A schematic illustration of the piezo-augmented photocatalytic process in the BST heterostructure. The data are presented as mean ± SD, and statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
To confirm the effect of BST NPs on intracellular oxidative stress, the reactive oxygen species (ROS) probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was utilized. US parameters were set at 1.0 MHz, 2.0 W/cm2, 50 % duty cycle, and 5 min, while NIR parameters were set at 1.0 W/cm2 and 5 min. Compared to the Blank group, the BST+US group and the BST+NIR group exhibited only a slight increase in intracellular ROS fluorescence, while the highest ROS fluorescence intensity was observed in the BST+US+NIR group (Fig. 3c). Compared with the Blank group (defined as 1.0), the fluorescence intensity of the BST+US, BST+NIR, and BST+US+NIR groups increased approximately 2.0, 2.6, and 3.9 times, respectively. These findings confirm that the combination of piezoelectric and SPR effects generates a large number of ROS (Fig. 3d).
To further verify the exact species of oxygen radicals produced in the piezo enhanced photodynamic therapy, 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethylpiperidine (TEMP) were used to capture •OH and 1O2 produced by BST, respectively (Fig. 3e and f). The electron spin resonance (ESR) results showed that both the BST+US group and the BST+NIR group exhibited weaker 1O2 signal peaks and almost no •OH signal peaks. Notably, the •OH and 1O2 signal intensities were greatest in the BST+US+NIR group. In summary, heterojunction BST NPs generate a large amount of generated •OH and 1O2 by virtue of the piezoelectric-enhanced LSPR effect, which is promising for local tumor therapy.
The enhanced piezoelectric-photocatalytic performance of heterojunction BST NPs is explained in detail through energy band diagrams (Fig. 3g). Based on SEM and TEM images, it is evident that Bi NPs are partially embedded in ST nanocubes, forming a BST heterostructure, as shown in the left panel of Fig. 3g. When NIR light illuminates BST (middle panel of Fig. 3g), the surface electrons of Bi NPs exhibit collective oscillation (i.e., LSPR) and decay into high-energy hot electrons. The generated hot electrons overcome the schottky barrier and transfer to the conduction band of ST. The remaining holes are captured by the solution's donors, initiating the oxidation reaction. When ultrasonic vibrations apply external stress, a built-in piezoelectric field (Pf) generates inside the piezoelectric ST NPs. The built-in piezoelectric field bends the conduction and valence bands downward, making BST more conducive to generating reactive oxygen species. Importantly, the reduction of the Schottky barrier (φ2) effectively suppresses SPR-induced high-energy hot-carrier recombination, leading to more free radical generation. Therefore, the induction of a built-in piezoelectric electric field in ST nanocrystals by ultrasonic treatment promotes charge carrier migration and inhibits photogenerated carrier recombination, enhancing ROS production. Piezo-Augmented photodynamic is expected to be useful for efficient local cancer treatment.
3.4. Preparation and characterization of BST/gelatin/OCS nanocomposite Hydrogel (BGO Hydrogel)
After bone tumor surgery, large bone defects and residual tumor cells are often present [50], making the construction of an injectable hydrogel that responds to the tumor microenvironment and delivers BST NPs an ideal method for bone tumor treatment. However, physically mixing BST NPs into hydrogels can degrade the stability of nanocomposite hydrogels. To address this issue, amino groups were modified on the surface of BST NPs using silane coupling agent modification (KH550) to obtain surface-functionalized nanoparticles, which were characterized by Fourier transform infrared (FT-IR) spectroscopy (Fig. S5a). The FT-IR Spectrometer showed that NH2-BST had newly added stretching vibration peaks of Si-O bonds, -NH2 bending vibration peaks and -CH3 stretching vibration absorption peaks at 1119 cm−1, 1570 cm−1 and 2923 cm−1, respectively, proving that the BST surface had successfully bonded the branched amino group. Additionally, the polymer employed in this study was composed of oxidized chondroitin sulfate (OCS) and gelatin which are polysaccharide naturally found in bone extracellular matrix, have been used as hydrogel scaffolds due to their biocompatibility, biodegradability, and bio-functionality [51,52]. To treat residual tumor cells after bone tumor surgery and promote bone regeneration, an injectable self-healing nanocomposite hydrogel was rationally prepared as a therapeutic platform for osteosarcoma (Fig. 4a). Strikingly, it was observed that OCS and gelatin do not form a gel at 37 °C (Fig. S4). However, when a 5 % (w/v) solution of NH2-BST was added to the OCS/gelatin mixture, it rapidly formed a hydrogel at 37 °C (Fig. S4). This phenomenon can be attributed to the dynamic Schiff base bond cross-linking between the -NH2 groups of NH2-BST and gelatin, and the -COH groups of OCS. In this experiment, nanocomposite hydrogels with different contents of BST were successfully prepared, named BGO-1 (1 % w/v), BGO-2 (5 % w/v) and BGO-3 (10 % w/v). The microstructure of BGO hydrogel was further investigated by SEM (Fig. 4a and S6). The lyophilized BGO hydrogel exhibited a porous structure, and BST NPs were observed on the pore walls. Moreover, the pore diameter decreased with the increase of BST intercalation (Fig. 4b and c and S6). This was mostly due to the amino-functionalized BST increasing the crosslink density of the BGO hydrogel network. The 3D laser confocal microscopy images of the BGO-2 hydrogel also showed that the BST nanoparticles (red fluorescence) were uniformly distributed inside the hydrogel (Fig. 4d). The effective fabrication of BGO hydrogels was examined further using FT-IR (Fig. S5b). The characteristic peaks appeared and disappeared at 1648 cm−1 and 1717 cm−1 in BGO-2 hydrogel, respectively, indicating the formation of Schiff base bonds. Meanwhile, the band at 560 cm−1 was attributed to the Ti-O stretching vibration of BST nanoparticles, indicating that BST was successfully embedded in BGO-2 hydrogel.
Fig. 4.
Physical Characterization of BGO Hydrogels. (a) Schematic diagram of the preparation of BGO hydrogel. (b) SEM images of BGO-2 hydrogels. (c) Pore diameter of BGO hydrogels with different contents of incorporated BST NPs. (d) Localization of BST nanoparticles (red fluorescence) in BGO-2 hydrogels using CLSM. (e) The average storage modulus of BGO hydrogels at 37 °C at 1 Hz (n = 3). (f) Gel time of BGO hydrogel (n = 3). (g) Shear-thinning behavior of BGO-2 hydrogels. (h) The strain amplitude sweep test of BGO-2 hydrogel. (i) Image of the macroscopic healing capacities of BGO-2 hydrogel. (j) Photocurrent of BGO hydrogel with different contents of incorporated BST. (k) ROS levels of BGO hydrogels with different BST contents. (l) ROS levels of BGO-2 hydrogels under different treatments. (m) Schematic illustration of BGO-2 hydrogels generating reactive oxygen species under ultrasound and near-infrared stimulation. The data are presented as mean ± SD, and statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
The mechanical properties of hydrogels can have an impact on cell behavior [53]. According to the rheological results, the storage modulus (G′) and loss modulus (G") of the hydrogels were found to enhance the increasing concentration of doped BST, with G′ being superior than G" throughout the concentration range (Fig. 4e and S7b). This suggests that the BGO nanocomposite hydrogel, with different BST contents incorporated, consistently behaves as a stable elastic solid. However, a longer gelation time can be unfavorable for surgical treatment, while a gel that sets too quickly may not be able to cover the damaged tissue effectively, which could impact the effectiveness of the treatment [54]. Therefore, it is crucial to determine an appropriate gelation time for the treatment of bone defects after bone tumor surgery. To this end, a time-sweep rheological investigation was carried out at a physiological temperature of 37 °C. The time point at which the loss modulus (G") value crosses the storage modulus (G′) is the gelation time of the BGO hydrogel. As shown in Fig. 4f and S7a, the gelation times of BGO-1 BGO-2 and BGO-3 were 220, 182 and 174 s, respectively. This confirms that the increase of BST concentration significantly reduces the gelation time of BGO nanocomposite hydrogels, indicating that BST works as a crosslinking site in the crosslinking reaction of hydrogels. Furthermore, the change in hydrogel viscosity with shear rate at 37 °C was analyzed, and it was found that the viscosity of the BGO-2 hydrogel decreases with the increasing of the shear rate, allowing the hydrogel to be continuously injected into PBS by a syringe while maintaining the gel state (Fig. 4g). This property enables the BGO-2 hydrogel to be precisely injected into the bone defect after bone tumor surgery using minimally invasive surgery. The self-healing properties of hydrogels are also crucial in matching complex morphological bone defects after bone tumor surgery. The Schiff base bonds between the amino-functionalized BST and the gelatin and OCS network (as shown in Fig. 4h and i) endow hydrogels with dynamic properties, resulting in both G′ and G" values of the BGO hydrogel increasing with strain ranging from 0.1 % to 1000 %, indicating the typical self-healing behavior of hydrogels. The self-healing ability of the BGO-2 hydrogel was further evaluated by a macroscopic healing test (Fig. 4i), where two disc-shaped hydrogels were cut and placed at 37 °C and then fully healed without any external intervention. Additionally, the healing hydrogels maintain their integrity under mechanical action. The injectable and self-healing properties of BGO-2 hydrogel make it a promising candidate for local bone tumor treatment and bone defect regeneration.
The degradation ability of hydrogels is related to the biological performance [55,56]. Hydrogel dry weight changes were further investigated under physiological conditions (i.e., in phosphate-buffered saline at 37 °C) (Fig. S8a). The results showed that BGO-1 was completely degraded in less than 3 days, while the degraded mass percentages of GO-2 and BGO-3 were close to 51.2 % and 49.9 %, respectively. In addition, the release profile of BST NPs from BGO-2 hydrogel was further investigated. As anticipated, BGO-2 hydrogels exhibited an accelerated release of BST NPs in response to acidic solutions (pH = 6.7, 5.0) compared to those in PBS solutions (pH = 7.4). Specifically, approximately 20 % of BST NPs were released within three days in PBS at pH 7.4, while 45.6 % and 59.1 % were released at pH = 6.7 and 5.0, respectively (Fig. S8b). The mechanism of the release kinetics can be attributed to the cleavage of the dynamic Schiff base bonds in the BGO-2 hydrogel under acidic conditions, leading to the disintegration of the three-dimensional structure of the hydrogel and consequently, the release of BST NPs encapsulated in the BGO-2 hydrogel network [57]. Subsequently, the in vivo degradation properties of BGO-2 nanocomposite hydrogels were further studied. The BGO-2 nanocomposite hydrogel was subcutaneously implanted on the dorsal region of rats. The nanocomposite hydrogel possesses dimensions of 1 cm in diameter and 0.2 cm in thickness. To assess the in vivo degradation of the BGO-2 hydrogel, tissue excision was conducted at the implantation site after intervals of 3, 5, and 7 days. Macroscopic images indicated that the BGO-2 hydrogel mass in vivo was significantly reduced on day 5 and completely degraded by day 7, relative to its mass on day 3 (Fig. S9a). H&E staining of the implantation site revealed a distinct boundary between the tissue and BGO-2 on days 3 and 5, while the BGO-2 hydrogel was completely absent by day 7 (Fig. S9b). These results on hydrogel degradation are consistent with the mechanical properties of the hydrogel, which can be mainly attributed to the enhancement of crosslink density by NH2-BST as a crosslink site.
Benefiting from the excellent piezoelectric-enhanced photogenerated carrier properties of the heterojunction BST NPs, we further investigated the photoelectrochemical properties of BGO nanocomposite hydrogels. The results presented in Fig. 4j, illustrate that the current density of BGO hydrogels increases the concentration of intercalated BST nanoparticles, which is consistent with the trend of reactive oxygen species (ROS) concentration produced by BGO nanocomposite hydrogels under ultrasonic and near-infrared responses (Fig. 4k). Specifically, the concentration of ROS produced by BGO nanocomposite hydrogels increases with the concentration of doped BST nanoparticles. Moreover, the results in Fig. 4l further support these findings by demonstrating that, after 10 min of ultrasound and light irradiation, the concentration of ROS produced by the BGO-2+US+NIR group was 2.7 times and 1.9 times higher than that of the BGO-2+US group and the BGO-2+NIR group, respectively. Taken together, these results indicate that the BGO hydrogel embedded with BST NPs not only exhibits excellent ROS generation ability, but also possesses injectable and self-healing properties, making it a promising candidate for local treatment of osteosarcoma and bone defect regeneration (Fig. 4m).
Fig. S10 depicts the temperature changes of BGO-2 hydrogels subjected to sequential ultrasound (US) and near-infrared (NIR) treatments. The temperature changes observed in the BGO+US group were consistent with those in the BLANK group and BGO-2 group, suggesting that US treatment did not induce temperature changes in the BGO-2 hydrogel. In the US+NIR group, the temperature increased by approximately 5.9 °C (from 26.3 °C to 32.2 °C). Notably, the temperatures of the BGO-2+NIR group and BGO-2+NIR+US group increased by 12.8 °C and 12 °C, respectively (changing from 26.4 °C to 39.2 °C and 26.8 °C–38.8 °C, respectively). This result substantiates that BGO-2 exhibits a responsive behavior to near-infrared light with a comparatively smaller temperature increase. Furthermore, we monitored in vivo temperature changes following BGO-2 hydrogel treatment (Fig. S10). Real-time temperature monitoring revealed that the local temperature increased from 31.2 to 37.0 °C after ultrasound and near-infrared treatment alone. Moreover, in the BGO-2+US+NIR group, the color of the treated area shifted from dark blue to yellow, accompanied by a corresponding temperature increase of 8.1 °C (from 30.8 °C to 38.9 °C). Combining the results of in vitro and in vivo experiments, it can be inferred that BGO-2 treated with ultrasound and NIR induces a modest temperature increase. Literature reports indicate that such a small temperature elevation lacks anticancer effects and does not result in tissue damage [58,59].
3.5. In vitro piezo-augmented photodynamic therapy
Encouraged by the efficient ROS generation capability of BGO-2 hydrogels, the anti-osteosarcoma cell performance of BGO-2 under US and NIR irradiation was assessed. Ultrasound therapy parameters used were: 1.0 MHz, 2.0 W/cm2, 50 % duty cycle, and 5 min of treatment. Additionally, the parameters of the NIR light were: 1.0 W/cm2, 5 min. In order to verify the spectral anti-osteosarcoma properties of BGO-2 hydrogel, three different osteosarcoma cells, Saos-2, 143B and UMR106 cell, were selected. As shown in Fig. 5a, neither the US+NIR nor the BGO group showed cytotoxicity in the three different cells. In addition, the BGO-2 hydrogel exhibited limited cytotoxicity against three different tumor cells when stimulated with ultrasound or NIR, indicating the poor effectiveness of piezodynamic and photodynamic treatment alone. Of note, under US and NIR irradiation, the cytotoxicity of BGO-2 hydrogel towards Saos-2, 143B, and UMR106 cells was 83.7 %, 82.3 %, and 85.9 %, respectively. This demonstrates that the piezo-augmented photodynamic therapy of BGO-2 hydrogels has a more significant anticancer effect than single-mode piezodynamic therapy or photodynamic therapy. The therapeutic efficacy was further assessed by calcein-AM and propidium iodide (PI) double staining (Fig. 5b). Distinguished from the Blank/US+NIR/BGO-2 groups with no significant decrease in cell viability, some tumor cells died (red fluorescence) in the BGO-2+US group and BGO-2+NIR group. As expected, almost all tumor cells died in the BGO-2+US+NIR group. Further, cell death pathways were investigated by fluorescent staining after co-staining with Hoechst and PI. The apoptosis staining images showed that the number of apoptotic cells increased with the addition of piezodynamic therapy and photodynamic therapy (Fig. 5c). More apoptotic cancer cells could be found on the surface of BGO-2+US+NIR group. This suggests that the piezo-augmented photodynamic therapy promotes apoptosis in cancer cells.
Fig. 5.
Potential antitumor effects of BGO-2 hydrogels under ultrasound and near-infrared irradiation in vitro. After incubating the cells on the hydrogel surface for 24 h, they underwent treatment with ultrasound and near-infrared irradiation. (a) Relative cell viability of Saos-2, UMR016, and 143B cancer cells after different treatments (n = 3). (b) Calcein-AM (green) and PI (red) co-staining images in 143B cells after different treatments. (c) Cell apoptosis staining of 143B cells after different treatments. Green fluorescence and red fluorescence represent living 143B cells and apoptotic cells, respectively. (d) Intracellular ROS level after different treatments. (e) Mitochondrial membrane potential measured by JC-1 staining after different treatments. (f) Quantitative analysis of intracellular ROS fluorescence using Image J software (n = 3). (g) Quantitative analysis of JC-1 monomer fluorescence using Image J software (n = 3). (h) Intracellular ATP level after different treatments (n = 3). The data are presented as mean ± SD, and statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
To investigate the cell killing mechanism of piezo-enhanced photodynamic therapy, intracellular ROS levels were detected (Fig. 5d). Compared with the US+NIR and BGO-2 groups, the BGO-2+US and BGO-2+NIR groups had increased intracellular ROS fluorescence. The fluorescence intensities of BGO-2+US and BGO-2+NIR groups were 2.0 times and 3.3 times that of Blank group, respectively (Fig. 5f). Notably, the BGO-2+US+NIR group had the highest ROS fluorescence intensity, which was 9.1, 3.4, and 2.1 times that of the Blank, BGO-2+US, and BGO-2+NIR groups, respectively. The experimental results of ROS in 143B cells were similar with those of BGO-2 hydrogel materials (Fig. 4k). This was mainly attributed to the effects of piezo-augmented photodynamic therapy of BGO-2 hydrogels.
Excessive intracellular ROS levels can induce cancer cell death by impairing mitochondrial respiration [60,61]. To assess mitochondrial function, changes in membrane potential were measured using JC-1 dye as a fluorescent indicator. In normal mitochondrial membranes, JC-1 dye forms aggregates with green fluorescence. Conversely, in depolarized and damaged mitochondrial membranes, JC-1 dye exists as a monomer and exhibits red fluorescence [62,63]. As shown in Fig. 5e and g, strong green fluorescence was observed in the blank group, US+NIR and BGO-2 groups, indicating that the mitochondrial function of these cells was intact. While cells treated with BGO-2+US or BGO-2+NIR exhibited only a modest increase in red fluorescence, the Piezo-augmented photodynamic therapy generated by BGO-2+US+NIR group induced the highest level of red fluorescence, signifying that the persistent and ample generation of ROS has a significant impact on cellular mitochondrial respiration. The state of the mitochondria often determines the intracellular adenosine triphosphate (ATP) content, which serves as a direct energy source for cells and affects the survival status of cancer cells [64]. Clearly, The BGO-2+US+NIR group had the lowest concentration of intracellular ATP, which was 0.08 times, 0.23 times, and 0.28 times that of the Blank, BGO-2+US, and BGO-2+NIR groups, respectively. The above results confirm that BGO-2 hydrogel can efficiently induce tumor cell death by virtue of piezo-augmented photodynamic therapy to generate reactive oxygen species under ultrasound and near-infrared light irradiation. This is expected to be used in the local treatment of osteosarcoma. Due to the physiological complexity of tumors, single therapies often fall short of achieving satisfactory results. The introduction of combination therapies can target various mechanisms and inhibit different pathways, thereby enhancing anticancer efficacy. Piezoelectrically enhanced photodynamic therapy holds promise for producing superior therapeutic effects by leveraging the combined benefits of the piezoelectric effect and plasmon resonance effect. Moreover, this approach allows for the reduction of ultrasound/light energy and sensitizer dosage, contributing to the mitigation of toxicity and side effects.
3.6. In vivo therapeutic efficacy in mice with different tumor models
Thanks to the excellent in vitro antitumor properties of BGO-2 hydrogel, this encourages further evaluation and understanding of its in vivo therapeutic effects. Notably, the combination of ultrasonic and near-infrared therapies (US+NIR group) exhibited no cytotoxicity in vitro cell studies, and most of the similar treatments described in the literature have shown no significant in vivo antitumor effects [65,66]. Therefore, in this in vivo tumor treatment experiment, tumor-bearing nude mice were randomly divided into 5 groups (n = 5): (1) Blank group (inject normal saline); (2) BGO-2 group; (3) BGO-2+US group; (4) BGO-2+NIR group; (5) BGO-2+US+NIR group. The in vivo antitumor activity of piezo-enhanced photodynamic therapy was evaluated following the treatment cycles shown in Fig. 6a. In in vivo anti-tumor experiments, the drug was administered when the tumor volume reached approximately 100 mm^3. The mice were then subjected to ultrasound and near-infrared light treatments every other day throughout the 18-day treatment period. The mice received ultrasound radiation (1.0 MHz, 2.0 W/cm2, 5 min, 50 % duty cycle) and near-infrared light irradiation (808 nm, 1.0 W/cm2, 5 min). It is noteworthy that the drug only required a single administration for the entire treatment duration.
Fig. 6.
In vivo osteosarcoma therapy of different tumor models using BGO-2 hydrogels. (a) Schematic illustration of piezo-augmented photodynamic therapy in vivo. (b) Schema of the PDX tumor model in mice. (c) average tumor growth curves of the PDX tumor model mice (n = 3). (d) Time dependent body weights of the PDX tumor model mice (n = 3). (e) Average tumor weight of the PDX tumor model (n = 3). (f) Microscopy images of H&E stained PDX tumor slices. (g) Schematic of the tibia osteosarcoma model in mice. (h) Average tumor growth curves of the tibia osteosarcoma model mice (n = 3). (i) Time dependent body weights of the tibia osteosarcoma model mice (n = 3). (j) Average tumor weight of the tibia osteosarcoma model mice (n = 3). (k) Microscopy images of H&E stained tibia osteosarcoma slices. The data are presented as mean ± SD, and statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
Human osteosarcoma tissue was utilized to develop patient-derived xenograft (PDX) tumor models in immunodeficient mice. These models were utilized to assess the effectiveness of piezo-enhanced photodynamic therapy induced by BGO-2 hydrogel (Fig. 6b). Based on the tumor growth curves during treatment (Fig. 6c and S10), there was no significant difference in tumor growth between the BGO-2 group and the Blank group, indicating a lack of therapeutic effect. In addition, the tumors of mice in the BGO-2+US group were moderately suppressed (mean tumor volume was 666.1 mm3), which was mainly attributed to the piezodynamic therapy of BGO-2 response to US. Although BGO-2+NIR treated with photodynamic therapy showed better tumor suppression effect (mean tumor volume was 12.5 mm3), there was still one mouse with incomplete tumor elimination (Figs. S10 and S11). Notably, BGO-2+US+NIR group exhibited the highest tumor suppressive effect, and almost all tumors in mice were eliminated (Figs. S12 and S13). At the end of treatment, the estimated tumor inhibition rate in the BGO-2+US+NIR group was 98.6 % (Fig. 6e), highlighting the potential of piezo-augmented photodynamic therapy in osteosarcoma treatment. The highly effective antitumor properties of BGO-2+US+NIR were further confirmed by the pathological hematoxylin and eosin (H&E) staining of tumor tissues (Fig. 6f). Fig. S14 further shows a substantial presence of BST nanoparticles (depicted in black) in the BGO-2+US+NIR group. These results suggest that although the hydrogel underwent complete degradation by day 7 (Fig. S9), the released BST NPs persisted within the tumor tissue. Importantly, these BST NPs exhibited outstanding in vivo anti-tumor properties in response to ultrasound and near-infrared light stimulation. Biosafety assessment results indicated that all groups exhibited insignificant fluctuations in mice body weight during treatment (Fig. 6d). After treatment with piezo-augmented photodynamic therapy, the BGO-2+US+NIR group exhibited the largest region of tumor necrosis in the residual tissue.
Furthermore, the effectiveness of our approach in animal tumor models that are more clinically relevant was also assessed. A highly metastatic orthotopic osteosarcoma model was established by inoculating 2 million osteosarcoma cells (143B cells) on the right tibial plateau of each nude mouse (Fig. 6g). The body weight of the mice did not decrease considerably throughout the treatment of tibial osteosarcoma (Fig. 6i), indicating that the piezo-augmented photodynamic therapy based on BGO-2 hydrogel has good safety, which is consistent with the in vivo safety results of the PDX model (Fig. 6d). Locally injected BGO-2 hydrogel could effectively and continuously inhibit tumor growth under synergistic NIR and US stimulation by virtue of its excellent piezo-augmented photodynamic therapy ability, while the tumors of the Blank group or BGO-2 hydrogel alone showed rapid growth (Fig. 6h–S15 and S16). Although completely eliminating the tibial bone tumors in situ is challenging, the tumor inhibition rate in the BGO-2+US+NIR group was as high as 67.6 % compared to the control group (Fig. 6j). The results of H&E staining further revealed that the tumor tissue treated with BGO-2+US+NIR exhibited substantial histological damage and cell necrosis (Fig. 6k). As in the treatment of the PDX model, we also observed the presence of BST NPs in the tumor tissue of tibial osteosarcoma mice (Fig. S17). Furthermore, lung histology in the mice from the BGO-2 and blank groups revealed a small osteosarcoma metastasis, but there was none in the mice from the BGO-2+US+NIR group (Fig. S18). The presented results above confirm that BGO-2+US+NIR exhibits excellent anti-osteosarcoma efficacy and high biological safety in both PDX and highly metastatic tibial osteosarcoma models, due to its piezo-enhanced photodynamic therapy.
In vivo biosafety is as important as tumor therapy [67,68]. The (H&E) staining results of the mice's major organs further revealed that there were no inflammatory lesions and no abnormal pathological changes after treatment in all groups (Fig. S18). The blood biochemical analysis and hemolysis experiments indicated the good biocompatibility and biosafety of BGO-2+US+NIR with piezo-augmented photodynamic therapy. The results showed similar outcomes between the BGO-2+US+NIR group and the Blank treatment group, with no statistically significant difference (Fig. S19). Furthermore, all treatment groups exhibited good hemocompatibility in the hemolysis experiments (Fig. S20). Taken together, these findings suggest that BGO-2+US+NIR is a highly biocompatible and safe strategy for antitumor therapy across different tumor models.
3.7. Intracellular electrical stimulation promotes osteogenic differentiation of BMSCs
For the postoperative treatment of bone malignancies, bone substitute materials need to possess not only outstanding anti-tumor characteristics, but also osteogenic properties that facilitate bone defect healing. Thanks to the suitable degradation properties of BGO-2 hydrogel, it is expected to promote osteogenic differentiation through piezoelectric stimulation, utilizing the exposed BST nanoparticles in response to ultrasound stimulation during the later stages of treatment. To evaluate the cytotoxicity of BST NPs, rat bone marrow mesenchymal stem cells (BMSCs) were co-cultured with nanoparticles at different concentrations from 0 to 1000 μg/ml for a predetermined time. As illustrated in Fig. S21b, the optical density (OD) values of BST nanoparticles within the concentration range of 50–1000 μg/ml did not exhibit a significant difference from those of the blank group. Fluorescent images of cell live/dead staining further revealed negligible dead cells in all groups, where green fluorescence indicated live cells and red fluorescence indicated dead cells (Fig. S21a). After ultrasonic treatment with different powers, the absorbance of CCK-8 was recorded. Fig. S22a showed the percentage of cell viability compared to blank samples. The results confirmed that, for BST NPs at a concentration of 100 μg/ml, they exhibited excellent biocompatibility at ultrasonic intensity below 1.0 w/cm2, while at an ultrasonic stimulation with an acoustic intensity of 2w/cm2, BST NPs (100 μg/ml) exhibited 79 % cell viability. In BST NPs at a concentration of 500 μg/ml, cell viability decreased with increasing ultrasound intensity. Therefore, unlike the ultrasonic parameters (2 W/cm2) utilized in piezo-enhanced photodynamic therapy, we opted for more moderate ultrasonic parameters (1 W/cm2) to activate BST NPs (100 μg/ml) and generate piezoelectric stimulation for the investigation of osteogenic differentiation. In addition, Fig. S22b demonstrated that the cell viability of BST+US group, BST+NIR group and BST+US+NIR group were 93 %, 75 %, 30 %, respectively. The fluorescent images of cell live/dead staining further revealed that the BST+NIR group and the BST+US+NIR group exhibited a significant number of dead cells (red fluorescence), whereas the BST+US group was consistent with the blank group, demonstrating a significant number of live cells (green fluorescence) (Fig. S22c). This conclusion may be attributed to the higher levels of ROS production in the BST+NIR and BST+US+NIR groups compared to the BST+US group, which potentially affected the cell viability of BMSCs.
To examine the distribution of BST NPs on BMSCs, the endocytosis of BST NPs by cells at various periods was characterized by TEM (Fig. 7a). After 1 h of co-culture, most of the BST NPs appeared on the edge of the cell membrane, which seemed to be preparing for entry into the cell. After 12 h of co-culture, almost all BST NPs were endocytosed by cells, creating the ability for BST to perform electrical stimulation within cells in response to ultrasound. Subsequently, the effect of intracellular electrical stimulation generated by BST under US on the morphology of adherent BMSCs was also investigated. The study of cytoskeleton staining (FITC-phalloidin/DAPI) showed that after 1 day of culture, BMSCs in the blank group exhibited a smaller cell spreading morphology. In contrast, the BMSCs of the BST+US group exhibited a polygonal shape (Fig. 7b and S19).
Fig. 7.
In vitro osteogenic differentiation of BMSCs via intracellular electrical stimulation. (a) TEM pictures of cellular uptake of BST NPs at 1 h and 12 h. (b) Fluorescence images of BMSCs were stained with the cytoskeleton (green) and nucleus (blue). (c) ALP staining of BMSCs after differential treatment for 7 days. (d) Quantitative analysis of ALP staining. (e) The expression levels of osteogenic genes (ALP, Runx-2, OPN, and OCN) in BMSCs. (f) Immunofluorescent staining of the osteogenesis-related protein Runx-2 (red) and nuclei (blue) of BMSCs. (g) Immunofluorescent staining of the OCN (red) and nuclei (blue) of BMSCs. (h) Fluorescence images depicting cytosolic Ca2+ concentrations via Fluo 4-AM staining after different treatments. (i) Quantitative analysis of fluorescence intensity of Runx-2 (n = 3). (j) Quantitative analysis of fluorescence intensity of OCN (n = 3). (k) Quantitative analysis of fluorescence intensity of calcium ions (n = 3). (l) Western blot assay of the phosphorylation levels of PI3K and AKT in BMSCs. (m) Schematic illustration of the molecular mechanism of intracellular electrical stimulation facilitating osteogenesis. The data are presented as mean ± SD, and statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
To evaluate the effect of intracellular electrical stimulation induced by BST under US irradiation on the osteogenic differentiation of BMSCs, the expression levels of three osteogenesis-related proteins, namely alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx-2), and osteopontin (OPN), were analyzed to assess the osteogenic differentiation efficiency. ALP staining results demonstrated that significantly higher ALP activity was detected in the BST+US group compared with the Blank and BST groups (Fig. 7c). Quantitative analysis of ALP staining confirmed that ALP activity in BST+US was 2.3, 2.4, and 1.46 times higher than in blank, US, and BST groups, respectively (Fig. 7d). These results suggest that the intracellular electrical stimulation generated by BST NPs under ultrasound can induce rapid early osteogenic differentiation of BMSCs. The expressions of other osteogenesis-related proteins, including Runx-2 and OPN, exhibited similar trends to the ALP protein level. The immunofluorescence images of Runx-2 in Fig. 7f showed that the red fluorescence was the strongest in the BST+US group, and most of the red fluorescence was distributed in the nucleus. In addition, the fluorescence intensity of Runx-2 in the BST+US group was 2.1, 1.9 and 2.3 times higher than that of Blank, US and BST, respectively. OPN immunofluorescence pictures also confirmed that the BST+US group exhibited the strongest red fluorescence, indicating that intracellular electrical stimulation most effectively promoted osteogenic differentiation (Fig. 7g and j). In addition to the previously mentioned osteogenesis-related proteins, the expression levels of osteogenesis-related genes were also assessed, including Alp, Runx-2, OCN, and OPN (Fig. 7e), to investigate the impact of intracellular electrical stimulation generated by BST under US irradiation on the osteogenic differentiation of BMSCs. Results showed that the expression of osteogenesis-related genes on day 7 was significantly higher in BST+US-treated cells compared to other groups, indicating the effectiveness of intracellular electrical stimulation in enhancing the osteogenic differentiation of BMSCs. Overall, these results demonstrat that endocytosed BST NPs create intracellular electrical stimulation in response to ultrasound, promoting cell spreading and osteogenic differentiation.
Bioelectrical signals consisting of changes in ionic currents or transitions in cell membrane potential play a crucial role in regulating cell differentiation, proliferation, and migration [69]. To evaluate the effect of BST NPs on intracellular bioelectrical signals in response to ultrasound, changes in intracellular calcium ions were investigated. As expected, cells treated with BST and exposed to US stimulation exhibited the highest intracellular calcium ion concentration (the strongest green fluorescence), which was 1.9 times that of the blank group (Fig. 7h and k). The results of the study suggested that the endocytosis of BST NPs has minimal impact on cellular bioelectrical signals. However, when ultrasonic cavitation-driven BST NPs were utilized for intracellular electrical stimulation, they could cause an elevation in intracellular calcium ion concentration.
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway is frequently associated with changes in cytoplasmic Ca2+ concentration [70]. The aforementioned pathway is involved in diverse signal transduction processes, such as cell proliferation, differentiation, and apoptosis [71]. Furthermore, it plays a crucial role in bone metabolism, promoting osteogenic differentiation and regulating bone regeneration, thus aiding in the prevention of osteoporosis [72,73]. The results of quantitative RT-PCR analysis were consistent with the findings mentioned above (Fig. S23). The gene expression levels of PI3K and AKT were upregulated when BST NPs were stimulated with US, providing further evidence of the potential of intracellular electrical stimulation to promote osteogenic differentiation. Furthermore, protein phosphorylation levels in the PI3K/AKT pathway were analyzed using Western blotting (Fig. 7l). The results revealed that the expression levels of p-PI3K and p-AKT proteins in the BST+US group were significantly higher than those in the other three groups, although there was no notable difference in PI3K and AKT proteins.
Combining intracellular calcium ions and PI3K/AKT pathway results, we summarized the mechanism by which intracellular electrical stimulation promotes osteogenic differentiation of BMSCs. Initially, the intracellular electrical stimulation generated by BST NPs endocytosed by BMSCs can change the intracellular bioelectrical state, including the increase of the intracellular calcium ion concentration. In addition, calcium ions, as a second messenger, can activate the PI3K/AKT signaling pathway, further increasing the stability and transcriptional activity of Runx-2. As a key transcription factor in the nucleus, Runx-2 regulates the expression of osteogenic marker genes such as ALP, OPN, and OCN (Fig. 7m).
3.8. The electrical stimulation generated by BST NPs coupled with ultrasound accelerates bone healing
Inspired by the excellent injectability and suitable degradation properties of BGO-2 hydrogel, it can serve as a delivery platform for BST NPs to accelerate bone healing. In order to assess the efficacy of bone regeneration through electrical stimulation generated by the BST NPs under US, a rat skull model featuring critical-sized bone defects (a diameter of 5 mm) was established (Fig. 8a). After injecting the hydrogel into the skull bone defect area, ultrasound treatment was administered to the rat skull every three days for a duration of 8 weeks. The ultrasonic parameters are: 1.0 MHz, 1.0 W/cm2, 5 min, 50 % duty cycle. Fig. 8b displayed the optical photographs of the implanted area and injectable BGO-2 hydrogel. Micro-CT images taken after 12 weeks of implantation (Fig. 8c) showed that the BGO-2+US group had noticeable new bone tissue over the entire defect area, while the other three groups had minimal bone formation. Quantitative morphological analysis of bone volume/tissue volume ratio (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and other parameters was performed to evaluate the growth of regenerating bone. It was found that the BV/TV ratio in the BGO-2+US group was significantly higher than that of the Blank, US, and BGO-2 groups at week 12, with values of 21.40 ± 1.86 % versus 11.72 ± 2.33 %, 11.97 ± 2.54 %, and 12.60 ± 4.39 %, respectively (Fig. 8d). Additionally, the BGO-2+US group showed the maximum increase in Tb.N (0.86 ± 0.12 mm−1) and Tb.Th (0.41 ± 0.04 mm) and the most significant decrease in Tb.Sp (0.62 ± 0.15 mm) (Fig. 8d). These results suggest that electrical stimulation of BST NPs delivered by the BGO-2 nanocomposite hydrogel coupled with ultrasound can increase bone anabolism and promote bone regeneration in vivo in bone defect areas.
Fig. 8.
In vivo newly formed bone ability via intracellular electrical stimulation. (a) Schematic illustration of the surgical procedure for a 5 mm calvarial defect in a rat. (b) Optical photos of hydrogels injected into a rat calvarial defect. (c) Micro-CT images of newly formed bone tissues in different groups at 8 weeks after implantation. (d) Quantitative analysis of BV/TV, Tb.N, Tb.Th, and Tb.Sp. (e) Histological analysis of bone regeneration in calvarial defects by H&E,MT, and immunohistochemical staining of Runx-2 and OCN at weeks 8 after surgery. NB: newly formed bone. The black arrow represents positive staining. (f) Quantitative analysis of relative regenerated tissue area, relative mineralized tissue area, and average optical density of OCN and Runx-2. The data are presented as mean ± SD, and statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons (*p < 0.05, **p < 0.01, ***p < 0.001).
Bone regeneration in bone defects was further analyzed by H&E and Masson staining (Fig. 8e). In the blank, US, and BGO-2 groups, the bone defect area was almost completely covered by fibrous tissue, and limited new bone was observed. However, in the BGO-2+US group, a large amount of new bone tissue was observed, almost filling the original bone defect area. Quantification of the sum of pink areas in H&E-stained images revealed that the BGO-2+US group had a greater amount of regenerated tissue, almost 3.37 times that of the blank group (Fig. 8f). Masson staining also showed similar results. After treatment with BGO-2 coupled with ultrasound, more mineralized tissue was observed in the bone defect area, that is, the blue part in the MT staining image, which was approximately 2.03 times that of the Blank group (Fig. 8g). These results demonstrate that electrical stimulation of BGO-2 in response to US accelerates bone regeneration.
As shown in the immunohistochemical images (Fig. 8e), the BGO-2+US group exhibited an increased expression of osteogenic-related proteins Runx-2 and OCN (black arrow), indicating the recruitment of more osteogenically differentiated active cells to the defect location for facilitating bone regeneration. The expression levels of Runx-2 and OCN were quantified by measuring the brown-stained areas (Fig. 8f). The analysis revealed that the BGO-2+US group had significantly higher expression levels of Runx-2 compared to the Blank, US, and BST groups, with values of 0.09 ± 0.006, 0.13 ± 0.004, 0.10 ± 0.01, and 0.16 ± 0.008, respectively. Similarly, the expression levels of OCN on the BGO-2+US group were apparently higher than that of Blank, US and BGO-2 groups, which were 0.18 ± 0.007 vs 0.13 ± 0.008, 0.12 ± 0.009 and 0.14 ± 0.016 (Fig. 8f). These findings suggest that electrical stimulation generated by BGO-2 in response to US enhances the expression of osteogenic-related proteins and create an electrical microenvironment for bone regeneration. The aforementioned results provide evidence that the BGO-2 nanocomposite hydrogel effectively delivers BST nanoparticles to the bone defect area, and when combined with ultrasound irradiation, creates an electrical stimulation microenvironment that accelerates bone tissue regeneration. These findings suggested that this approach has potential for use in the treatment of bone defects following bone tumor surgery.
During the repair of skull defects, the electrical stimulation induced by BST NPs enhances the expression of the Runx-2 protein in BMSCs (Fig. 8e). Runx-2, a pivotal osteogenic transcription factor, promotes the differentiation of BMSCs into osteoblasts. Osteoblasts are the key cells responsible for bone formation and play a pivotal role in new bone growth. On the one hand, osteoblasts secrete crucial extracellular matrix proteins like type I collagen and OCN. Significantly, type I collagen aids in the deposition of calcium in the form of hydroxyapatite, providing essential structural support for bones. These new bone tissues consist of osteoblasts and bone extracellular matrix [74]. Hence, BST NPs can expedite bone defect healing in response to electrical stimulation generated by ultrasound.
4. Conclusion
In summary, we developed a novel piezo-enhanced NIR photocatalytic nanoheterojunction and integrated it into an injectable biopolymer hydrogel for combined anti-osteosarcoma and osteogenesis therapy. Through material characterization and finite element simulation calculations, we thoroughly explored the structure-property relationship of BST nanoheterostructures. It is worth noting that the integration of ST nanoparticles with piezoelectric effect and Bi metal nanoparticles with LSPR effect resulted in the following: 1) BST heterojunction has narrow energy band gap; 2) the introduction of a built-in piezoelectric field to regulate charge carrier migration behavior; 3) a BST heterojunction responsive to the NIR band; and 4) piezo-augmented NIR responsive photodynamic therapy. Acid-responsive BST NPs composite hydrogels with excellent mechanical properties were synthesized using dynamic covalent amide bonds. The resulting hydrogel exhibits remarkable filling and retention abilities in the bone defect area, and effectively releases BST NPs in response to the acidic tumor microenvironment (pH < 6.7), thereby eliminating osteosarcoma cells. Importantly, piezo-enhanced photodynamic therapy resulted in tumor inhibition rates of 98.6 % and 67.6 % in the in vivo PDX model and in the tibial orthotopic osteosarcoma model, respectively. Additionally, exposed BST NPs after BGO hydrogel degradation responded to mild-intensity ultrasound to generate piezoelectric stimulation, effectively activating the PI3K/AKT pathway, promoting osteogenic differentiation of BMSCs, and accelerating bone healing. Overall, this work highlights a promising innovative and effective strategy for the development of an integrated osteosarcoma treatment and therapy to promote bone defect healing.
According to the evaluation criteria in solid tumors (RECIST) guidelines, a reduction of at least 30 % in the sum of the longest diameters of target lesions indicates the alleviation of tumor progression. Complete disappearance of all target lesions implies the complete alleviation of tumor progression [75]. The tumor inhibition rates of piezoelectric-enhanced near-infrared photodynamic therapy in the tibial osteosarcoma model and PDX model were 67.6 % and 98.6 %, respectively, indicating that the therapy can effectively alleviate tumor progression. Additionally, this hydrogel is expected to be seamlessly integrated into clinical treatment strategies for osteosarcoma. During neoadjuvant chemotherapy before surgery, BGO hydrogel is combined with chemotherapy drugs (such as methotrexate, cisplatin, etc.) to reduce tumor size, thereby enhancing the clarity of the resection margin and improving surgical efficiency. Following the surgical removal of tumor tissue, the injection of BGO hydrogel is employed to prevent tumor recurrence, and electrical stimulation accelerates bone defect regeneration. In the ongoing progress of this research, the metabolism time of BST NPs in the body needs further clarification. Additionally, the intensity of near-infrared light faces a gradual attenuation trend in deep osteosarcoma, which may weaken the therapeutic effect of BST NPs.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Cairong Xiao: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Renxian Wang: Writing – review & editing, Investigation, Data curation. Rumin Fu: Writing – review & editing, Investigation, Formal analysis. Peng Yu: Software. Jianxun Guo: Visualization, Software. Guangping Li: Methodology, Formal analysis. Zhengao Wang: Visualization, Methodology. Honggang Wang: Formal analysis, Data curation. Jingjun Nie: Methodology, Investigation. Weifeng Liu: Methodology. Jinxia Zhai: Resources, Investigation. Changhao Li: Methodology, Investigation. Chunlin Deng: Project administration, Funding acquisition. Dafu Chen: Methodology, Investigation, Funding acquisition. Lei Zhou: Writing – review & editing, Writing – original draft, Funding acquisition. Chengyun Ning: Writing – review & editing, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Key R&D Program of China (Nos. 2021YFC2400402 and 2021YFC2400405) the National Natural Science Foundation of China (Nos. 52072127, 52173275, 52201297, 51932002), Beijing Municipal Health Commission (BJRITO-RDP-2023, PXM 2020_026275_000002 and BMHC-2021-6), the Natural Science Foundation of Guangdong Province (2021A0505030083 and 202201010228), the Guangdong Basic and Applied Basic Research Foundation (2023A1515010784) and the China Postdoctoral Science Foundation (No. 2023M743979).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2024.01.003.
Contributor Information
Dafu Chen, Email: chendafujst@126.com.
Lei Zhou, Email: zhoul@gzhmu.edu.cn.
Chengyun Ning, Email: imcyning@scut.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fletcher C.D. The evolving classification of soft tissue tumours-an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2–11. doi: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]
- 2.Valery P.C., Laversanne M., Bray F. Bone cancer incidence by morphological subtype: a global assessment, Cancer. Causes. Control. 2015;26(8):1127–1139. doi: 10.1007/s10552-015-0607-3. [DOI] [PubMed] [Google Scholar]
- 3.Ritter J., Bielack S.S. Osteosarcoma, Ann. Oncol. 2010;21(Suppl 7):vii320–v325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 4.Yang C., Tian Y., Zhao F., Chen Z., Su P., Li Y., Qian A. Bone microenvironment and osteosarcoma metastasis. Int. J. Mol. Sci. 2020;21(19) doi: 10.3390/ijms21196985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakoff M.S., Bielack S.S., Meltzer P., Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J. Clin. Oncol. 2015;33(27):3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill J., Gorlick R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021;18(10):609–624. doi: 10.1038/s41571-021-00519-8. [DOI] [PubMed] [Google Scholar]
- 7.Kansara M., Teng M.W., Smyth M.J., Thomas D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer. 2014;14(11):722–735. doi: 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- 8.Misaghi A., Goldin A., Awad M., Kulidjian A.A. Osteosarcoma: a comprehensive review. Sicot j. 2018;4:12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Lovell J.F., Yoon J., Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 10.Sun B., Bte Rahmat J.N., Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials. 2022;291 doi: 10.1016/j.biomaterials.2022.121875. [DOI] [PubMed] [Google Scholar]
- 11.Pham T.C., Nguyen V.N., Choi Y., Lee S., Yoon J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021;121(21):13454–13619. doi: 10.1021/acs.chemrev.1c00381. [DOI] [PubMed] [Google Scholar]
- 12.Yang D., Gulzar A., Yang G., Gai S., He F., Dai Y., Zhong C., Yang P. Au nanoclusters sensitized black TiO2− x nanotubes for enhanced photodynamic therapy driven by near‐infrared light. Small. 2017;13(48) doi: 10.1002/smll.201703007. [DOI] [PubMed] [Google Scholar]
- 13.Hou Z., Zhang Y., Deng K., Chen Y., Li X., Deng X., Cheng Z., Lian H., Li C., Lin J. UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano. 2015;9(3):2584–2599. doi: 10.1021/nn506107c. [DOI] [PubMed] [Google Scholar]
- 14.Mao C., Zhu W., Xiang Y., Zhu Y., Shen J., Liu X., Wu S., Cheung K.M.C., Yeung K.W.K. Enhanced near-infrared photocatalytic eradication of MRSA biofilms and osseointegration using oxide perovskite-based P-N heterojunction. Adv. Sci. 2021;8(15) doi: 10.1002/advs.202002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia H.R., Zhu Y.X., Duan Q.Y., Chen Z., Wu F.G. Nanomaterials meet zebrafish: toxicity evaluation and drug delivery applications. J. Contr. Release. 2019;311–312:301–318. doi: 10.1016/j.jconrel.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Wang P., Ao Y., Chen J., Gao X., Jia B., Ma T. Directing charge transfer in a chemical‐bonded BaTiO3@ ReS2 Schottky heterojunction for piezoelectric enhanced photocatalysis. Adv. Mater. 2022;34(29) doi: 10.1002/adma.202202508. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Wang L., Yu X., Zhang J., Yang R., Zhang X., Ji Y., Wu M., Deng L., Li L. Piezoelectric‐effect‐enhanced full‐spectrum photoelectrocatalysis in p–n heterojunction. Adv. Funct. Mater. 2019;29(41) [Google Scholar]
- 18.Zhang C., Lei D., Xie C., Hang X., He C., Jiang H.L. Piezo‐photocatalysis over metal–organic frameworks: promoting photocatalytic activity by piezoelectric effect. Adv. Mater. 2021;33(51) doi: 10.1002/adma.202106308. [DOI] [PubMed] [Google Scholar]
- 19.Basu M., Garg N., Ganguli A.K. A type-II semiconductor (ZnO/CuS heterostructure) for visible light photocatalysis. J. Mater. Chem. A. 2014;2(20):7517–7525. [Google Scholar]
- 20.Liu Y.-L., Wu J.M. Synergistically catalytic activities of BiFeO3/TiO2 core-shell nanocomposites for degradation of organic dye molecule through piezophototronic effect. Nano Energy. 2019;56:74–81. [Google Scholar]
- 21.Wu X., Li H., Wang X., Jiang L., Xi J., Du G., Ji Z. Ferroelectric enhanced photoelectrochemical water splitting in BiFeO3/TiO2 composite photoanode. J. Alloys Compd. 2019;783:643–651. [Google Scholar]
- 22.Xu M.L., Lu M., Qin G.Y., Wu X.M., Yu T., Zhang L.N., Li K., Cheng X., Lan Y.Q. Piezo‐photocatalytic synergy in BiFeO3@ COF Z‐scheme heterostructures for high‐efficiency overall water splitting. Angew Chem. Int. Ed. Engl. 2022;61(44) doi: 10.1002/anie.202210700. [DOI] [PubMed] [Google Scholar]
- 23.Kang Y., Lei L., Zhu C., Zhang H., Mei L., Ji X. Piezo-photocatalytic effect mediating reactive oxygen species burst for cancer catalytic therapy. Mater. Horiz. 2021;8(8):2273–2285. doi: 10.1039/d1mh00492a. [DOI] [PubMed] [Google Scholar]
- 24.Cong C., Rao C., Ma Z., Yuan Y., Wang D., Zhang X., He Y., Lou H., Gao D. Coupling piezo-photocatalysis to imitate lymphoid reflux for enhancing antitumor hydrodynamics therapy. Chem. Eng. J. 2022;450 [Google Scholar]
- 25.Wang K., Han C., Li J., Qiu J., Sunarso J., Liu S. The mechanism of piezocatalysis: energy band theory or screening charge effect? Angew. Chem. 2022;134(6) doi: 10.1002/anie.202110429. [DOI] [PubMed] [Google Scholar]
- 26.Tu S., Guo Y., Zhang Y., Hu C., Zhang T., Ma T., Huang H. Piezocatalysis and piezo‐photocatalysis: catalysts classification and modification strategy, reaction mechanism, and practical application. Adv. Funct. Mater. 2020;30(48) [Google Scholar]
- 27.Lin J., Hu J., Qiu C., Huang H., Chen L., Xie Y., Zhang Z., Lin H., Wang X. In situ hydrothermal etching fabrication of CaTiO3 on TiO2 nanosheets with heterojunction effects to enhance CO 2 adsorption and photocatalytic reduction. Catal. Sci. Technol. 2019;9(2):336–346. [Google Scholar]
- 28.Mao C., Zhu W., Xiang Y., Zhu Y., Shen J., Liu X., Wu S., Cheung K.M., Yeung K.W.K. Enhanced near‐infrared photocatalytic eradication of MRSA biofilms and osseointegration using oxide perovskite‐based P–N heterojunction. Adv. Sci. 2021;8(15) doi: 10.1002/advs.202002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Koo S., Kim J.H., Huang X., Kong N., Zhang L., Zhou J., Xue J., Harris M.B., Tao W. Nanoscale materials-based platforms for the treatment of bone-related diseases. Matter. 2021;4(9):2727–2764. [Google Scholar]
- 30.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5(8):584–603. [Google Scholar]
- 31.Shao R., Wang Y., Li L., Dong Y., Zhao J., Liang W. Bone tumors effective therapy through functionalized hydrogels: current developments and future expectations. Drug Deliv. 2022;29(1):1631–1647. doi: 10.1080/10717544.2022.2075983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1(12):1–17. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer D.S., Shodeinde A.B., Beckman D.W., Luu B.C., Hodges H.R., Peppas N.A. Cytocompatibility, membrane disruption, and siRNA delivery using environmentally responsive cationic nanogels. J. Contr. Release. 2021;332:608–619. doi: 10.1016/j.jconrel.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li I.-C., Moore A.N., Hartgerink J.D. “Missing tooth” multidomain peptide nanofibers for delivery of small molecule drugs. Biomacromolecules. 2016;17(6):2087–2095. doi: 10.1021/acs.biomac.6b00309. [DOI] [PubMed] [Google Scholar]
- 35.Dai Z., Zhang Q., Li X., Chen Q., Chen J., Wang M., Chen H. In situ forming pH/ROS-responsive niche-like hydrogel for ultrasound-mediated multiple therapy in synergy with potentiating anti-tumor immunity. Mater. Today. 2023;65:62–77. [Google Scholar]
- 36.Merino S., Martin C., Kostarelos K., Prato M., Vázquez E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano. 2015;9(5):4686–4697. doi: 10.1021/acsnano.5b01433. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y., Wang Y., Long L., Hu C., Kong Q., Wang Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Contr. Release. 2022;341:147–165. doi: 10.1016/j.jconrel.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Feng Q., Xu J., Zhang K., Yao H., Zheng N., Zheng L., Wang J., Wei K., Xiao X., Qin L. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS Cent. Sci. 2019;5(3):440–450. doi: 10.1021/acscentsci.8b00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W., Cheng S., Wang X., Zhang Y., Chen L., Zhang L. Noncompressible hemostasis and bone regeneration induced by an absorbable bioadhesive self‐healing hydrogel. Adv. Funct. Mater. 2021;31(22) [Google Scholar]
- 40.Christensen S.T., Elam J.W., Rabuffetti F.A., Ma Q., Weigand S.J., Lee B., Seifert S., Stair P.C., Poeppelmeier K.R., Hersam M.C. Controlled growth of platinum nanoparticles on strontium titanate nanocubes by atomic layer deposition. Small. 2009;5(6):750–757. doi: 10.1002/smll.200801920. [DOI] [PubMed] [Google Scholar]
- 41.Fan L., Liu C., Chen X., Zheng L., Zou Y., Wen H., Guan P., Lu F., Luo Y., Tan G. Exosomes‐loaded Electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via Immunoregulation and enhancement of myelinated axon growth. Adv. Sci. 2022;9(13) doi: 10.1002/advs.202105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing Z., Ni R., Wang J., Lin X., Fan D., Wei Q., Zhang T., Zheng Y., Cai H., Liu Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021;6(12):4542–4557. doi: 10.1016/j.bioactmat.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang L., Kang X., Sang Y., Liu H. One‐dimensional ferroelectric nanostructures: synthesis, properties, and applications. Adv. Sci. 2016;3(7) doi: 10.1002/advs.201500358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang B., Iocozzia J., Zhao L., Zhang H., Harn Y.W., Chen Y., Lin Z. Barium titanate at the nanoscale: controlled synthesis and dielectric and ferroelectric properties. Chem. Soc. Rev. 2019;48(4):1194–1228. doi: 10.1039/c8cs00583d. [DOI] [PubMed] [Google Scholar]
- 45.Xiao C., Fan L., Zhou S., Kang X., Guan P., Fu R., Li C., Ren J., Wang Z., Yu P., Wang Y., Deng C., Zhou L., Ning C. One-dimensional ferroelectric nanoarrays with wireless switchable static and dynamic electrical stimulation for selective regulating osteogenesis and antiosteosarcoma. ACS Nano. 2022;16(12):20770–20785. doi: 10.1021/acsnano.2c07900. [DOI] [PubMed] [Google Scholar]
- 46.Fu R., Tu L., Guan Y., Wang Z., Deng C., Yu P., Tan G., Ning C., Zhou L. Intrinsically piezoelectric elastomer based on crosslinked polyacrylonitrile for soft electronics. Nano Energy. 2022;103 [Google Scholar]
- 47.Chen Q., Cheng X., Long H., Rao Y. A short review on recent progress of Bi/semiconductor photocatalysts: the role of Bi metal. Chin. Chem. Lett. 2020;31(10):2583–2590. [Google Scholar]
- 48.Zhou X., Shen B., Lyubartsev A., Zhai J., Hedin N. Semiconducting piezoelectric heterostructures for piezo-and piezophotocatalysis. Nano Energy. 2022 [Google Scholar]
- 49.Li J., Xie G., Jiang J., Liu Y., Chen C., Li W., Huang J., Luo X., Xu M., Zhang Q. Enhancing photodegradation of Methyl Orange by coupling piezo-phototronic effect and localized surface plasmon resonance. Nano Energy. 2023;108 [Google Scholar]
- 50.Xiang H., Yang Q., Gao Y., Zhu D., Pan S., Xu T., Chen Y. Cocrystal strategy toward multifunctional 3D‐printing scaffolds enables NIR‐activated photonic osteosarcoma hyperthermia and enhanced bone defect regeneration. Adv. Funct. Mater. 2020;30(25) [Google Scholar]
- 51.Liu D., Nikoo M., Boran G., Zhou P., Regenstein J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015;6:527–557. doi: 10.1146/annurev-food-031414-111800. [DOI] [PubMed] [Google Scholar]
- 52.Sharma R., Kuche K., Thakor P., Bhavana V., Srivastava S., Mehra N.K., Jain S. Chondroitin Sulfate: emerging biomaterial for biopharmaceutical purpose and tissue engineering. Carbohydr. Polym. 2022;286 doi: 10.1016/j.carbpol.2022.119305. [DOI] [PubMed] [Google Scholar]
- 53.Brandl F., Sommer F., Goepferich A. Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials. 2007;28(2):134–146. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Delplace V., Nickerson P.E., Ortin‐Martinez A., Baker A.E., Wallace V.A., Shoichet M.S. Nonswelling, ultralow content inverse electron‐demand diels–alder hyaluronan hydrogels with tunable gelation time: synthesis and in vitro evaluation. Adv. Funct. Mater. 2020;30(14) [Google Scholar]
- 55.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang K., Feng Q., Fang Z., Gu L., Bian L. Structurally dynamic hydrogels for biomedical applications: pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021;121(18):11149–11193. doi: 10.1021/acs.chemrev.1c00071. [DOI] [PubMed] [Google Scholar]
- 57.Dai Z., Zhang Q., Li X., Chen Q., Chen J., Wang M., Chen H. In situ forming pH/ROS-responsive niche-like hydrogel for ultrasound-mediated multiple therapy in synergy with potentiating anti-tumor immunity. Mater. Today. 2023;65:62–77. [Google Scholar]
- 58.Urano M. Kinetics of thermotolerance in normal and tumor tissues: a review. Cancer Res. 1986;46(2):474–482. [PubMed] [Google Scholar]
- 59.Emami B., Song C.W. Physiological mechanisms in hyperthermia: a review. Int. J. Radiat. Oncol. Biol. Phys. 1984;10(2):289–295. doi: 10.1016/0360-3016(84)90015-4. [DOI] [PubMed] [Google Scholar]
- 60.Jain I.H., Calvo S.E., Markhard A.L., Skinner O.S., To T.L., Ast T., Mootha V.K. Genetic screen for cell fitness in high or low oxygen highlights mitochondrial and lipid metabolism. Cell. 2020;181(3):716–727.e11. doi: 10.1016/j.cell.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chouchani E.T., Kazak L., Jedrychowski M.P., Lu G.Z., Erickson B.K., Szpyt J., Pierce K.A., Laznik-Bogoslavski D., Vetrivelan R., Clish C.B., Robinson A.J., Gygi S.P., Spiegelman B.M. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532(7597):112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suski J.M., Lebiedzinska M., Bonora M., Pinton P., Duszynski J., Wieckowski M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2012;810:183–205. doi: 10.1007/978-1-61779-382-0_12. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y., Liu J., He M., Dong Q., Zhang L., Xu Z., Kang Y., Xue P. Platinum–titania Schottky junction as nanosonosensitizer, glucose scavenger, and tumor microenvironment-modulator for promoted cancer treatment. ACS Nano. 2022;16(8):12118–12133. doi: 10.1021/acsnano.2c02540. [DOI] [PubMed] [Google Scholar]
- 64.Krall A.S., Mullen P.J., Surjono F., Momcilovic M., Schmid E.W., Halbrook C.J., Thambundit A., Mittelman S.D., Lyssiotis C.A., Shackelford D.B., Knott S.R.V., Christofk H.R. Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metabol. 2021;33(5):1013–1026.e6. doi: 10.1016/j.cmet.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Wang Y., Zhen W., Wang Y., Zhang S., Zhao Y., Song S., Wu Z., Zhang H. Defect modified zinc oxide with augmenting sonodynamic reactive oxygen species generation. Biomaterials. 2020;251 doi: 10.1016/j.biomaterials.2020.120075. [DOI] [PubMed] [Google Scholar]
- 66.Hu D., Pan M., Yang Y., Sun A., Chen Y., Yuan L., Huang K., Qu Y., He C., Wei Q. Trimodal sono/photoinduced focal therapy for localized prostate cancer: single‐drug‐based nanosensitizer under dual‐activation. Adv. Funct. Mater. 2021;31(50) [Google Scholar]
- 67.Wan M., Li T., Chen H., Mao C., Shen J. Biosafety, functionalities, and applications of biomedical micro/nanomotors. Angew Chem. Int. Ed. Engl. 2021;60(24):13158–13176. doi: 10.1002/anie.202013689. [DOI] [PubMed] [Google Scholar]
- 68.Pei Z., Lei H., Cheng L. Bioactive inorganic nanomaterials for cancer theranostics. Chem. Soc. Rev. 2023;52(6):2031–2081. doi: 10.1039/d2cs00352j. [DOI] [PubMed] [Google Scholar]
- 69.Levin M. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell. 2021;184(8):1971–1989. doi: 10.1016/j.cell.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 70.Wang C., Lin K., Chang J., Sun J. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34(1):64–77. doi: 10.1016/j.biomaterials.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 71.Ediriweera M.K., Tennekoon K.H., Samarakoon S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin. Cancer Biol. 2019;59:147–160. doi: 10.1016/j.semcancer.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 72.Ma Y., Ran D., Zhao H., Song R., Zou H., Gu J., Yuan Y., Bian J., Zhu J., Liu Z. Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141638. [DOI] [PubMed] [Google Scholar]
- 73.Abdurahman A., Li X., Li J., Liu D., Zhai L., Wang X., Zhang Y., Meng Y., Yokota H., Zhang P. Loading-driven PI3K/Akt signaling and erythropoiesis enhanced angiogenesis and osteogenesis in a postmenopausal osteoporosis mouse model. Bone. 2022;157 doi: 10.1016/j.bone.2022.116346. [DOI] [PubMed] [Google Scholar]
- 74.Salhotra A., Shah H.N., Levi B., Longaker M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L., Verweij J., Van Glabbeke M., van Oosterom A.T., Christian M.C. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.