Abstract
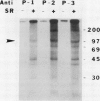
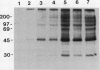
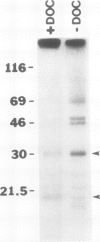
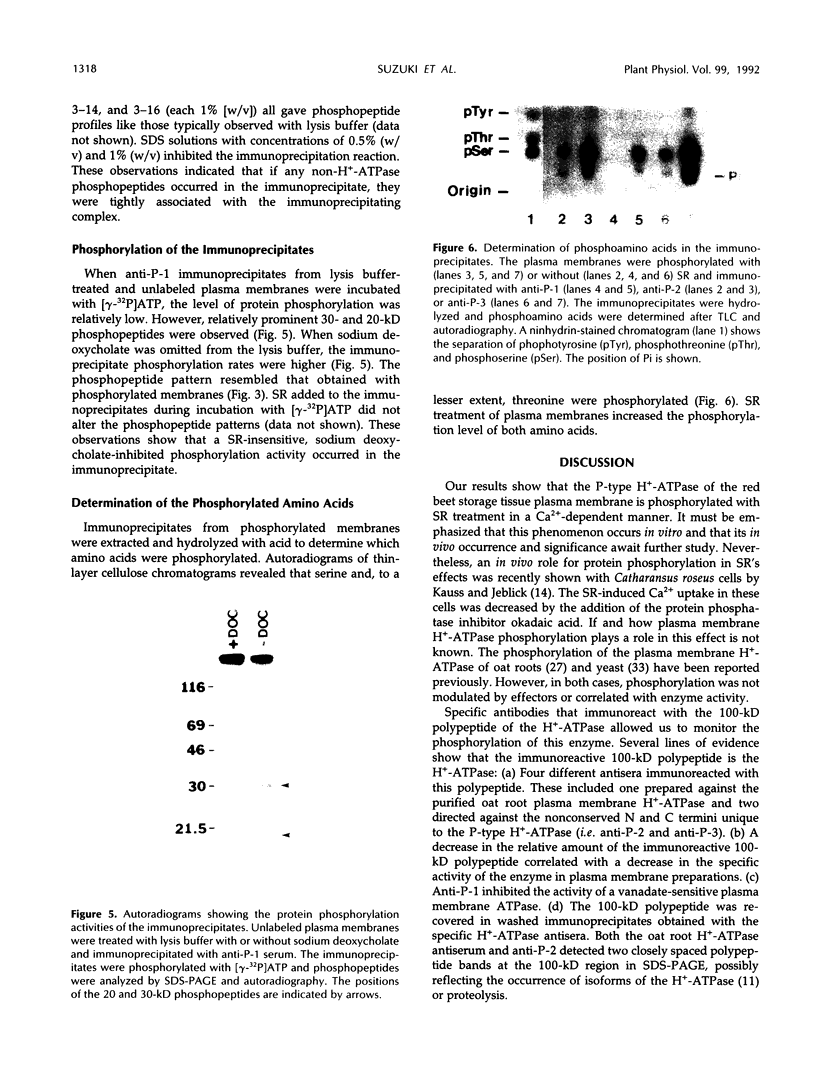
The syringomycin-stimulated in vitro protein phosphorylation of the plasma membrane H+-ATPase of red beet (Beta vulgaris L.) storage tissue was investigated. Peptides representing the H+-ATPase N and C termini and nucleotide binding site (P-2, P-3, and P-1, respectively) were synthesized, and rabbit antisera against each were produced. In western immunoblots of purified plasma membranes, these antisera immunoreacted with the 100-kilodalton polypeptide of the H+-ATPase and with other smaller polypeptides. The smaller polypeptides appeared to be degraded forms of the intact 100-kilodalton polypeptide. Immunoprecipitation experiments showed that plasma membranes treated with syringomycin had increased protein phosphorylation rates of the 100-kilodalton polypeptide. Optimal phosphorylation levels were achieved with 25 micromolar free Ca2+. Phosphoserine and phosphothreonine were detected in the immunoprecipitates. Washed immunoprecipitates generated with anti-P-1 possessed protein phosphorylation activity. This immunoprecipitate activity was not stimulated by syringomycin, but it was inhibited when plasma membranes were treated with sodium deoxycholate before immunoprecipitation. The findings show that syringomycin stimulates the phosphorylation of the plasma membrane H+-ATPase and that specific protein kinase(s) are probably associated with the enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., Raden D., Racker E., Katchalski-Katzir E. Phosphorylation of synthetic random polypeptides by protein kinase P and other protein-serine (threonine) kinases and stimulation or inhibition of kinase activities by microbial toxins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1408–1411. doi: 10.1073/pnas.85.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwai A. P., Takemoto J. Y. Bacterial phytotoxin, syringomycin, induces a protein kinase-mediated phosphorylation of red beet plasma membrane polypeptides. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6755–6759. doi: 10.1073/pnas.84.19.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwai A. P., Zhang L., Bachmann R. C., Takemoto J. Y. Mechanism of Action of Pseudomonas syringae Phytotoxin, Syringomycin : Stimulation of Red Beet Plasma Membrane ATPase Activity. Plant Physiol. 1987 Jan;83(1):39–43. doi: 10.1104/pp.83.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blowers D. P., Trewavas A. J. Rapid cycling of autophosphorylation of a ca-calmodulin regulated plasma membrane located protein kinase from pea. Plant Physiol. 1989 Aug;90(4):1279–1285. doi: 10.1104/pp.90.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Roti-Roti J. L. Target molecular size of the red beet plasma membrane ATPase. Plant Physiol. 1985 Jul;78(3):642–644. doi: 10.1104/pp.78.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhugga K. S., Ray P. M. Isoelectric Focusing of Plant Plasma Membrane Proteins : Further Evidence that a 55 Kilodalton Polypeptide Is Associated with beta-1,3-Glucan Synthase Activity from Pea. Plant Physiol. 1991 Apr;95(4):1302–1305. doi: 10.1104/pp.95.4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamo K. Purification and Properties of the Plasma Membrane H-Translocating Adenosine Triphosphatase of Phaseolus mungo L. Roots. Plant Physiol. 1986 Apr;80(4):818–824. doi: 10.1104/pp.80.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Memon A. R., Chen Q. Y., Boss W. F. Inositol phospholipids activate plasma membrane ATPase in plants. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1295–1301. doi: 10.1016/0006-291x(89)90814-0. [DOI] [PubMed] [Google Scholar]
- Mott K. A., Takemoto J. Y. Syringomycin, a bacterial phytotoxin, closes stomata. Plant Physiol. 1989 Aug;90(4):1435–1439. doi: 10.1104/pp.90.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M., Serrano R., Larsson C. Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H(+)-ATPase. J Biol Chem. 1991 Oct 25;266(30):20470–20475. [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M., Ulvskov P., Larsson C. Effect of detergents on the H(+)-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochim Biophys Acta. 1990 Jan 29;1021(2):133–140. doi: 10.1016/0005-2736(90)90025-j. [DOI] [PubMed] [Google Scholar]
- Pardo J. M., Serrano R. Structure of a plasma membrane H+-ATPase gene from the plant Arabidopsis thaliana. J Biol Chem. 1989 May 25;264(15):8557–8562. [PubMed] [Google Scholar]
- Putnam-Evans C. L., Harmon A. C., Cormier M. J. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990 Mar 13;29(10):2488–2495. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Schaller G. E., Sussman M. R. Isolation and sequence of tryptic peptides from the proton-pumping ATPase of the oat plasma membrane. Plant Physiol. 1988 Feb;86(2):512–516. doi: 10.1104/pp.86.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre A., Bachmann R. C., Ballio A., Bossa F., Grgurina I., Iacobellis N. S., Marino G., Pucci P., Simmaco M., Takemoto J. Y. The structure of syringomycins A1, E and G. FEBS Lett. 1989 Sep 11;255(1):27–31. doi: 10.1016/0014-5793(89)81054-3. [DOI] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita Y., Abdel-Ghany M., Raden D., Nelson N., Racker E. Polypeptide-dependent protein kinase from bakers' yeast. Proc Natl Acad Sci U S A. 1987 Feb;84(4):925–929. doi: 10.1073/pnas.84.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]