Summary
Background
The incidence of gastric cancer (GC) decreased in past decades, which was thought largely attributable to risk factors control, yet China still accounts for 44% of global GC burdens. We aimed to estimate changing trajectories of proportions of GC burdens attributable to modifiable risk factors from 2000 to 2050 in China, to inform future targeted preventive strategies.
Methods
The incidence and new cases of GC were predicted to 2050 using Bayesian age-period-cohort model based on incidence data by anatomical subsites drawn from 682 cancer registries from National Central Cancer Registry. Population attributable fractions (PAFs) were calculated based on prevalence of risk factors and relative risks with GC. Temporal trends of PAFs were described by sex and categories of risk factors using joinpoint analysis.
Findings
We observed declining trends of PAFs of Helicobacter pylori (H. pylori) infection, smoking, pickled vegetable and alcohol consumption, but increasing trends of PAFs of unhealthy body mass index and diabetes for GC in China. The combined PAFs of these risk factors were estimated to decrease by 10.57% from 2000 to 2050 for GC. We estimated there will be 279,707 GC (122,796 cardia gastric cancer [CGC] and 156,911 non-cardia gastric cancer [NCGC]) cases in 2050. Out of these cases, 70.18% of GC cases could be attributable to modifiable risk factors, while H. pylori infection was predicted to be responsible for 40.7% of CGC and 62.1% of NCGC cases in 2050.
Interpretation
More than half of GC remained attributable to modifiable risk factors in China. Continued effective strategies on risk factors control are needed to reduce the burden of this highly life-threatening cancer in future.
Funding
Beijing Nova Program (No. Z201100006820069), CAMS Innovation Fund for Medical Sciences (CIFMS, grant No. 2021-I2M-1-023), CAMS Innovation Fund for Medical Sciences (CIFMS, grant No. 2021-I2M-1-010), Talent Incentive Program of Cancer Hospital Chinese Academy of Medical Sciences (Hope Star).
Keywords: Population attributable fraction, Gastric cancer, Cardia gastric cancer, Non-cardia gastric cancer, Modifiable risk factor, Changing trend
Research in context.
Evidence before this study
We searched PubMed, Google Scholar, Web of Science, and China National Knowledge Infrastructure, without language restrictions, for articles from database inception to June 1, 2022, using the terms “population attributable fraction”, “gastric cancer”, “cardia gastric cancer”, “non-cardia gastric cancer”, “modifiable risk factor”, and “changing trend”. We found only one study that estimated the effect of changing trends in exposure to lifestyle risk factors on the burden of gastrointestinal cancer in China, with a lack of burden trends attributable to several important risk factors for gastric cancer (GC), such as Helicobacter pylori (H. pylori) infection. No study to date has investigated the changing trajectories of the proportions of cancer burden attributable to important risk factors for gastric cancer (GC) in the Chinese population.
Added value of this study
To our knowledge, this study is the first to evaluate the population attributable fractions (PAFs) trajectories of important modifiable risk factors for GC overall and by anatomical subsite in China. The combined PAF of selected modifiable risk factors has decreased by 6.19% for GC from 2000 to 2020, and is expected to decrease by 4.38% from 2020 to 2050 under the existing health policy. Though declining trends of PAFs of H. pylori infection, smoking, pickled vegetable, and alcohol consumption were observed, these factors remained major contributors for GC burdens. H. pylori infection is predicted to continue to contribute to 40.7% of cardia gastric cancer (CGC) and 62.1% of non-cardia gastric cancer (NCGC) cases in 2050. Having an unhealthy body mass index (BMI) and suffering from diabetes are expected to contribute to an increasing burden of GC in the coming decades. We predicted there would be 279,707 GC (122,796 CGC and 156,912 NCGC) cases in 2050 taking account into declining trends of age-standardized incidence and population aging, among which 70.18% of GC cases were attributable to modifiable risk factors.
Implications of all the available evidence
Our study provides evidence that great achievement has been made on GC prevention in China for past decades, yet half of GC remained attributable to modifiable risk factors. Continued effective strategies on risk factors control are needed to reduce the burden of this highly life-threatening cancer in future.
Introduction
Gastric cancer (GC) is anatomically subdivided into cardia gastric cancer (CGC) and non-cardia gastric cancer (NCGC).1 CGC cancers arise in the proximal stomach, near the esophageal-gastric junction, whereas NCGC cancers arise in more distal regions of the stomach. It ranks as the 5th most common cancer site worldwide, among which China accounts for 70% and 50% of new CGC and NCGC cases worldwide.2 In past decades, there was a global declining trend of the incidence for GC,3 which was thought largely attributable to effective control of important modifiable risk factors including eradication of Helicobacter pylori (H. pylori) infection, nutrition improvement, effective control of body mass index (BMI), restriction on smoking and alcohol consumption et al.4 With the global population aging and champions on risk factors control, it's critical to investigate the extent to which GC burden changes attributable to the distribution shifts of modifiable risk factors and guide future GC prevention and control.
The population attributable fraction (PAF) is the proportion of new cancer cases in the population due to risk factor exposure, and depends on the prevalence of the factor in the population and the strength of its association with related cancer. PAF is often used to describe the cancer burden that could be avoided by eliminating or reducing exposure to the risk factor. Periodically evaluation of PAFs of risk factors in a certain population could provide important evidence for the assessment of public health strategies on risk factor control for past period. For example, it was estimated that the PAF of smoking for GC remained stable at around 13% and 3% for males and females respectively from 2012 to 2020 in the US,5 the PAF of infectious agents increased from 3.3% in 2000 to 4.0% in 2015 in France,6,7 but decreased by 4.8% from 2002 to 2018 globally.8,9 However, few studies have investigated the changing trajectories of the PAFs of GC burdens attributable to modifiable risk factors in the Chinese populations, which suffered severe GC burdens and accounted for 44% of the global GC cases.1 Moreover, CGC and NCGC have both distinct but also shared etiological pathways. Smoking and heavy alcohol consumption have been associated with CGC and NCGC, increasing body weight and gastroesophageal reflux disease (GERD) have been associated with CGC in high-income countries, whereas H. pylori infection was thought to be the most important risk factor for NCGC. Given these differences, but also similarities in epidemiological characteristics, there is increasing interest in studying the PAF of GC by subsites.2
Therefore, this study aimed to evaluate the PAF changing trajectories of important modifiable risk factors for GC in China from 2000 to 2050, and to provide reference data for future targeted strategies on GC prevention and control.
Methods
Estimation on gastric cancer burden in China
All GC data were obtained from the Chinese National Central Cancer Registry (NCCR), which collected data from population-based cancer registries in all provinces across China and published Chinese national cancer statistics. This study used the national registry data in 2016, which were obtained from 682 cancer registries in 31 provinces (autonomous regions and municipalities) and the Xinjiang Production and Construction Corps (excluding Hong Kong, Macao Special Administrative Region and Taiwan Province), covering more than 470 million (34.48% of Chinese population) population in both urban and rural areas. The detailed quality control was summarized in the Supplementary Methods. Crude incidence rates for CGC and NCGC were calculated by sex (male/female), area (urban/rural) and 5-year age groups (from 0–4 to 85+). The incidence rates were multiplied by the corresponding population for each stratum and then summed to estimate the number of new cases. Age-standardized rates were calculated using the Segi world standard population. We also estimated the incidence rates for CGC and NCGC from 2000 to 2015, using data from the 682 cancer registries in 2016 and calibrated by high-quality data from 22 cancer registries with continuous surveillance data for years 2000–2015 (Supplementary Methods).10
The Bayesian age-period-cohort (BAPC) model was used to predict the incidence rates of CGC and NCGC, which combined prior knowledge with observational data and was less dependent on parametric assumptions (Supplementary Methods for details). Previous studies have indicated that the effects of age and birth cohort are approximately linearly related to GC incidence in both males and females in China, while period effect shows a constant trend over time.10,11 Therefore, the random walk 2 (RW2) prior was used for age and cohort effects and the random walk 1 (RW1) prior was used for period effect in our study. Then the incidence rates were combined with the population size, age and sex structure of the Chinese population obtained from World Population Prospects 2022 to predict the burden of CGC and NCGC.12 We further divided the contribution to changes in predicted cases of CGC and NCGC into risk changes and population changes, including age structure and population size, following the methods described by Moller et al., which calculates changes in the number of cases by combining observed and expected periods of incidence, and population size with each other.13
Risk factors and the RRs of gastric cancer
Our study included six risk factors with robust evidence on an association with GC, including H. pylori infection, smoking, alcohol consumption, pickled vegetable, unhealthy BMI, and diabetes.14 The detailed definitions were summarized in the Supplementary Methods. We assume there was an average latency of 10 years between changes in risk factor exposure and cancer occurrence at the population level. Prevalence data of these risk factors were collected since 1990 at a five-year interval to estimate the attributable burden of CGC and NCGC from year 2000 onwards.15 Prevalence data of risk factors were obtained from Chinese nationally representative surveys (Supplementary Methods for details).
We obtained relative risks (RRs) and 95% confidence intervals (CIs) for the above-mentioned risk factors from large-scale meta-analyses, or representative large-scale cohort studies in Chinese population. Alternatively, we used data from Asian populations or other populations if the Chinese data were not available (see Supplementary Table S1). For non-sex-specific risk factors (including H. pylori infection, alcohol consumption, pickled vegetable, diabetes and unhealthy BMI, the selection criteria were summarized in the Supplementary Methods), we used the overall RR for both males and females.
In addition, we conducted a comprehensive systematic review on the prevalence of H. pylori infection in China from 1990 to 2015 to estimate the attributable burden of H. pylori infection for GC. The search strategy, quality assessment and sensitivity analysis were described in detail in Supplementary Figure S1 and Supplementary Methods.
Attributable burden of risk factors on gastric cancer
We calculated the PAFs and 95% CIs for the modifiable risk factors applicable to the Chinese population using the Levin's formula, by combining the prevalence and RRs for modifiable risk factors related with GC (Supplementary Methods).16, 17, 18 Temporal trends in PAFs for modifiable risk factors from 2000 to 2025 were estimated by joinpoint regression (version 4.9.0.1, https://surveillance.cancer.gov/joinpoint/). Based on the APC and the linear trend of last decade, we extrapolated the change trend of the PAF to 2050. The model attenuated the slope by 25% and 50% in the second and third projection periods, respectively, and by 75% in the fourth and fifth projection periods, as recommended by the empirical validation of previous studies.13
All statistical analyses were completed using R software (version 4.0.3).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or waiting of the report.
Results
Time trends of incidence and new cases of GC from 2000 to 2050
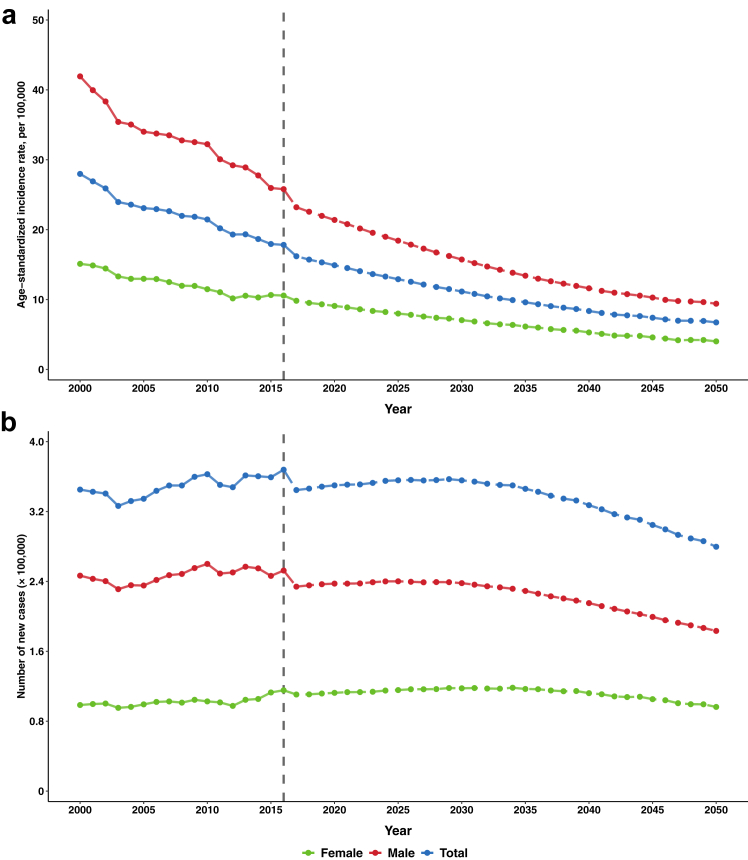
The estimated trends of incidence rates and new cases of GC overall and anatomical subsites from 2000 to 2050 are presented in Fig. 1 and Supplementary Figure S2. Overall, the incidence rate of GC showed a decreasing trend, and the rates were higher in NCGC than that of CGC and in males than that in females. According to the observed data from 2000 to 2016, we predicted that the incidence rates of GC (17.82 vs. 6.73 per 100,000) would continue to decrease from 2016 to 2050 in both CGC (7.24 vs. 2.93 per 100,000) and NCGC (10.59 vs. 3.80 per 100,000) (Table 1). However, we did not observe paralleled declining trends of the annual incident case numbers with those of the incidence rates GC from 2000 to 2016, with the contrary combined effects of risk factors control and demographic shift of population aging. Supplementary Figure S3 shows the tradeoff effects of risk factor control on the reductive proportions of the case numbers and demographic changes of population aging on the increased proportions of the case numbers of GC overall and anatomical subsites from 2020 to 2050. It is predicted that there will be a decrease in the number of new cases for GC from 367,896 in 2016 to 279,707 in 2050 (including both CGC and NCGC from 150,280 and 217,615 to 122,795 and 156,911, respectively). This is a comprehensive result of the continued effective impact of risk factor control in current health policies, as well as the negative impact of population aging on the cancer burden (Table 1). We present the age-specific trends of the incidence rates and 95% CIs of CGC and NCGC from 2000 to 2050 in Supplementary Figure S4, stratified by sex and 5-year age group. The age-specific incidence of GC in both males and females is expected to continue declining in individuals over the age of 55.
Fig. 1.
Trends of the age-standardized incidence rates (a) and number of new cases (b) of GC in males and females in China, 2000–2050. Notes: The changing trends of the adjusted incidence rates from 2000 to 2016 were statistically significant for GC overall (AAPC = −2.6%, P < 0.001) and for males (AAPC = −2.7%, P < 0.001) and females (AAPC = −2.4%, P < 0.001) in GC. Projected incidence rates for 2017–2050 based on adjusted incidence rate of GC from 2000 to 2016. Rates were age-adjusted to the world standard population. Abbreviations: GC, gastric cancer.
Table 1.
Changes of incidence rates and number of new cases of GC overall and anatomical subsites between 2016 and 2050, considering effects from risk factors and demographics changes.
| Age-standardized incidence rate (1/105) |
Number of new cases |
Change between 2016 and 2050 (%) |
|||||
|---|---|---|---|---|---|---|---|
| 2016 | 2050 | 2016 | 2050 | Total change of new cases | Change due to risk factors | Change due to demographics | |
| GC | |||||||
| Male | 25.79 | 9.41 | 252,475 | 183,366 | −27.37 | −136.19 | 108.82 |
| Female | 10.57 | 4.01 | 115,421 | 96,340 | −16.53 | −126.33 | 109.80 |
| Both | 17.82 | 6.73 | 367,896 | 279,707 | −23.97 | −133.10 | 109.13 |
| CGC | |||||||
| Male | 11.29 | 4.20 | 110,595 | 83,085 | −24.87 | −143.85 | 118.97 |
| Female | 3.56 | 1.65 | 39,686 | 39,710 | 0.06 | −129.68 | 129.74 |
| Both | 7.24 | 2.93 | 150,281 | 122,796 | −18.29 | −140.10 | 121.82 |
| NCGC | |||||||
| Male | 14.50 | 5.21 | 141,880 | 100,281 | −29.32 | −130.23 | 100.91 |
| Female | 7.01 | 2.36 | 75,735 | 56,630 | −25.22 | −124.57 | 99.35 |
| Both | 10.59 | 3.80 | 217,615 | 156,911 | −27.89 | −128.26 | 100.37 |
Abbreviations: GC, gastric cancer; CGC, cardia gastric cancer; NCGC, non-cardia gastric cancer.
Time trends of the prevalence of risk factors and the associations between main risk factors and GC
The meta-analysis of H. pylori infection prevalence included 116 studies and found a significant decrease in the prevalence of H. pylori infection from 1990 to 2015 (Supplementary Figure S5). After excluding studies with the low Loney scores (<5 points, n = 7), the prevalence of H. pylori infection remained on the similar decreasing trend (Supplementary Figure S6). Additionally, prevalence of other important modifiable risk factors and relative risks with GC are summarized in Supplementary Table S1. The prevalence of smoking and alcohol consumption was higher in males than that in females and declined significantly from 1990 to 2015 in both sexes. As well, the proportion of pickled vegetable consumption decreased by approximately 10% in both sexes in 2015 compared to 1990. For both sexes, we observed a reduction on the prevalence of underweight, but a significant increase on overweight, obese, and diabetes from 1990 to 2015.
Disease burden and time trends of GC attributable to main risk factors from 2000 to 2050
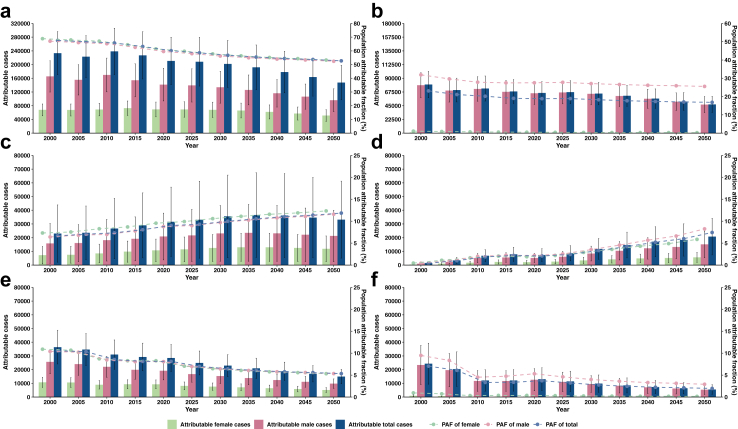
Tables 2 and 3 present the PAFs for the selected modifiable risk factors and the attributable cases for GC from 2000 to 2050 at a 5-year interval. We observed decreasing trends of the PAFs of H. pylori infection, smoking, pickled vegetable and alcohol consumption, while increasing trends of the PAFs of unhealthy BMI and diabetes (Fig. 2). The combined PAFs of above-mentioned risk factors for GC were expected to decline by 10.57% from 2000 to 2050. The greatest reduction on GC case numbers from 2000 to 2050 was attributable to H. pylori infection, followed by tobacco control, restriction of pickled vegetable and alcohol consumption. In contrast, having an unhealthy BMI and suffering from diabetes were estimated to contribute to an increased cases number of GC.
Table 2.
PAFs of modifiable risk factors for GC from 2000 to 2050.
| Risk factor | PAF (95% CIs) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | 2020 | 2025 | 2030 | 2035 | 2040 | 2045 | 2050 | |
| Male | |||||||||||
| H. pyloria | 67.09 (48.58, 85.60) | 66.06 (47.31, 84.80) | 65.24 (46.30, 84.18) | 62.59 (43.09, 82.08) | 59.56 (39.53, 79.60) | 57.99 (37.78, 78.20) | 56.24 (36.70, 75.79) | 55.00 (35.95, 74.06) | 54.02 (35.37, 72.66) | 53.38 (34.99, 71.77) | 52.42 (34.32, 70.51) |
| Smokingb | 31.90 (23.55, 40.25) | 29.67 (21.65, 37.69) | 27.87 (20.14, 35.59) | 27.49 (19.83, 35.16) | 27.54 (19.87, 35.21) | 27.78 (20.07, 35.50) | 27.10 (19.57, 34.62) | 26.59 (19.21, 33.98) | 26.18 (18.91, 33.45) | 25.92 (18.72, 33.12) | 25.57 (18.47, 32.67) |
| Pickled vegetablec | 10.41 (6.89, 13.92) | 10.21 (6.76, 13.67) | 8.48 (5.55, 11.40) | 8.07 (5.28, 10.87) | 8.07 (5.28, 10.87) | 6.97 (4.52, 9.41) | 6.43 (4.17, 8.68) | 6.05 (3.93, 8.18) | 5.76 (3.74, 7.78) | 5.58 (3.62, 7.54) | 5.35 (3.47, 7.23) |
| Alcohold | 9.48 (3.71, 15.26) | 8.35 (3.20, 13.50) | 4.53 (1.62, 7.43) | 4.75 (1.71, 7.80) | 5.31 (1.93, 8.70) | 4.61 (1.65, 7.57) | 4.00 (1.43, 6.57) | 3.60 (1.29, 5.92) | 3.31 (1.18, 5.43) | 3.14 (1.12, 5.14) | 2.92 (1.04, 4.79) |
| Unhealthy BMIe | 6.42 (0.51, 12.32) | 6.82 (1.11, 12.53) | 6.99 (1.14, 12.83) | 7.78 (1.28, 14.28) | 8.78 (1.61, 15.95) | 9.00 (1.08, 16.93) | 9.70 (1.16, 18.24) | 10.26 (1.23, 19.29) | 10.76 (1.29, 20.23) | 11.10 (1.33, 20.87) | 11.59 (1.39, 21.79) |
| Diabetesf | 0.42 (0.15, 0.69) | 0.98 (0.34, 1.62) | 1.97 (0.70, 3.25) | 2.25 (0.80, 3.69) | 2.17 (0.78, 3.57) | 2.54 (0.91, 4.16) | 3.53 (1.26, 5.78) | 4.55 (1.63, 7.47) | 5.68 (2.04, 9.31) | 6.62 (2.38, 10.86) | 8.25 (2.96, 13.53) |
| All above | 83.07 (64.99, 94.53) | 81.88 (63.28, 93.91) | 80.03 (60.88, 92.95) | 78.59 (58.40, 92.12) | 77.24 (56.06, 91.28) | 76.13 (54.22, 90.63) | 75.03 (53.07, 89.60) | 74.38 (52.32, 88.95) | 73.97 (51.80, 88.53) | 73.78 (51.50, 88.32) | 73.60 (51.05, 88.14) |
| Female | |||||||||||
| H. pylori | 68.89 (51.48, 86.31) | 67.97 (50.36, 85.59) | 67.10 (49.22, 84.97) | 64.45 (45.95, 82.96) | 61.44 (42.31, 80.57) | 59.71 (40.27, 79.15) | 57.80 (38.91, 76.69) | 56.38 (37.87, 74.89) | 55.17 (36.96, 73.38) | 54.32 (36.28, 72.37) | 53.29 (35.51, 71.08) |
| Smoking | 1.09 (0.33, 1.85) | 0.87 (0.26, 1.48) | 0.70 (0.21, 1.19) | 0.57 (0.17, 0.97) | 0.50 (0.15, 0.86) | 0.44 (0.13, 0.75) | 0.36 (0.11, 0.62) | 0.32 (0.09, 0.55) | 0.29 (0.08, 0.49) | 0.27 (0.08, 0.46) | 0.24 (0.07, 0.42) |
| Pickled vegetable | 10.91 (7.24, 14.57) | 10.68 (7.08, 14.27) | 8.72 (5.72, 11.72) | 8.28 (5.42, 11.14) | 8.28 (5.42, 11.14) | 7.05 (4.58, 9.52) | 6.47 (4.20, 8.74) | 6.07 (3.94, 8.20) | 5.76 (3.74, 7.78) | 5.57 (3.62, 7.52) | 5.33 (3.46, 7.19) |
| Alcohol | 0.95 (0.32, 1.59) | 0.74 (0.25, 1.23) | 0.33 (0.10, 0.56) | 0.37 (0.12, 0.62) | 0.37 (0.12, 0.62) | 0.31 (0.10, 0.52) | 0.25 (0.08, 0.42) | 0.21 (0.07, 0.36) | 0.19 (0.06, 0.31) | 0.17 (0.06, 0.29) | 0.15 (0.05, 0.26) |
| Unhealthy BMI |
7.32 (0.91, 13.73) | 7.55 (1.38, 13.72) | 8.28 (1.70, 14.86) | 8.70 (1.89, 15.50) | 9.55 (2.24, 16.87) | 9.92 (2.13, 17.71) | 10.59 (2.28, 18.90) | 11.12 (2.39, 19.84) | 11.58 (2.49, 20.67) | 11.90 (2.56, 21.24) | 12.35 (2.66, 22.04) |
| Diabetes | 0.45 (0.16, 0.75) | 1.09 (0.38, 1.80) | 1.64 (0.58, 2.71) | 2.05 (0.73, 3.37) | 1.90 (0.67, 3.13) | 2.19 (0.78, 3.60) | 2.89 (1.03, 4.75) | 3.59 (1.28, 5.89) | 4.31 (1.53, 7.08) | 4.89 (1.74, 8.04) | 5.86 (2.09, 9.63) |
| All above | 74.95 (55.77, 90.32) | 74.27 (54.91, 89.82) | 73.19 (53.36, 89.20) | 71.12 (50.36, 87.84) | 68.90 (47.16, 86.31) | 67.25 (44.79, 85.22) | 65.94 (43.50, 83.74) | 65.07 (42.58, 82.77) | 64.42 (41.83, 82.05) | 64.01 (41.28, 81.63) | 63.66 (40.73, 81.21) |
| Both sex | |||||||||||
| H. pylori | 67.61 (49.41, 85.80) | 66.63 (48.22, 85.04) | 65.77 (47.13, 84.41) | 63.17 (43.99, 82.36) | 60.17 (40.43, 79.91) | 58.55 (38.59, 78.51) | 56.76 (37.43, 76.09) | 55.47 (36.60, 74.34) | 54.41 (35.92, 72.91) | 53.71 (35.44, 71.98) | 52.72 (34.73, 70.71) |
| Smoking | 23.11 (16.92, 29.29) | 21.13 (15.30, 26.95) | 20.18 (14.50, 25.85) | 19.03 (13.65, 24.42) | 18.85 (13.53, 24.17) | 18.90 (13.59, 24.21) | 18.25 (13.13, 23.37) | 17.72 (12.75, 22.68) | 17.31 (12.46, 22.16) | 17.06 (12.28, 21.84) | 16.85 (12.13, 21.56) |
| Pickled vegetable | 10.55 (6.99, 14.11) | 10.35 (6.85, 13.85) | 8.55 (5.60, 11.49) | 8.14 (5.32, 10.95) | 8.14 (5.32, 10.96) | 6.99 (4.54, 9.45) | 6.44 (4.18, 8.70) | 6.06 (3.93, 8.18) | 5.76 (3.74, 7.78) | 5.58 (3.62, 7.53) | 5.34 (3.47, 7.22) |
| Alcohol | 7.05 (2.74, 11.36) | 6.09 (2.32, 9.86) | 3.34 (1.19, 5.49) | 3.38 (1.21, 5.54) | 3.72 (1.35, 6.10) | 3.21 (1.15, 5.28) | 2.76 (0.98, 4.53) | 2.46 (0.88, 4.04) | 2.24 (0.80, 3.68) | 2.11 (0.75, 3.47) | 1.97 (0.70, 3.23) |
| Unhealthy BMI |
6.67 (0.62, 12.72) | 7.04 (1.19, 12.88) | 7.35 (1.30, 13.41) | 8.07 (1.47, 14.66) | 9.03 (1.81, 16.25) | 9.30 (1.42, 17.18) | 9.99 (1.53, 18.45) | 10.55 (1.62, 19.48) | 11.04 (1.70, 20.38) | 11.38 (1.76, 21.00) | 11.85 (1.83, 21.88) |
| Diabetes | 0.43 (0.15, 0.71) | 1.01 (0.35, 1.67) | 1.88 (0.67, 3.10) | 2.18 (0.78, 3.59) | 2.09 (0.74, 3.43) | 2.43 (0.87, 3.98) | 3.32 (1.19, 5.44) | 4.23 (1.51, 6.93) | 5.21 (1.86, 8.55) | 6.03 (2.16, 9.89) | 7.43 (2.66, 12.19) |
| All above | 80.75 (62.36, 93.33) | 79.62 (60.80, 92.70) | 78.09 (58.75, 91.89) | 76.24 (55.87, 90.77) | 74.56 (53.20, 89.68) | 73.24 (51.16, 88.87) | 72.02 (49.90, 87.66) | 71.23 (49.03, 86.86) | 70.7 (48.38, 86.31) | 70.41 (47.97, 86.01) | 70.18 (47.50, 85.76) |
Abbreviations: PAF, population attributable fraction; GC, gastric cancer; CIs, confidence intervals; H. pylori, helicobacter pylori; BMI, body mass index.
The positive status of H. pylori infection as defined by enzyme-linked immunosorbent assay (ELISA), immunoblotting or urea breath test (UBT).
Currently smoking, accumulated up to 100 cigarettes, and still smoking.
Exclude those who did not use or consumed pickled vegetable minimally.
Regular alcohol consumption, drinking alcohol at least three times a week.
Unhealthy BMI included underweight (BMI < 18.5) and overweight/obesity (BMI > 25.0).
The diabetes as defined by the American Diabetes Association criteria.
Table 3.
Attributable cases of modifiable risk factors for GC from 2000 to 2050.
| Risk factor | Attributable cases (95% CIs) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | 2020 | 2025 | 2030 | 2035 | 2040 | 2045 | 2050 | |
| Male | |||||||||||
| H. pyloria | 165,459 (119,816, 211,102) | 155,469 (111,353, 199,584) | 169,670 (120,414, 218,926) | 154,181 (106,157, 202,204) | 141,472 (93,896, 189,048) | 139,269 (90,725, 187,813) | 133,884 (87,353, 180,414) | 126,020 (82,365, 169,674) | 116,239 (76,113, 156,365) | 106,455 (69,780, 143,129) | 96,112 (62,933, 129,292) |
| Smokingb | 78,674 (58,077, 99,262) | 69,826 (50,952, 88,702) | 72,470 (52,377, 92,557) | 67,733 (48,852, 86,618) | 65,401 (47,194, 83,628) | 66,724 (48,199, 85,255) | 64,500 (46,592, 82,413) | 60,929 (44,013, 77,850) | 56,343 (40,700, 71,991) | 51,688 (37,338, 66,043) | 46,883 (33,867, 59,904) |
| Pickled vegetablec | 25,665 (16,992, 34,328) | 24,038 (15,909, 32,172) | 22,050 (14,434, 29,647) | 19,892 (13,007, 26,779) | 19,178 (12,541, 25,818) | 16,730 (10,855, 22,599) | 15,298 (9926, 20,664) | 13,869 (8998, 18,734) | 12,398 (8044, 16,747) | 11,132 (7223, 15,037) | 9811 (6366, 13,252) |
| Alcohold | 23,389 (9149, 37,633) | 19,658 (7531, 31,772) | 11,769 (4213, 19,323) | 11,711 (4213, 19,216) | 12,619 (4584, 20,664) | 11,078 (3963, 18,180) | 9527 (3408, 15,634) | 8259 (2954, 13,554) | 7120 (2547, 11,685) | 6252 (2236, 10,260) | 5351 (1914, 8782) |
| Unhealthy BMIe | 15,822 (1259, 30,385) | 16,056 (2618, 29,494) | 18,169 (2968, 33,370) | 19,161 (3143, 35,180) | 20,849 (3813, 37,886) | 21,624 (2593, 40,655) | 23,090 (2768, 43,411) | 23,512 (2819,44,205) | 23,154 (2776, 43,532) | 22,138 (2654, 41,622) | 21,256 (2549, 39,964) |
| Diabetesf | 1031 (370, 1702) | 2302 (800, 3813) | 5134 (1820, 8452) | 5536 (1971, 9090) | 5165 (1853, 8479) | 6093 (2185, 9990) | 8391 (3010, 13,759) | 10,436 (3743, 17,112) | 12,224 (4385, 20,044) | 13,210 (4738, 21,661) | 15,134 (5428, 24,816) |
| All above | 204,849 (160,266, 233,127) | 192,698 (148,929, 221,023) | 208,119 (158,337, 241,728) | 193,605 (143,877, 226,935) | 183,459 (133,141, 216,796) | 182,820 (130,217, 217,657) | 178,612 (126,326, 213,274) | 170,414 (119,876, 203,799) | 159,170 (111,461, 190,503) | 147,136 (102,699, 176,127) | 134,964 (93,612, 161,621) |
| Female | |||||||||||
| H. pylori | 67,876 (50,720, 85,032) | 67,487 (49,995, 84,980) | 68,894 (50,542, 87,246) | 72,770 (51,877, 93,663) | 69,111 (47,594, 90,627) | 69,038 (46,565, 91,511) | 68,018 (45,784, 90,252) | 65,909 (44,274, 87,543) | 61,847 (41,437, 82,256) | 57,169 (38,178, 76,160) | 51,344 (34,212, 68,475) |
| Smoking | 1072 (325, 1823) | 866 (258, 1469) | 718 (216, 1222) | 642 (192, 1095) | 566 (169, 967) | 507 (150, 867) | 429 (127, 735) | 373 (111, 638) | 320 (95, 549) | 281 (83, 481) | 235 (70, 402) |
| Pickled vegetable | 10,744 (7133, 14,355) | 10,600 (7029, 14,168) | 8953 (5873, 12,034) | 9345 (6119, 12,577) | 9310 (6096, 12,530) | 8151 (5295, 11,007) | 7614 (4947, 10,283) | 7098 (4611, 9585) | 6458 (4196, 8722) | 5864 (3810, 7919) | 5132 (3334, 6930) |
| Alcohol | 938 (315, 1567) | 733 (248, 1221) | 339 (103, 575) | 418 (135, 700) | 417 (135, 697) | 357 (116, 601) | 292 (94, 491) | 247 (80, 416) | 208 (67, 350) | 180 (58, 303) | 149 (48,250) |
| Unhealthy BMI | 7209 (896, 13,523) | 7495 (1370, 13,621) | 8502 (1746, 15,258) | 9819 (2138, 17,499) | 10,746 (2521, 18,970) | 11,475 (2468, 20,482) | 12,458 (2680, 22,237) | 12,995 (2795, 23,196) | 12,982 (2792, 23,173) | 12,522 (2693, 22,351) | 11,899 (2559, 21,238) |
| Diabetes | 447 (158, 739) | 1082 (377, 1787) | 1689 (596, 2783) | 2311 (824, 3805) | 2138 (754, 3521) | 2535 (902, 4162) | 3405 (1211, 5590) | 4192 (1491, 6883) | 4833 (1719, 7934) | 5152 (1832, 8457) | 5648 (2009, 9273) |
| All above | 73,842 (54,944, 88,990) | 73,734 (54,518, 89,175) | 75,145 (54,788, 91,591) | 80,293 (56,857, 99,167) | 77,494 (53,045, 97,074) | 77,759 (51,785, 98,535) | 77,594 (51,189, 98,541) | 76,069 (49,783, 96,757) | 72,218 (46,887, 91,977) | 67,371 (43,441, 85,910) | 61,333 (39,243, 78,242) |
| Both sex | |||||||||||
| H. pylori | 233,335 (170,536, 296,134) | 222,956 (161,348, 284,564) | 238,564 (170,956, 306,172) | 226,951 (158,034, 295,868) | 210,583 (141,490, 279,676) | 208,307 (137,290, 279,324) | 201,902 (133,137, 270,666) | 191,929 (126,640, 257,218) | 178,086 (117,551, 238,621) | 163,623 (107,958, 219,289) | 147,456 (97,145, 197,767) |
| Smoking | 79,746 (58,402, 101,084) | 70,692 (51,210, 90,171) | 73,187 (52,592, 93,779) | 68,375 (49,044, 87,713) | 65,967 (47,362, 84,595) | 67,231 (48,349, 86,122) | 64,929 (46,720, 83,148) | 61,302 (44,123, 78,489) | 56,663 (40,795, 72,539) | 51,969 (37,421, 66,525) | 47,118 (33,936, 60,306) |
| Pickled vegetable | 36,409 (24,125, 48,683) | 34,638 (22,939, 46,340) | 31,002 (20,307, 41,681) | 29,237 (19,127, 39,356) | 28,488 (18,637, 38,347) | 24,881 (16,150, 33,606) | 22,912 (14,873, 30,947) | 20,967 (13,610, 28,319) | 18,856 (12,240, 25,468) | 16,996 (11,032, 22,956) | 14,943 (9700, 20,182) |
| Alcohol | 24,327 (9465, 39,200) | 20,391 (7779, 32,993) | 12,108 (4316, 19,898) | 12,129 (4348, 19,916) | 13,036 (4719, 21,361) | 11,435 (4078, 18,781) | 9819 (3502, 16,125) | 8507 (3034, 13,970) | 7329 (2614, 12,035) | 6432 (2295, 10,563) | 5500 (1962, 9032) |
| Unhealthy BMI | 23,031 (2155, 43,908) | 23,552 (3988, 43,115) | 26,671 (4713, 48,628) | 28,980 (5281, 52,679) | 31,595 (6335, 56,856) | 33,099 (5061, 61,136) | 35,548 (5448, 65,648) | 36,508 (5614, 67,401) | 36,137 (5569, 66,705) | 34,660 (5348, 63,973) | 33,155 (5108, 61,202) |
| Diabetes | 1478 (528, 2441) | 3385 (1177, 5600) | 6823 (2416, 11,235) | 7848 (2795, 12,895) | 7303 (2606, 12,000) | 8628 (3087, 14,153) | 11,796 (4221, 19,349) | 14,628 (5235, 23,995) | 17,057 (6104, 27,978) | 18,362 (6571, 30,119) | 20,782 (7438, 34,089) |
| All above | 278,691 (215,210, 322,117) | 266,432 (203,447, 310,198) | 283,264 (213,125, 333,319) | 273,897 (200,734, 326,102) | 260,953 (186,186, 313,870) | 260,579 (182,002, 316,192) | 256,206 (177,516, 311,815) | 246,483 (169,659, 300,556) | 231,387 (158,348, 282,479) | 214,507 (146,139, 262,037) | 196,297 (132,856, 239,863) |
Abbreviations: GC, gastric cancer; CIs, confidence intervals; H. pylori, helicobacter pylori; BMI, body mass index.
The positive status of H. pylori infection as defined by enzyme-linked immunosorbent assay (ELISA), immunoblotting or urea breath test (UBT).
Currently smoking, accumulated up to 100 cigarettes, and still smoking.
Exclude those who did not use or consumed pickled vegetable minimally.
Regular alcohol consumption, drinking alcohol at least three times a week.
Unhealthy BMI included underweight (BMI <18.5) and overweight/obesity (BMI>25.0).
The diabetes as defined by the American Diabetes Association criteria.
Fig. 2.
Time trends of population attributable fraction and estimated number of modifiable risk factors for GC in males and females in China. Notes: The scatter and bar represent estimated attributable fraction and cases from 2000 to 2050 for (a) H. pylori, (b) Smoking, (c) Unhealthy BMI, (d) Diabetes, (e) Pickled vegetable, and (f) Alcohol consumption, respectively. Error bar represents the 95% CIs for attributable cases. The two y-axes on either side represent the attributable cases (left side) and the population attributable fraction (right side), respectively. Abbreviations: CGC, cardia gastric cancer; NCGC, non-cardia gastric cancer; H. pylori, Helicobacter pylori; BMI, body mass index; CIs, confidence intervals; PAF: population attributable fraction.
By anatomical subsite, sex and region, the PAFs and attributable cases of H. pylori infection were higher in NCGC than in CGC from 2000 to 2050, and the trends of the attributable burden of other risk factors were similar for NCGC and CGC (Table 4, Supplementary Tables S4 and S5). The trends of PAFs of each risk factor were similar for males and females in GC, with higher PAFs of smoking and alcohol consumption in males. And the number of attributed cases for each risk factor was higher in males than in females (Supplementary Figure S7). The overall PAFs of modifiable risk factors of CGC/NCGC were both higher in rural areas than in urban areas, with higher PAFs for H. pylori infection, smoking, pickled vegetable and unhealthy BMI in rural areas, but higher PAFs for diabetes in urban areas (Supplementary Tables S6 and S7). In sensitivity analyses, we excluded several controversial risk factors of GC, including diabetes and low BMI, and found quite comparable estimates for a combined PAF of 73.36% for GC (Supplementary Tables S8 and S9).
Table 4.
Population attributable fraction and attributable cases of modifiable risk factors for CGC and NCGC from 2000 to 2050.
| Risk factor | 2000 | 2005 | 2010 | 2015 | 2020 | 2025 | 2030 | 2035 | 2040 | 2045 | 2050 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CGC | |||||||||||
| The PAF (95% CIs) of H. pyloria | 56.13 (30.95, 81.30) | 54.90 (29.59, 80.22) | 54.01 (28.61, 79.40) | 51.14 (25.59, 76.68) | 48.11 (22.58, 73.63) | 46.45 (21.02, 71.88) | 44.62 (20.19, 69.05) | 43.30 (19.59, 67.01) | 42.24 (19.11, 65.36) | 41.57 (18.81, 64.32) | 40.67 (18.40, 62.94) |
| The attributable cases (95% CIs) of H. pyloria | 78,422 (43,246, 113,598) | 73,730 (39,737, 107,723) | 79,463 (42,098, 116,829) | 75,154 (37,609, 112,698) | 71,038 (33,349, 108,727) | 70,377 (31,844, 108,909) | 67,992 (30,765, 105,218) | 64,381 (29,131, 99,631) | 59,593 (26,965, 92,222) | 54,902 (24,842, 84,962) | 49,945 (22,599, 77,291) |
| The PAF (95% CIs) of all risk factors | 74.38 (49.18, 91.44) | 72.93 (47.27, 90.59) | 70.96 (44.80, 89.49) | 68.92 (41.90, 88.04) | 67.31 (39.71, 86.72) | 65.86 (37.67, 85.69) | 64.56 (36.54, 84.27) | 63.73 (35.75, 83.34) | 63.17 (35.17, 82.69) | 62.89 (34.83, 82.35) | 62.81 (34.60, 82.13) |
| The attributable cases (95% CIs) of all risk factors | 103,928 (68,719, 127,769) | 97,938 (63,481, 121,654) | 104,410 (65,911, 131,678) | 101,285 (61,581, 129,393) | 99,403 (58,646, 128,057) | 99,788 (57,085, 129,844) | 98,385 (55,684, 128,416) | 94755 (53,156, 123,909) | 89,129 (49,622, 116,667) | 83,069 (45,999, 108,771) | 77,122 (42,493, 100,858) |
| NCGC | |||||||||||
| The PAF (95% CIs) of H. pylori | 75.42 (61.97, 88.86) | 74.49 (60.70, 88.27) | 73.79 (59.77, 87.82) | 71.51 (56.73, 86.28) | 68.97 (53.45, 84.50) | 67.53 (51.63, 83.43) | 65.86 (50.35, 81.37) | 64.64 (49.41, 79.86) | 63.64 (48.65, 78.62) | 63.00 (48.16, 77.84) | 62.14 (47.51, 76.78) |
| The attributable cases (95% CIs) of H. pylori | 154,913 (127,290, 182,536) | 149,226 (121,611, 176,841) | 159,101 (128,858, 189,343) | 151,797 (120,425, 183,169) | 139,545 (108,141, 170,949) | 137,931 (105,446, 170,415) | 133,910 (102,372, 165,448) | 127,548 (97,508, 157,587) | 118,493 (90,586, 146,399) | 108,721 (83,116, 134,326) | 97,511 (74,546, 120,476) |
| The PAF (95% CIs) of all risk factors | 85.08 (71.32, 94.61) | 84.10 (69.86, 94.11) | 82.95 (68.28, 93.52) | 81.31 (65.55, 92.66) | 79.85 (63.04, 91.84) | 78.72 (61.16, 91.23) | 77.62 (59.92, 90.20) | 76.89 (59.04, 89.52) | 76.40 (58.39, 89.05) | 76.16 (58.03, 88.81) | 75.95 (57.59, 88.59) |
| The attributable cases (95% CIs) of all risk factors | 174,763 (146,490, 194,348) | 168,494 (139,966, 188,544) | 178,854 (147,213, 201,641) | 172,612 (139,153, 196,709) | 161,550 (127,540, 185,813) | 160,791 (124,917, 186,348) | 157,821 (121,831, 183,400) | 151,728 (116,503, 176,647) | 142,258 (108,727, 165,812) | 131,438 (100,140, 153,266) | 119,175 (90,363, 139,005) |
Abbreviations: H. pylori, helicobacter pylori; PAF, population attributable fraction; CGC, cardia gastric cancer; NCGC, non-cardia gastric cancer.
The positive status of H. pylori infection as defined by enzyme-linked immunosorbent assay (ELISA), immunoblotting or urea breath test (UBT).
Table 5 tabulates the estimated burden of GC overall and by anatomical subsites in different scenarios of risk factor exposure. According to the NCCR data, we estimated there were 349,988 GC cases (147,670 CGC and 202,318 NCGC cases) in 2020, among which 260,953 (99,403 CGC and 161,550 NCGC) were attributable to the above-mentioned modifiable risk factors. However, if China has not implemented the existing health policy on risk factor control in past 20 years, which means the combined PAFs in 2020 would be similar as that in 2000, there would be 19,781 more cases for GC (9749 more CGC and 10,032 more NCGC cases). Otherwise, if these modifiable risk factors have been controlled at the theoretical minimum-risk exposure distribution (TMRED), the number of GC would have been reduced to 89,035 (including 48,268 CGC and 40,767 NCGC cases). Similarly, we predict that there would be 10,835 GC (4782 CGC and 6053 NCGC cases) could be avoided in 2050 if continued to implement the existing health policy on risk factors control, and the number of GC would further decrease to 83,410 (45,674 and 37,737 for CGC and NCGC, respectively) in 2050 if China made greatest effects to keep these risk factors at the TMRED level.
Table 5.
Current and projected new cases for GC overall and anatomical subsites in different scenarios and the avoided cases from 2000 to 2050.
| Subtype | 2020 |
2050 |
Avoided cases from 2000 to 2020g | Avoided cases from 2020 to 2050h | ||||
|---|---|---|---|---|---|---|---|---|
| Scenario 1a | Currentb | Scenario 2c | Scenario 1d | Projectione | Scenario 2f | |||
| GC | ||||||||
| Male | 250,883 | 237,512 | 54,053 | 189,991 | 183,367 | 48,403 | 13,371 | 6,624 |
| Female | 118,887 | 112,476 | 34,982 | 100,551 | 96,340 | 35,008 | 6,411 | 4,211 |
| Total | 369,770 | 349,988 | 89,035 | 290,542 | 279,707 | 83,410 | 19,781 | 10,835 |
| CGC | ||||||||
| Male | 114,192 | 107,092 | 31,282 | 86,160 | 83,085 | 27,343 | 7,100 | 3,074 |
| Female | 43,227 | 40,578 | 16,986 | 41,418 | 39,710 | 18,330 | 2,649 | 1,708 |
| Total | 157,419 | 147,670 | 48,268 | 127,577 | 122,796 | 45,674 | 9,749 | 4,782 |
| NCGC | ||||||||
| Male | 136,691 | 130,420 | 22,771 | 103,831 | 100,281 | 21,059 | 6,270 | 3,550 |
| Female | 75,660 | 71,898 | 17,996 | 59,133 | 56,630 | 16,678 | 3,762 | 2,503 |
| Total | 212,351 | 202,318 | 40,767 | 162,965 | 156,912 | 37,737 | 10,032 | 6,053 |
Abbreviations: GC, gastric cancer; CGC, cardia gastric cancer; NCGC: non-cardia gastric cancer; PAF, population attributable fraction; TMRED, theoretical minimum-risk exposure distribution.
The part of calculation of the number of cases attributed to modifiable risk factors based on the combined PAF maintained in 2000.
The part of calculation of the number of cases attributed to modifiable risk factors based on the combined PAF in 2020.
Calculation of the number of cases based on the combined PAF under TMRED of modifiable risk factors in 2020.
The part of calculation of the number of cases attributed to modifiable risk factors based on the combined PAF maintained in 2020.
The part of calculation of the number of cases attributed to modifiable risk factors based on the PAF projections in 2050 according to current trends.
Calculation of the attributed cases based on the combined PAF under TMRED of modifiable risk factors in 2050.
Difference in the number of attributable cases between scenario 1 and current situation in 2020.
Difference in the number of attributable cases between scenario 1 and projection situation in 2050.
Discussion
This study is the first to evaluate the PAF trajectories of important modifiable risk factors for GC overall and by anatomical subsite in China. We observed declining trends of the PAFs of H. pylori infection, smoking, pickled vegetable and alcohol consumption, but increasing trends of the PAFs of unhealthy BMI and diabetes for GC in China from 2000 to 2050. Although there was an overall declining trend of the age-standardized incidence rate of GC overall and by anatomical subsite from 2000 to 2050, we predict there will be 279,707 GC cases (122,796 CGC and 156,911 NCGC cases) in China in 2050 taking account into the population aging, among which 70.18% of GC cases were attributable to potentially modifiable risk factors.
H. pylori infection, which was associated with factors such as hygienic conditions, and socioeconomic status, has been classified as a Group I carcinogen by IARC.19 Our findings confirmed that H. pylori infection will remain as the leading risk factor of GC in China in the coming decades, though it presented a declining prevalence among the Chinese population. The declining trend was in accordance with a previous estimation for the prevalence of H. pylori infection from 63.8% in 1983–1994 to 46.7% in 2006–2018.20 The declining trend is due to the joint efforts of academic groups and the public, including the issuance of a series of expert consensus on the prevention and control of H. pylori, such as the National Consensus Report on the Management of H. pylori Infection, Chinese Consensus Opinions on Chronic Gastritis, National Consensus on the Integrated Treatment of H. pylori by Traditional Chinese and Western Medicines, and Expert Consensus Opinions on the Eradication of H. pylori and Prevention and Control of Gastric Cancer etc., which provide guidelines for the prevention and control of H. pylori and GC.21 Other factors, such as the westernization of lifestyles (individual serving) and improvements in socioeconomic and sanitation (hygienic drinking water sources), also partly contributed to the declining trend of H. pylori infection in China.22,23 In addition, we found a higher prevalence and PAF of H. pylori infection in rural areas compared to that in urban areas. Per capita household income in urban areas was triple that of rural areas, and there was a higher population with a secondary or higher education level in urban areas, which may partly contribute to the health and socioeconomic inequalities between urban and rural areas.24 Similar trends have been observed in Japan. With the improved hygiene and economic recovery in past decades, the prevalence of H. pylori infection has declined rapidly by the birth cohort effect in Japan, and the recent prevalences in young birth cohorts were comparable to the level in the Western countries, and lower than those reported in South Korea and China.25,26 Effective implementation of a national public health program focused on cancer prevention and control from an early period has also greatly contributed to a significant decline in the incidence of GC in Japan.27 In contrary to the controversial results on the association of H. pylori infection with CGC in Western populations, most Asian studies showed a positive association between H. pylori and CGC, though more modest than that of NCGC.28 Evidence from multiplex serology indicates diverse associations between individual antigens and CGC and NCGC, as well as distinct pathogenic effects for a single H. pylori virulence factor across different ethnicities.29 Evidence from both in vivo and in vitro studies demonstrated a greater carcinogenic effect of the East Asian-type CagA protein when compared to the Western-type.30 Available evidence suggested two distinct etiologies for CGC.1 One type resembled NCGC, was the result of atrophic gastritis due to H. pylori infection and concentrated in East Asian populations. Another type was associated with GERD, was concentrated in Western populations, resembled features of esophageal adenocarcinoma, and H. pylori infection could reduce the extent of GERD by suppressing gastric acid secretion. Thus, we estimated that there were 48% of CGC and 69% of NCGC attributable to H. pylori in 2020 in China by using separate RRs from a representative case-cohort study in Chinese population.31 Moreover, the PAF of H. pylori will remain as 62% and 41% in 2050 under the current strategy on risk factor control. More robust efforts focused on the prevention and control of H. pylori infection are urgently needed to help reduce the burden of GC in the coming future. Eradication of H. pylori was reported as an effective strategy for preventing GC by IARC in 2014.32 The results of a systematic review and meta-analysis that included 10 randomized controlled trials (9 of which were conducted in East Asia) showed that H. pylori-positive individuals who have received eradication therapy reduced the risk of developing GC by half compared with those who received placebo or no treatment (RR: 0.54, 95% CI: 0.40–0.72).33 And a large intervention trial in China suggested that acceptable levels of H. pylori eradication therapy (72.9% overall eradication rate) are feasible as a strategy for gastric cancer prevention.34 Meanwhile, serologic screening for H. pylori and eradication of infection in people over 50 years of age in areas with a high burden of GC has been shown to be cost-effective,35 but the increase in antibiotic resistance and the unknown results of antibiotic use in large populations warrant caution in considering expanded eradication strategies.36 In addition to the above-mentioned “testing/screening and treatment” strategies, the novel concept of “whole family-based H. pylori infection control and management strategy” introduced by the government has facilitated the curb transmission and clinical practice in managing H. pylori infection.21
Smoking was also a major contributing risk factor to the GC burden. The latest global burden of disease study noted the age-standardized prevalence of daily smoking contributed to 25% of the disease burden among males and 5.4% among females worldwide.37 Some Chinese reported the PAF of smoking for GC ranging from 17% to 31% in males and 0.9%–4% in females, which was comparable to our estimations for a PAF of 28% for males and 0.5% for females in 2020.38,39 It should be noted that the actual burden of smoking would be even higher because we did not include second-hand smoking and electronic cigarette smoking in the present estimation. We observed a decreasing trend of the PAF for smoking from 2000 to 2050, which indicates a further reduction of GC burden in the coming future following established models of the Western countries. Smoking prevalence among middle-aged men in the UK halved between 1990 and 1950, and among adults in the US dropped by 67% in 2017 compared to 1965.40,41 The burden of smoking-related cancers, including lung cancer and GC, has likewise declined in these countries.37 In addition, the PAF of smoking was much higher in males than in females, which was mainly due to the higher prevalence of smoking in males caused by the socio-cultural characteristics of Chinese society that were quite tolerant of male smoking.42 Though considerable progress has been made in China on tobacco control,43 there were still 17% of GC were predicted attributable to smoking in 2050. More strict policies including restrictions on smoking in indoor public places and higher taxes on tobacco products are needed to meet the objective of reducing the smoking prevalence to 20% in “Healthy China 2030” Program.44
We found declining trends of the PAFs of pickled vegetable and alcohol consumption, which were in line with a global estimation on their PAFs of total cancer burden. The association of pickled vegetable with GC has been extensively investigated in Asia,45 and our result supported an even stronger role of pickled vegetable on GC by summarizing the prevalence data from recent representative national surveys and estimating RRs from the credible meta-analysis of Asian populations.39 However, the estimated PAF of pickled vegetable on GC was lower than that of Korea during the same period, probably due to the difference on the traditional dietary habits on vegetable preservation and cooking.46 For past decades, widespread using of household refrigerators in China allowed healthier preservation of vegetables, and continued education significantly reduced the consumption of pickled vegetables, which reduced the N-nitroso compounds that may be produced during food preparation.47 Alcohol consumption increased the risk of GC, especially in East Asians.48 Certain polymorphisms of aldehyde dehydrogenase 2 (ALDH2) in East Asian populations were associated with the flushing response caused by the rapid accumulation of the carcinogen acetaldehyde.49 And reliance on self-reported information of drinking status and lack of stratification of H. pylori status might lead to an underestimation of the association.50 One interpretation was that the damaging effects of bacteria on the gastric mucosa enhanced the genotoxic effects of acetaldehyde.51 Compared to the PAF of alcohol consumption for GC in western populations such as the US (1%), our study found about 4% of GC in China were currently attributable to alcohol consumption, which was higher in males than in females.52 The sex difference might be related to the traditional culture in China for more social activities among Chinese men. The public health measures mentioned in the “Medium-to-Long Term for the Prevention and Treatment of Chronic Diseases in China” could provide references for reducing the burden of GC attributable to these risk factors in the future, which involves limiting alcohol consumption, prohibiting the sale of alcohol to adolescents, and public education on healthy dietary structure including reducing the intake of pickled vegetable and salt-processed food and increasing the intake of fresh vegetables and fruits.53
It was noteworthy for the rising burden of GC attributable to unhealthy BMI and diabetes over the next 30 years. In contrast with some previous studies that only considered the role of overweight and obesity on GC burden,18,39,54 we assessed the GC burden attributable to unhealthy BMI comprised of the dual contributions from overweight/obesity and underweight in China, considering a U-shaped association between BMI and GC risk in Asian populations.55 The observed increasing prevalence of overweight and obesity in China was consistent with the recent trends in other Asian populations and globally.54,56,57 This was considered related to the growth of sedentary behavior and transformation of dietary structure in Chinese population.54,58 Meanwhile, the Chinese dietary pattern that has shifted to intake higher fat, sugar and dietary energy also played an important role on the increasing prevalence of diabetes.59 The latest data showed that the prevalence of diabetes in China (12.4% in 2018) was higher than the global level (8.3% in 2019), and lower than those in developed countries (US: 14.6% in 2018, Korea: 13.7% in 2016). The lower rates of awareness, treatment and control of diabetes in China compared with the US indicated that the coming times would be a critical period for diabetes control in China.60 Furthermore, diabetes and GC shared some important risk factors including smoking, obesity, etc.61 Previous studies have also reported diabetes significantly increased risk of GC after H. pylori eradication or after higher salt intake due to diabetes-induced taste impairment.62 These interactions between risk factors, which were calculated conservatively in our study, suggested that the burden of GC attributable to these factors remained underestimated. The Chinese government has also started to actively address these challenges by incorporating diabetes management into the National Basic Public Health Service Project since 2009,63 and by continuously emphasizing maintenance of normal body weight, reduction of oil use, and control of salt intake in the “Healthy Lifestyles for All 2017–2025 Action Protocol” and the “Healthy China 2030”,64 providing reliable guidance on measures to tackle the growing prevalence of unhealthy BMI and diabetes in China from an economic and health perspective.
Our results revealed an opposite role of risk factor control and demographic shift on the burden of GC, indicating that approximately 82% of the effects of risk factor control in GC would be deducted by the demographic changes. A comparative study on the latest cancer statistics between China and US found that increasing population size and aging were the leading determinants of incremental cancer burden in both countries, and even the role of population aging had the potential to overtake population size in foreseeable future.65 China was considered one of the fastest-growing aging population among the 57 countries with serious aging problems in the future in the GBD projection study, which will complicate the reduction of the cancer burden through risk factor control.66 Our estimates were derived from the population projection of the World Population Prospects 2022, which provides the latest estimates on one-year age group of the population for each single year and shows more visible changes in the precise year of occurrence for the impact of natural disasters or policy adjustments on fertility and mortality. In addition, although the universal two-child policy was announced in 2015 and three-children policy was announced in 2021 in China, the birth rate only slightly increased from 2016 to 2017 and continued to decrease after 2017 which was in line with the age-conditional changing trend of the Chinese population in past decades (Supplementary Figure S8). So, we assume the impact of two-child and three-children policy was partly reflected in the present population projections, and the future potential changes were acceptable in the forecasting analysis. Therefore, in addition to incorporating healthy aging strategies into prevention activities of GC, emphasis on risk factor prevention and control to some extent offsets the negative impact of the irreversible process of population aging on the disease burden of GC. The decline in age-specific incidence of GC among older groups suggested by our results may reflect variations in risk factors among different age groups. For instance, young people were more likely to have obesity/overweight caused by unhealthy lifestyle,67 which elevated the risk of GC in younger age groups. The H. pylori screening and eradication strategy showed greater benefit in older groups. Therefore, it is essential to implement prevention strategies that target various risk factors to reduce the incidence of gastric cancer in different age groups. Our results also demonstrated that the implementation of the existing health policy on risk factor control can reduce the disease burden of GC from past to future, and even more than half of the number of GC cases can be avoided under a simulated scenario with more vigorous risk factor control instruments. Meanwhile, risk factor control as one of the primary prevention measures was feasible and low cost, which helped to rationalize the allocation of scarce healthcare resources and reduced the economic burden of GC.68
This study has some strengths. First, we used up-to-date representative data from 682 cancer registries covering about 470 million Chinese population to estimate burden of GC overall and by anatomical subsite. Second, to our knowledge, our study was the first to estimate and predict the trend of attributable burden of important modifiable risk factors for GC in China, overall and by sex and anatomical subsite, according to high-quality data from cancer registries with continuous surveillance in China. Thirdly, we used the BAPC model applying random wandering prior of different orders to the age, period and cohort parameters to avoid the identifiability problems of traditional APC models due to the co-linearity of parameters. Fourthly, we analyzed and predicted future GC cancer burdens that could be attributable to the demographic shift and evolution of risk factors, adding important evidence to help the policymakers allocate health resources for future GC cancer prevention and control. The study also has some limitations. Firstly, certain recognized risk factors, including the consumption of red and processed meat, low intake of vegetables and fruits, high salt intake, infection with the Epstein–Barr virus, autoimmune diseases, and medication use, were not covered in the present study since there is a lack of reliable prevalence data. Secondly, our study also lacked data on the quantity of ethanol consumption, which may bias the results due to the misclassification of very light drinkers who may not have an elevated risk of gastric cancer. Third, the PAF analyses by anatomical subsite mainly focused on H. pylori infection due to the lack of subsite specific RRs for other risk factors. In addition, we did not consider the interaction of risk factors due to lack of reliable RR estimates for interactive effects. These may lead to some bias in the estimates on the attributable burden of gastric cancer and the results should be interpreted with caution. Finally, the precision of H. pylori-associated PAF estimates might be affected by the use of H. pylori prevalence from meta-analyses in different periods instead of longitudinal surveillance data from a large population-based cohort, which was not available in the real world. Collaboration with other large prospective cohorts would be explored in the future to add surveillance data on H. pylori infection and to reduce bias due to differences in detection methods and populations studied.
In conclusion, great achievement has been made on GC prevention in China for past decades, yet more than half of GC remained attributable to modifiable risk factors. Continued effective strategies on risk factor control are needed, including enhancing H. pylori screening and treatment, tobacco control and maintaining healthy BMI to reduce the burden of this highly life-threatening cancer in future.
Contributors
Study concept and design: Shaoming Wang, Wenqiang Wei, Jie He. Funding acquisition: Shaoming Wang, Wenqiang Wei. Data collection and quality control: Feifan He, Jianhua Gu, Rongshou Zheng, Xinqing Li. Data analysis: Feifan He, Rongshou Zheng, Shaoming Wang. Manuscript draft: Feifan He, Shaoming Wang, Jianhua Gu. Manuscript review and editing: Hongmei Zeng, Kexin Sun, Ru Chen, Li Li, Bingfeng Han, Shaoming Wang, Wenqiang Wei, Jie He. All authors read and approved the final manuscript.
Data sharing statement
All the data used in this study were obtained from the public database, except for the incidence data of gastric cancer from the Chinese National Central Cancer Registry, and the sources of the public database were provided in the Supplementary Methods. The R programmes and the incidence data of gastric cancer in China will be available from the corresponding author upon reasonable request (weiwq@cicams.ac.cn).
Declaration of interests
All authors declare that no conflict of interest is directly related to the research.
Acknowledgements
We sincerely thank for the all staff from the local cancer registries who have made great contributions to data collection, supplements and auditing.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.101003.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Ferlay J., van Berge Henegouwen M.I., Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 3.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C.T., Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 4.Thrift A.P., El-Serag H.B. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleteiro B., Castro C., Morais S., Ferro A., Lunet N. Worldwide burden of gastric cancer attributable to tobacco smoking in 2012 and predictions for 2020. Dig Dis Sci. 2015;60(8):2470–2476. doi: 10.1007/s10620-015-3624-x. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization IARC . International Agency for Research on Cancer; Lyon, France: 2007. Attributable causes of cancer in France in the year 2000. [Google Scholar]
- 7.Soerjomataram I., Shield K., Marant-Micallef C., et al. Cancers related to lifestyle and environmental factors in France in 2015. Eur J Cancer. 2018;105:103–113. doi: 10.1016/j.ejca.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 8.de Martel C., Ferlay J., Franceschi S., et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 9.de Martel C., Georges D., Bray F., Ferlay J., Clifford G.M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z. Union Medical College; 2019. Trend and prediction of burden of stomach cancer in China [硕士]: Chinese Academy of medical Sciences and peking. [Google Scholar]
- 11.Shi J.F., Cao M., Wang Y., et al. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 2021;148(5):1051–1065. doi: 10.1002/ijc.33313. [DOI] [PubMed] [Google Scholar]
- 12.Pretorius R.G., Zhang W.H., Belinson J.L., et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191(2):430–434. doi: 10.1016/j.ajog.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 13.Møller B., Weedon-Fekjær H., Hakulinen T., et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev. 2002;11(Suppl 1):S1–S96. [PubMed] [Google Scholar]
- 14.WCRF/AICR . 2018. Continuous update project expert report 2018: diet, nutrition, physical activity and stomach cancer. [Google Scholar]
- 15.Islami F., Goding Sauer A., Miller K.D., et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 16.Barendregt J.J., Veerman J.L. Categorical versus continuous risk factors and the calculation of potential impact fractions. J Epidemiol Community Health. 2010;64(3):209–212. doi: 10.1136/jech.2009.090274. [DOI] [PubMed] [Google Scholar]
- 17.Ezzati M., Vander Hoorn S., Rodgers A., Lopez A.D., Mathers C.D., Murray C.J.L. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet. 2003;362(9380):271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M., Sawada N., Matsuda T., et al. Attributable causes of cancer in Japan in 2005--systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23(5):1362–1369. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]
- 19.Parsonnet J., Friedman G.D., Vandersteen D.P., et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 20.Li M., Sun Y., Yang J., et al. Time trends and other sources of variation in Helicobacter pylori infection in mainland China: a systematic review and meta-analysis. Helicobacter. 2020;25(5) doi: 10.1111/hel.12729. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X.Z., Lyu N.H., Zhu H.Y., et al. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: the time to change practice for related disease prevention. Gut. 2023;72(5):855–869. doi: 10.1136/gutjnl-2022-328965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen X., Wen D., Yang Y., Chen Y., Wang G., Shan B. Urban-rural disparity in helicobacter pylori infection-related upper gastrointestinal cancer in China and the decreasing trend in parallel with socioeconomic development and urbanization in an endemic area. Ann Glob Health. 2017;83(3–4):444–462. doi: 10.1016/j.aogh.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy P., Johansson S., Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. doi: 10.1186/s13099-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., Geng G., Zhang Q., et al. Inequality of household consumption and air pollution-related deaths in China. Nat Commun. 2019;10(1):4337. doi: 10.1038/s41467-019-12254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C., Nishiyama T., Kikuchi S., et al. Changing trends in the prevalence of H. pylori infection in Japan (1908-2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-15490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017;20(Suppl 1):3–7. doi: 10.1007/s10120-016-0658-5. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Ren N., Zhang B., et al. Gastric cancer incidence trends in China and Japan from 1990 to 2019: disentangling age-period-cohort patterns. Cancer. 2023;129(1):98–106. doi: 10.1002/cncr.34511. [DOI] [PubMed] [Google Scholar]
- 28.Wang S.M., Roth M.J., Murphy G.A., et al. Serologic profile of antiparietal cell antibodies, pepsinogens, and H. pylori and risk of upper gastrointestinal cancer: a nested case-control study in China. Cancer Epidemiol Biomarkers Prev. 2019;28(12):2022–2029. doi: 10.1158/1055-9965.EPI-19-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao P., Kartsonaki C., Butt J., et al. Helicobacter pylori multiplex serology and risk of non-cardia and cardia gastric cancer: a case-cohort study and meta-analysis. Int J Epidemiol. 2023;52(4):1197–1208. doi: 10.1093/ije/dyad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Hafa F., Wang T., Ndifor V.M., Jin G. Association between Helicobacter pylori antibodies determined by multiplex serology and gastric cancer risk: a meta-analysis. Helicobacter. 2022;27(3) doi: 10.1111/hel.12881. [DOI] [PubMed] [Google Scholar]
- 31.Yang L., Kartsonaki C., Yao P., et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. 2021;6(12):e888–e896. doi: 10.1016/S2468-2667(21)00164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group I.W. 2013. Helicobacter pylori eradication as a strategy for preventing gastric cancer. [Google Scholar]
- 33.Ford A.C., Yuan Y., Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69(12):2113–2121. doi: 10.1136/gutjnl-2020-320839. [DOI] [PubMed] [Google Scholar]
- 34.Pan K.F., Zhang L., Gerhard M., et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65(1):9–18. doi: 10.1136/gutjnl-2015-309197. [DOI] [PubMed] [Google Scholar]
- 35.Areia M., Carvalho R., Cadime A.T., Rocha Gonçalves F., Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013;18(5):325–337. doi: 10.1111/hel.12050. [DOI] [PubMed] [Google Scholar]
- 36.Thung I., Aramin H., Vavinskaya V., et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collaborators GBDT Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389(10082):1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J.B., Jiang Y., Wei W.Q., Yang G.H., Qiao Y.L., Boffetta P. Estimation of cancer incidence and mortality attributable to smoking in China. Cancer Causes Control. 2010;21(6):959–965. doi: 10.1007/s10552-010-9523-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen W., Xia C., Zheng R., et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Global Health. 2019;7(2):e257–e269. doi: 10.1016/S2214-109X(18)30488-1. [DOI] [PubMed] [Google Scholar]
- 40.Peto R., Darby S., Deo H., Silcocks P., Whitley E., Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T.W., Asman K., Gentzke A.S., et al. Tobacco product use among adults - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. doi: 10.15585/mmwr.mm6744a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y.H. Korean adolescents' smoking behavior and its correlation with psychological variables. Addict Behav. 2005;30(2):343–350. doi: 10.1016/j.addbeh.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Qi F., Xu Z., Zhang H., et al. Predicting the mortality of smoking attributable to cancer in Qingdao, China: a time-series analysis. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Galea G. Healthy China 2030: an opportunity for tobacco control. Lancet. 2019;394(10204):1123–1125. doi: 10.1016/S0140-6736(19)32048-3. [DOI] [PubMed] [Google Scholar]
- 45.Lin S.H., Li Y.H., Leung K., Huang C.Y., Wang X.R. Salt processed food and gastric cancer in a Chinese population. Asian Pac J Cancer Prev. 2014;15(13):5293–5298. doi: 10.7314/apjcp.2014.15.13.5293. [DOI] [PubMed] [Google Scholar]
- 46.Park Y., Ki M. Population attributable fraction of Helicobacter pylori infection-related gastric cancer in Korea: a meta-analysis. Cancer Res Treat. 2021;53(3):744–753. doi: 10.4143/crt.2020.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2(7924):58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 48.Rota M., Pelucchi C., Bertuccio P., et al. Alcohol consumption and gastric cancer risk-A pooled analysis within the StoP project consortium. Int J Cancer. 2017;141(10):1950–1962. doi: 10.1002/ijc.30891. [DOI] [PubMed] [Google Scholar]
- 49.Ishioka K., Masaoka H., Ito H., et al. Association between ALDH2 and ADH1B polymorphisms, alcohol drinking and gastric cancer: a replication and mediation analysis. Gastric Cancer. 2018;21(6):936–945. doi: 10.1007/s10120-018-0823-0. [DOI] [PubMed] [Google Scholar]
- 50.Wang S., Freedman N.D., Loftfield E., Hua X., Abnet C.C. Alcohol consumption and risk of gastric cardia adenocarcinoma and gastric noncardia adenocarcinoma: a 16-year prospective analysis from the NIH-AARP diet and health cohort. Int J Cancer. 2018;143(11):2749–2757. doi: 10.1002/ijc.31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collatuzzo G., Pelucchi C., Negri E., et al. Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: a pooled analysis in the Stomach cancer Pooling (StoP) Project. Int J Cancer. 2021;149(6):1228–1238. doi: 10.1002/ijc.33678. [DOI] [PubMed] [Google Scholar]
- 52.Wang S.M., Katki H.A., Graubard B.I., et al. Population attributable risks of subtypes of esophageal and gastric cancers in the United States. Am J Gastroenterol. 2021;116(9):1844–1852. doi: 10.14309/ajg.0000000000001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong L.Z. China's medium-to-long term plan for the prevention and treatment of chronic diseases (2017-2025) under the healthy China initiative. Chronic Dis Transl Med. 2017;3(3):135–137. doi: 10.1016/j.cdtm.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y., Li Y., Giovannucci E. Potential impact of time trend of lifestyle risk factors on burden of major gastrointestinal cancers in China. Gastroenterology. 2021;161:1830. doi: 10.1053/j.gastro.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Jang J., Wang T., Cai H., et al. The U-shaped association between body mass index and gastric cancer risk in the Helicobacter pylori Biomarker Cohort Consortium: a nested case-control study from eight East Asian cohort studies. Int J Cancer. 2020;147(3):777–784. doi: 10.1002/ijc.32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura T., Nakamura Y., Saitoh S., et al. Relationship between socioeconomic status and the prevalence of underweight, overweight or obesity in a general Japanese population: NIPPON DATA2010. J Epidemiol. 2018;28 Suppl 3(Suppl 3):S10–S16. doi: 10.2188/jea.JE20170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malik V.S., Willet W.C., Hu F.B. Nearly a decade on - trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol. 2020;16(11):615–616. doi: 10.1038/s41574-020-00411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai F., Wang H., Du S., et al. Prospective study on nutrition transition in China. Nutr Rev. 2009;67(Suppl 1):S56–S61. doi: 10.1111/j.1753-4887.2009.00160.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang L., Peng W., Zhao Z., et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. 2021;326(24):2498–2506. doi: 10.1001/jama.2021.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L., Gao P., Zhang M., et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian T., Zhang L.Q., Ma X.H., Zhou J.N., Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes. 2012;120(4):217–223. doi: 10.1055/s-0031-1297969. [DOI] [PubMed] [Google Scholar]
- 62.Tseng C.H. The relationship between diabetes mellitus and gastric cancer and the potential benefits of metformin: an extensive review of the literature. Biomolecules. 2021;11(7) doi: 10.3390/biom11071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Francis D.O., Maynard C., Weymuller E.A., Reiber G., Merati A.L., Yueh B. Reevaluation of gastroesophageal reflux disease as a risk factor for laryngeal cancer. Laryngoscope. 2011;121(1):102–105. doi: 10.1002/lary.21165. [DOI] [PubMed] [Google Scholar]
- 64.Tan X., Liu X., Shao H. Healthy China 2030: a vision for health care. Value Health Reg Issues. 2017;12:112–114. doi: 10.1016/j.vhri.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Xia C., Dong X., Li H., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vollset S.E., Goren E., Yuan C.W., et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396(10258):1285–1306. doi: 10.1016/S0140-6736(20)30677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnold M., Park J.Y., Camargo M.C., Lunet N., Forman D., Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69(5):823–829. doi: 10.1136/gutjnl-2019-320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang H., Zou K., He J., Zhang Y. Current situation and prospect of primary prevention of cancer in China. Chin J Oncol. 2022;44(9):942–949. doi: 10.3760/cma.j.cn112152-20220209-00083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.