Abstract
Individuals with clinical manifestations of lymphatic filariasis may be currently infected or not. Twenty-five individuals from a Wuchereria bancrofti-endemic area of Brazil were classified as being asymptomatic microfilaremic individuals, antigenemic individuals with clinical filariasis, or nonantigenemic individuals with clinical filariasis. Intracellular cytokine staining of mitogen-stimulated peripheral blood mononuclear cells (PBMC) showed that the frequency of either gamma interferon (IFN-γ)- or interleukin-4 (IL-4)-producing cells was higher in the nonantigenemic individuals with clinical filariasis than in the asymptomatic microfilaremic individuals (geometric means, 22.1 versus 10.7% [P = 0.02] and 2.9 versus 1.4% [P = 0.01], respectively). When the asymptomatic microfilaremic individuals and antigenemic individuals with clinical filariasis were grouped together to constitute all actively infected individuals, the frequency of IFN-γ-producing cells was also lower than in the nonantigenemic individuals with clinical filariasis (P = 0.04). Likewise, the frequency of IL-4-producing cells in the actively infected individuals was also lower than in the nonantigenemic individuals with clinical filariasis (P = 0.02). No differences in the frequency of IFN-γ-, IL-4-, or IL-5-producing cells in purified CD4 T lymphocytes were found among the groups. These findings suggest that the presence of antigenemia, which is an indicator of current active infection, is closely associated with the frequency of IFN-γ- and IL-4-producing cells in lymphatic filariasis. The differences found in the frequency of cytokine-producing cells among the three groups appear to be due to a subset of cells other than CD4 T cells.
Lymphatic filariasis, which is caused by the helminths Wuchereria bancrofti and Brugia malayi, affects approximately 120 million people worldwide (23). In classifying the clinical manifestations of infection, clinicians have most often used two polar groupings. Infected individuals without any outwardly discernible disease on clinical examination but with microfilaremia have been called asymptomatic microfilaremic individuals; at the other pole, those with any transient or permanent clinical evidence of lymphatic obstruction have been called individuals with chronic pathology. This latter group of individuals has generally been assumed to be amicrofilaremic and free of current infection. However, a metaanalysis of 25 earlier studies as well as recent prospective measurements of circulating filarial antigen (CAg) as a determinant of current active infection in individuals classified as having chronic pathology have made it clear that these individuals form a heterogeneous group with an active infection rate of between 15 and 60% (1, 3, 9, 11, 24).
The pathogenesis of lymphatic obstructive disease is thought to be in large part immunologically mediated (13). When, for the purpose of immunological investigation, patients have been classified as above based on clinical status alone, asymptomatic microfilaremic individuals have been shown to manifest some degree of in vitro immunologic hyporesponsiveness to filarial antigen in comparison to those with chronic pathology, who have been shown to have increased in vitro immunologic reactivity to parasite antigen (15, 19, 28–30, 32, 35). Thus, the published literature has generally not taken into account any immunological differences that may exist between individuals with chronic pathology who are currently infected (positive circulating antigenemia) and those who are not. This distinction is relevant in light of recent data indicating that the profile of immunologic responses in patients with lymphatic filariasis is more closely related to the presence of circulating parasite antigen in those individuals than to the relatively insensitive determination by a clinician of whether a patient has any overt manifestations of lymphatic insufficiency. In particular, individuals who have circulating filarial antigen, whether they are asymptomatic or have clinical manifestations of the disease, seem to have a diminished capacity to produce parasite-specific gamma interferon (IFN-γ) compared to individuals with chronic pathology but without circulating antigenemia (4, 5, 20).
It is now also clear that essentially all individuals with filariasis, even those who are clinically asymptomatic, have some degree of underlying pathology (6, 11–13, 27). Thus we feel it is no longer appropriate to use the term “pathology” to segregate different groups of patients.
For the reasons presented above, we have begun to classify patients by accounting for clinical status as well as for infection status by measuring circulating adult parasite antigenemia in serum, which is a more sensitive indicator of active infection than microfilaria counts. Therefore, three patient groups become important for detailed immunological study: asymptomatic microfilaremic individuals; antigenemic individuals with clinical filariasis, irrespective of microfilaria status; and nonantigenemic individuals with clinical filariasis. The latter two groups comprise the same individuals that would have previously been grouped together as individuals with chronic pathology.
We have previously reported initial data on cytokine responses in these three patient groups by examination of supernatants and mRNA from bulk lymphocyte cultures. To better understand the distinct type 1 and type 2 cytokine responses in the three patient groups, we have now examined the frequency and subset of cells producing a specific cytokine by analyzing fresh, ex vivo cytokine-producing cells in both unfractionated peripheral blood mononuclear cells (PBMC) and purified CD4 T cells. To date, cell frequency analysis from individuals with bancroftian filariasis in areas of endemicity had been done only according to the previously discussed bipolar grouping of patients. Cytokine responses were closely associated with the presence or absence of active infection, and a role for non-CD4 cells in cytokine production by filariasis patients is suggested.
MATERIALS AND METHODS
Study population.
Standardized histories were obtained, and physical examinations were done on 25 study participants resident in two communities of Olinda, Brazil, in which W. bancrofti is endemic. Microfilaria counts by Nuclepore (Corning-Costar, Cambridge, Mass.) filtration of 3 ml of night blood were as described previously (10). Subjects were classified into three groups as previously described (4). Asymptomatic microfilaremic individuals included subjects with detectable microfilaremia who had no current or previous history of adenolymphangitis, erysipelas, cellulitis, or limb swelling and no physical stigmata of lymphatic dysfunction. Antigenemic individuals with clinical filariasis included subjects who had a clinical spectrum of lymphatic pathology ranging from acute filarial fever to chronic lymphedema or elephantiasis, had positive antifilarial immunoglobulin G, and also had current active infection as determined by CAg in serum (TropBio, Townsville, Queensland, Australia) (26). Nonantigenemic individuals with clinical filariasis included subjects with the same clinical manifestations as the antigenemic individuals with clinical filariasis but who had undetectable levels of CAg in serum. Seven North American subjects were used as healthy controls. Although not examined in this study, we have previously documented near-universal infection with intestinal helminths in filariasis-endemic communities in Recife and so have assumed our three groups to be matched in this respect. Study patients who were otherwise free of any intercurrent illness had received no diethylcarbamazine therapy within the previous 5 years. Standard diethylcarbamazine treatment was given to every patient after the study.
Stimulation and fixation of PBMC.
PBMC were isolated by Ficoll-diatrizoate gradient centrifugation from heparinized venous blood. For detection of intracellular cytokine, PBMC (2 × 106/ml) were cultured for 6 h in C-RPMI (RPMI 1640, 10 mM HEPES, 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10% fetal calf serum [FCS]) at 37°C and 5% CO2 with or without the presence of phorbol myristate acetate (PMA; 50 ng/ml; Calbiochem, La Jolla, Calif.) and ionomycin (1 μg/ml; Sigma, St. Louis, Mo.). Monensin (1 μM; Calbiochem) was included in all the cultures to inhibit cytokine secretion. After the 6 h of incubation, the cells were treated with DNase (20 μg/ml; Boehringer Mannheim, Indianapolis, Ind.) for 5 min at 37°C, washed, and fixed with 4% paraformaldehyde at 37°C for 5 min as described previously (7). Fixed cells were then immediately washed with ice-cold phosphate-buffered saline (PBS)–1% bovine serum albumin and stored frozen in PBS–1% bovine serum albumin–10% dimethyl sulfoxide prior to staining. In some experiments, CD4 T cells were negatively selected from fresh PBMC as described elsewhere (14), using a cocktail of subset-specific antibodies followed by two rounds of immunomagnetic negative selection with goat anti-mouse Fc antibody-conjugated magnetic beads (PerSeptive Diagnostics, Cambridge, Mass., and Dynal, Great Neck, N.Y.). Purity of every sample was assessed by flow cytometry with a fluorescein isothiocyanate-labeled anti-CD4 antibody (Becton Dickinson, San Jose, Calif.) and found to be 91.5% ± 4.7% CD4+. Purified CD4 T cells were stimulated, fixed, and frozen under the same conditions as the unfractionated PBMC.
Single-cell analysis of intracellular cytokines.
Previously fixed cells were washed twice with staining buffer (PBS–1% FCS–0.1% sodium azide); 2 × 105 cells/tube were pelleted by centrifugation (250 × g) and resuspended in 50 μl of permeabilization buffer (PBS–1% FCS–0.1% sodium azide–0.1% saponin). A previously titrated optimal dose of a combination of two fluorochrome-conjugated antibodies, fluorescein isothiocyanate-labeled mouse anti-human IFN-γ and phycoerythrin-labeled mouse anti-human interleukin-4 (IL-4) or IL-5 (PharMingen, San Diego, Calif.), was added, and cells were incubated at 4°C for 30 min. Irrelevant isotype-matched controls (PharMingen) were used in every experiment. Cells were then washed in permeabilization buffer, resuspended in staining buffer, and analyzed on a FACScan flow cytometer (Becton Dickinson). Ten thousand events were acquired per sample. The net frequency of IL-4 or IL-5-producing cells in a specific cell population was determined by subtracting the frequency of spontaneously producing cells in medium-stimulated cultures from the total frequency of stained cells upon mitogen stimulation. The net frequency of IFN-γ-producing cells was the average of the total frequencies found in two samples (IFN-γ/IL-4 and IFN-γ/IL-5) for that patient upon stimulation minus the average of the frequencies found in the respective medium-stimulated samples.
Cytokine measurements in supernatants.
For assessment of cytokine production in culture supernatants, unfractionated PBMC and purified CD4 T cells were stimulated with PMA-ionomycin for 72 h in the absence of monensin. Enzyme-linked immunosorbent assay kits for IFN-γ, IL-4, and IL-5 (Biosource, Camarillo, Calif.) had limits of detection of 15.6, 7.8, and 7.8 pg/ml, respectively.
Statistical analysis.
The nonparametric Mann-Whitney test was used to compare differences in the frequency of intracellular cytokine staining in the three patient groups. Values of less than 0.05 were considered significant. Correlations were performed by simple regression analysis.
RESULTS
Study population.
Table 1 shows the characteristics of the three patient groups. As previously reported for this area of Brazil (11), nonantigenemic individuals with clinical filariasis were significantly older (median age, 49 years) than asymptomatic microfilaremic individuals or antigenemic individuals with clinical filariasis (median ages, 25.5 and 30 years, respectively). Prevalence of W. bancrofti microfilaremia in males is well known to be significantly greater than in females between the ages of 15 and 54 in areas surrounding the one studied (1). Antigenemic individuals with clinical filariasis had statistically indistinguishable numbers of microfilaria in the blood or levels of circulating antigen compared with asymptomatic microfilaremic individuals.
TABLE 1.
Characteristics of study population
| Patient groupa | Median age (yr) (range) | Geometric mean (range)
|
|
|---|---|---|---|
| Microfilaria counts/ml | Circulating antigen (U) | ||
| Asymptomatic microfilaremic individuals (6 M, 6 F) | 25.5 (13–48) | 262 (17–1,550) | 1,007.1 (167.9–4,375)b |
| Antigenemic individuals with clinical filariasis (7 M) | 30 (17–53) | 214 (33–3,666) | 741.3 (221.1–1,195) |
| Nonantigenemic individuals with clinical filariasis (3 M, 3 F) | 49 (28–60) | 0 | <39 |
M, male; F, female.
P = 0.04 and P = 0.02 compared with antigenemic individuals with clinical filariasis and nonantigenemic with clinical filariasis, respectively.
Frequency of cytokine-producing cells in human lymphatic filariasis.
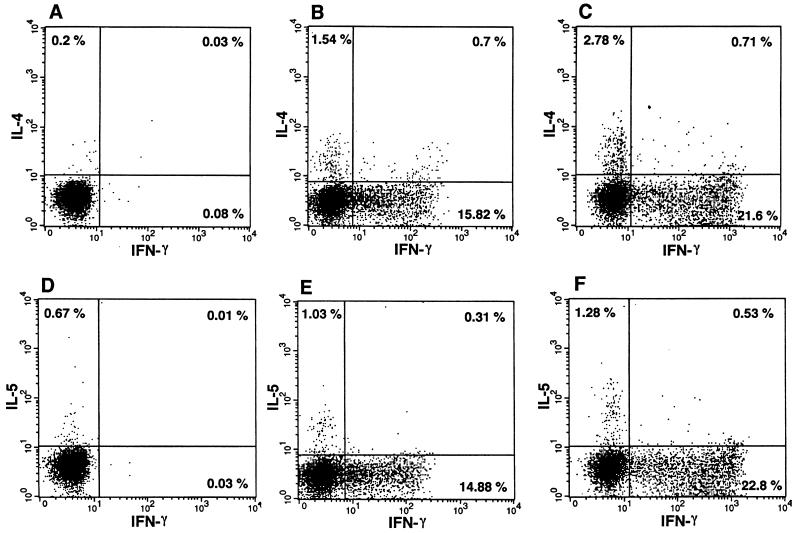
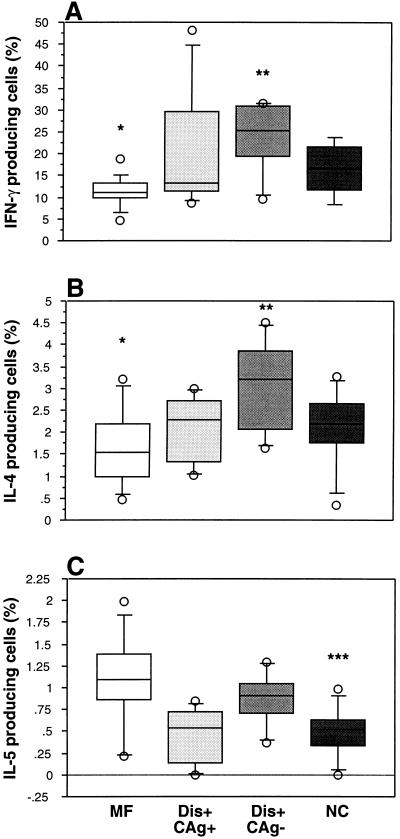
To determine whether there were differences in the frequency of IFN-γ-, IL-4-, or IL-5-producing cells in the three patient groups, fresh PBMC were stimulated with PMA-ionomycin or medium alone for 6 h in the presence of monensin and subsequently fixed. Cells were fluorescently stained with either IFN-γ and IL-4 or IFN-γ and IL-5 and analyzed by flow cytometry. Figure 1 illustrates representative two-parameter dot plots for an asymptomatic microfilaremic individual and a nonantigenemic individual with clinical filariasis. The frequencies of IFN-γ-producing and IL-4-producing, but not IL-5-producing, cells are lower in the asymptomatic microfilaremic individual than in the nonantigenemic individual with clinical filariasis. As a group, asymptomatic microfilaremic individuals had significantly lower frequencies of IFN-γ-producing cells compared to nonantigenemic individuals with clinical filariasis (geometric mean = 10.71%, range = 4.5 to 18.8 versus 22.1% [9.6 to 31.5%], P = 0.01) (Fig. 2A). The frequency of IL-4-producing cells in asymptomatic microfilaremic individuals was also lower than in nonantigenemic individuals with clinical filariasis (geometric mean = 1.4%, range = 0.45 to 3.2 versus 2.9% [1.6 to 4.5%], P = 0.02) (Fig. 2B). Nonantigenemic individuals with clinical filariasis also had significantly higher frequencies of stained cells for IFN-γ or IL-4 compared to the asymptomatic microfilaremic individuals and antigenemic individuals with clinical filariasis taken together as a group (i.e., all actively infected individuals; P = 0.04 and P = 0.02, respectively) (Fig. 2A and B). The individuals in the control (healthy) group did not differ significantly from any of the patient groups when the frequencies of IFN-γ- or IL-4-producing cells were considered. The frequency of IL-5-producing cells did not differ among the patient groups (Fig. 2C). The individuals in the control group had significantly lower frequencies of IL-5-producing cells compared to individuals in two of three patient groups.
FIG. 1.
Representative two-parameter dot plots of IFN-γ-, IL-4-, and IL-5-producing cells from medium- or PMA-ionomycin-stimulated cultures of PBMC. A and D, medium stimulation of PBMC from a nonantigenemic individual with clinical filariasis; B and E, mitogen stimulation of PBMC from an asymptomatic microfilaremic individual; C and F, mitogen stimulation of PBMC from a nonantigenemic individual with clinical filariasis. Quadrant statistics were set on the basis of the corresponding medium-stimulated cultures.
FIG. 2.
Percentages of IFN-γ (A)-, IL-4 (B)-, and IL-5 (C)-producing cells in PMA-ionomycin-stimulated PBMC from asymptomatic microfilaremic individuals (MF), antigenemic individuals with clinical filariasis (Dis+ CAg+), nonantigenemic individuals with clinical filariasis (Dis+ CAg−), and normal control individuals (NC). Box plots display the 25th, 50th, and 75th percentiles of the cytokine responses. Percentage of IFN-γ-producing cells in all patient groups is the average of results from two separate samples. ∗, P < 0.05 compared to the Dis+ CAg− group; ∗∗, P < 0.05 compared to the actively infected group taken together (MF and Dis+ CAg+); ∗∗∗, P < 0.05 compared to the MF, Dis+ CAg−, or actively infected group.
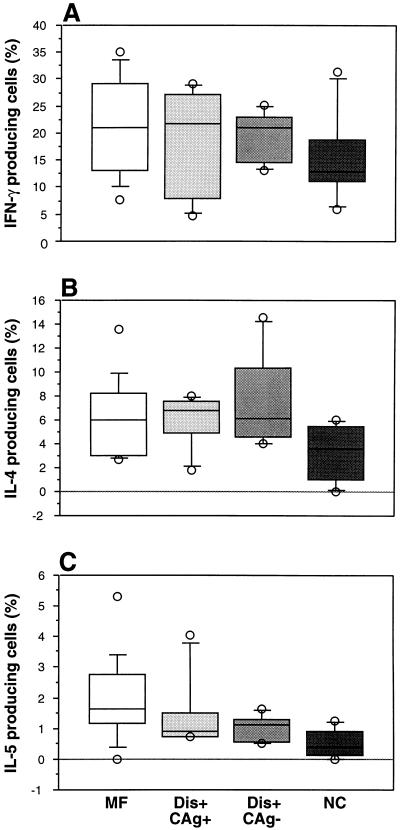
To determine whether there were differences in the frequencies of cytokine-producing cells in negatively selected CD4 T cells, as had been found for unfractionated PBMC, CD4 T cells were also mitogen stimulated for 6 h. No significant differences in the frequency of cytokine-producing cells were found among the groups for either IFN-γ, IL-4, or IL-5 (Fig. 3).
FIG. 3.
Percentages of IFN-γ (A)-, IL-4 (B)-, and IL-5 (C)-producing cells in PMA-ionomycin-stimulated CD4 T cells from asymptomatic microfilaremic individuals (MF), antigenemic individuals with clinical filariasis (Dis+ CAg+), nonantigenemic individuals with clinical filariasis (Dis+ CAg−), and normal control individuals (NC). Box plots display the 25th, 50th, and 75th percentiles of the cytokine responses. Percentage of IFN-γ-producing cells in all patient groups is the average of results from two separate samples.
Concomitant production of type 1 and type 2 cytokines.
As shown in Fig. 1, a small percentage of PBMC were doubly positive for both IFN-γ and IL-4 and, to a lesser extent, both IFN-γ and IL-5 (Table 2). This subset of double-positive cells accounted for 14 to 53% of the total IL-4-producing cells and 3 to 95% of the total IL-5-producing cells and was found in all groups, with no significant difference in frequency among the groups.
TABLE 2.
Percentages of IFN-γ/IL-4 and IFN-γ/IL-5 double-positive PBMC in persons with lymphatic filariasis
| Patient group (n) | Geometric mean (range)
|
|||
|---|---|---|---|---|
| IFN-γ/IL-4 double-positive | IFN-γ/IL-5 double-positive | |||
| Medium control | Mitogen stimulation | Medium control | Mitogen stimulation | |
| Asymptomatic microfilaremic individuals (11) | 0.08 (0.02–0.23) | 0.69 (0.20–2.14) | 0.02 (0.01–0.06) | 0.12 (0.05–0.30) |
| Antigenemic individuals with clinical filariasis (7) | 0.06 (0–0.26) | 0.68 (0.19–1.81) | 0.04 (0–0.09) | 0.25 (0.10–0.72) |
| Nonantigenemic individuals with clinical filariasis (5) | 0.12 (0.07–0.30) | 1.04 (0.65–2.10) | 0.05 (0.03–0.09) | 0.29 (0.07–0.65) |
The frequency of Th2 cytokine-producing cells correlates with levels of secretion in CD4 T cells.
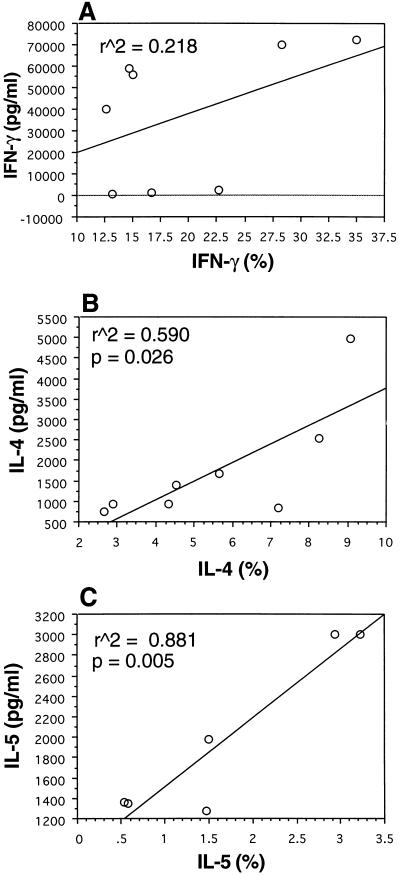
To assess whether frequencies of cytokine-producing cells correlated with secretion of cytokine in culture supernatants, unfractionated PBMC and CD4 T cells were cultured with PMA-ionomycin in the absence of monensin for 72 h in parallel with the cultures for flow cytometric analysis. Linear regression analysis showed a direct correlation between the frequency of CD4 T cells producing IL-4 and IL-5 and the amounts of IL-4 and IL-5 secreted into the culture supernatants (r2 = 0.59, P = 0.026; r2 = 0.88, P = 0.005, respectively) (Fig. 4B and C). However, no correlation was found between the frequency of CD4 T cells producing IFN-γ and the amount of IFN-γ secretion in culture supernatants (Fig. 4A). When those same parameters were examined in unfractionated PBMC, no correlation was found between the frequency of cytokine-producing cells and the levels of cytokine secretion in culture supernatants for IFN-γ, IL-4, or IL-5 (data not shown).
FIG. 4.
Correlation of frequency of IFN-γ (A)-, IL-4 (B)-, and IL-5 (C)-producing cells with the levels of secreted cytokine in culture supernatants from PMA-ionomycin-stimulated CD4 T cells of subjects in the three patient groups. Correlations are a result of linear regression analysis.
DISCUSSION
The profile of cytokine responses is an important determinant of pathology in a number of helminth infections. Experimental data from murine models of onchocerciasis and schistosomiasis have shown that deleterious immunopathology is associated with increased levels of IL-4 and/or IL-5 and therefore a Th2-like response (31, 36). In bancroftian filariasis, the few available studies of antigen-specific cytokine responses in human subjects have used purely clinical criteria for patient classification, which places individuals at one of two poles of the disease spectrum. Individuals with asymptomatic microfilaremia who are obviously actively infected have been placed at one pole, while individuals with chronic pathology, who have been considered to be uninfected, have been placed at the other pole. When this classification has been used, the data have been interpreted as suggesting an association between a Th2-like response and the presence of asymptomatic microfilaremia (16, 21). These studies in fact show that the frequency of IL-4-producing cells and levels of IL-4 production in culture supernatants are equivalent when the asymptomatic microfilaremic individuals are compared to those with chronic pathology. Though these studies demonstrate an increased IL-4/IFN-γ ratio in asymptomatic microfilaremic individuals, this is due to a significantly lower frequency of IFN-γ-secreting cells and lesser IFN-γ production in these individuals rather than any increased production of Th2 cytokines compared to individuals with chronic pathology.
Interpretation of these earlier data on cytokine responses in persons with lymphatic filariasis may, however, have been confounded by failure to consider differences in the circulating antigen status of individuals classified clinically as having chronic pathology. The long-held concept that clinical filariasis is uniformly associated with amicrofilaremia has been dispelled by an elegant metaanalysis of 25 studies done between 1945 and 1982, which showed that persons with and those without microfilaremia are equally likely to have clinical manifestations of the disease (24). In this regard, we and others have recently shown that in bulk lymphocyte culture, the pattern of cytokine secretion in persons with bancroftian filariasis is most closely associated with the presence or absence of active infection (i.e., circulating antigen status) irrespective of the presence or absence of evidence of lymphatic insufficiency on clinical examination (4, 5, 20). Reinterpretation of earlier immunologic studies in light of this new information is difficult since the presence or absence of circulating antigen or even of microfilaremia in the lymphatic pathology individuals in these studies is infrequently documented (16, 37). It is also possible that microfilaremic individuals were excluded from those classified in earlier immunologic studies as having chronic pathology because they did not fall neatly into the pathology-equals-amicrofilaremia paradigm in force at the time. Earlier studies did not always document treatment status. Since antecedent diethylcarbamazine therapy frequently clears circulating antigenemia (22), the major determinant of the cytokine response in filariasis, it is clear that this must be carefully controlled for in immunological studies.
In the present study, we have investigated fresh, ex vivo cytokine production at the single-cell level in persons with lymphatic filariasis who were carefully classified according to both clinical status and circulating antigen status. Individuals were classified into three groups: asymptomatic microfilaremic individuals, antigenemic individuals with clinical filariasis (includes microfilaremic and amicrofilaremic individuals), and nonantigenemic individuals with clinical filariasis. Both unfractionated PBMC and purified CD4 T cells were analyzed by flow cytometry for intracellular cytokine production. In unfractionated PBMC, the frequencies of both IFN-γ- and IL-4-producing cells were significantly lower in the asymptomatic microfilaremic individuals when grouped alone or in the actively infected individuals taken together (asymptomatic microfilaremic individuals and antigenemic individuals with clinical filariasis) than in the nonantigenemic individuals with clinical filariasis (Fig. 2A and B). These results agree with previous findings of decreased IFN-γ production in asymptomatic microfilaremic individuals (5, 16, 20) but do not agree with one earlier study where polyclonal activation of PBMC by anti-CD2 and recombinant IL-2 of asymptomatic microfilaremic individuals and individuals with lymphatic pathology stimulated statistically equivalent amounts of IFN-γ (38). The heterogeneity of those patients classified as having lymphatic pathology, discussed above, may account for the inability to distinguish cytokine profiles of asymptomatic microfilaremic individuals from those of individuals with lymphatic pathology. Alternatively, these discrepancies may be attributed to the lack of correlation (Fig. 4) between the frequency of IFN-γ-producing cells and the IFN-γ levels present in culture supernatants (discussed below). The frequencies of IFN-γ- or IL-4-producing cells in control individuals did not differ significantly from those individuals in the three patient groups. Nevertheless, the median frequencies for both IFN-γ or IL-4 clearly fell between the relative downregulation seen in the asymptomatic microfilaremic individuals and the relative upregulation seen in the nonantigenemic individuals with clinical filariasis.
Nonantigenemic individuals with clinical filariasis were older (mean = 43 years) than the individuals in the other two patient groups (means = 25 and 31 years). Older age in individuals with advanced clinical disease is consistent with all cross-sectional studies that have been done in filariasis-endemic areas and reflects the natural progression of what is a chronic long-lived infection (15, 37). Extremes of age have been associated with changes in polyclonal immune responses in both humans and animals. However, the literature on age-related increases or decreases in the production of cytokines such as IFN-γ and IL-4 is conflicting and inconsistent (25), and the differences in mean age between our groups were small relative to the comparative groups described in the aging immune system literature. The studies that have associated increasing age with changes in production of cytokines have generally attributed this to the well-documented replacement of virgin T cells by memory cells with increasing immunologic experience (2, 34). All individuals in our study, who live in an impoverished community within an already underdeveloped area of the Third World, are constantly exposed from the time of birth to a large antigenic burden from a spectrum of infectious agents. They therefore develop a relatively complete complement of memory T cells early in childhood so that the small age difference in our study groups is unlikely by itself to account for any of our findings. No less important is that we found no correlation between the frequencies of IFN-γ-, IL-4-, or IL-5-producing cells and age in any of the three patient groups examined individually or when all study subjects were grouped together (data not shown).
We found no differences in the frequency of any cytokine studied between any of the patient groups when purified CD4 T cells were examined (Fig. 3). When frequencies of cytokine-producing cells, regardless of patient group, were compared with the levels of cytokine production in culture supernatants, levels of IL-4 and IL-5 correlated with the frequencies of IL-4- and IL-5-producing CD4 T cells. No correlation was found for IFN-γ, in agreement with the findings of Elson et al. in patients with a spectrum of acute helminth infections (7). This suggests that the amount of IFN-γ produced in the supernatant by each individual cell varies within the same subpopulation of cells. However, it is possible that a minor contamination with non-CD4 T cells which may actively secrete IFN-γ in varied amounts was present in the cultures. No correlation was found between cell frequency and supernatant levels of any cytokine examined in the unfractionated PBMC. This lack of correlation, along with the similar frequencies of cytokine-producing cells between patient groups in purified CD4 T cells, suggests that a subset of cells which are non-CD4 T cells are involved in the differences of cytokine expression and levels of secretion among patient groups.
CD8 T cells are good candidates to be important sources of cytokine in lymphatic filariasis. We have reported the striking and consistent finding of a CD8 T-cell infiltrate in the tissue biopsies from individuals with clinical filariasis (10). Moreover, we have found that CD8 T cells can be a major source of IL-5 production in patients who have cleared infection (4). Persons with clinical pathology have elevated levels of soluble CD8 molecules and of CD8+ HLA-DR+ T cells in their circulation (17, 18). Of course, the possibility that these differences are a result of differential cytokine production by non-CD3 T cells in the lymphocyte gate cannot be discarded at the moment. Prospective field work will examine the role of CD8 T cells in more detail.
Simultaneous production of IFN-γ and IL-4 was found in PBMC from almost all of the subjects. These double-positive cells were significantly increased when mitogen stimulation was compared to medium stimulation (0.2 to 2.14% and 0.02 to 0.3%, respectively). Simultaneous production of IFN-γ and IL-5 was also found in subjects from all of the patient groups. However, the intensity of fluorescence of these double-positive cells was very low and usually just above the limits of the gates, and at this point we cannot draw any conclusions on the relevance of this subset of cells. These Th0-like cells have generally been considered the common precursors of Th1 and Th2 cytokines and appear transiently after activation of naive T cells (33). However, a recent study by Elson et al. (7) reported the coexpression of IFN-γ and IL-4 in the activated CD4+ CD27− cells, which have been shown to be increased in subjects with lymphatic filariasis compared with healthy subjects, and which are functionally differentiated T cells (8, 38). This finding suggested that Th0 cells can be as differentiated as Th1- or Th2-like cells. The importance of these Th0-like cells in filarial disease is not clear at this point and needs to be better characterized in the three patient groups upon antigenic stimulation.
In conclusion, we have found that there are differences in the frequency of cytokine-producing cells when filariasis patients are grouped in a scheme which takes both clinical and circulating antigen status into account. Cytokine responses do not appear to fall into the distinct Th1/Th2/Th0 paradigm, and the downregulation of the immune response, which is closely associated with the presence or absence of circulating filarial antigen, irrespective of clinical status, includes type 1 and type 2 cytokines. The frequency of IFN-γ-producing cells in particular seems to be markedly diminished in antigenemic individuals. The differences found in the frequency of cytokine-producing cells among the three patient groups are most likely due to a subset of cells which appear to be non-CD4 T cells. Studies are under way to investigate the role of CD8 T cells in similar systems in more detail.
ACKNOWLEDGMENTS
This work was supported by grant AI-31552 from the National Institutes of Health.
We thank Eridan Coutinho, Freddie Abath, and André Furtado for the continuing support of CPqAM in carrying out these studies, as well as Mineo Nakasawa and Janaína Miranda for technical assistance in Brazil. We also thank Tom Nutman for the kind gift of the antibodies used in the CD4-negative selection protocol. We thank Tracey McGuire for technical support with the flow cytometry, and we thank Adam Plier for helpful discussions and support.
REFERENCES
- 1.Albuquerque M F M, Marzochi M C, Sabroza P C, Braga M C, Padilha T, Silva M C M, Silva M R F, Schindler H C, Maciel M A, Souza W, Furtado A F. Bancroftian filariasis in two urban areas of Recife, Brazil: pre-control observations on infection and disease. Trans R Soc Trop Med Hyg. 1995;89:373–377. doi: 10.1016/0035-9203(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 2.al-Rayes H, Pachas W, Mirza N, Ahern D J, Geha R S, Vercelli D. IgE regulation and lymphokine patterns in aging humans. J Allergy Clin Immunol. 1992;90:630–636. doi: 10.1016/0091-6749(92)90136-p. [DOI] [PubMed] [Google Scholar]
- 3.Chanteau S, Glaziou P, Luquiaud P, Plichart C, Moulia-Pelat J P, Cartel J L. Og4C3 circulating antigen, anti-Brugia malayi IgG and IgG4 titers in Wuchereria bancrofti infected patients, according to their parasitological status. Trop Med Parasitol. 1994;45:255–257. [PubMed] [Google Scholar]
- 4.de Almeida A B, Maia e Silva M C, Maciel M A, Freedman D O. The presence or absence of active infection, not clinical status, is most closely associated with cytokine responses in lymphatic filariasis. J Infect Dis. 1996;173:1453–1459. doi: 10.1093/infdis/173.6.1453. [DOI] [PubMed] [Google Scholar]
- 5.Dimock K A, Eberhard M L, Lammie P J. Th1-like antifilarial immune responses predominate in antigen-negative persons. Infect Immun. 1996;64:2962–2967. doi: 10.1128/iai.64.8.2962-2967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dissanayake S, Watawana L, Piessens W F. Lymphatic pathology in Wuchereria bancrofti microfilaraemic infections. Trans R Soc Trop Med Hyg. 1995;89:517–521. doi: 10.1016/0035-9203(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 7.Elson L H, Nutman T B, Metcalfe D D, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+CD27− lymphocyte subpopulation. J Immunol. 1995;154:4294–4301. [PubMed] [Google Scholar]
- 8.Elson L H, Shaw S, Van Lier R A, Nutman T B. T cell subpopulation phenotypes in filarial infections: CD27 negativity defines a population greatly enriched for Th2 cells. Int Immunol. 1994;6:1003–1009. doi: 10.1093/intimm/6.7.1003. [DOI] [PubMed] [Google Scholar]
- 9.Faris R, Ramzy R M, Gad A M, Weil G J, Buck A A. Community diagnosis of Bancroftian filariasis. Trans R Soc Trop Med Hyg. 1993;87:659–661. doi: 10.1016/0035-9203(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 10.Freedman D O, Berry R S. Rapid diagnosis of Bancroftian filariasis by acridine orange staining of centrifuged parasites. Am J Trop Med Hyg. 1992;47:787–793. doi: 10.4269/ajtmh.1992.47.787. [DOI] [PubMed] [Google Scholar]
- 11.Freedman D O, de Almeida Filho P J, Besh S, Maia e Silva M C, Braga C, Maciel A. Lymphoscintigraphic analysis of lymphatic abnormalities in symptomatic and asymptomatic human filariasis. J Infect Dis. 1994;170:927–933. doi: 10.1093/infdis/170.4.927. [DOI] [PubMed] [Google Scholar]
- 12.Freedman D O, Horn T D, Maia e Silva C, Braga C, Maciel A. Predominant CD8+ infiltrate limb biopsies of individuals with filarial lymphedema and elephantiasis. Am J Trop Med Hyg. 1995;53:633–638. [PubMed] [Google Scholar]
- 13.Freedman, D. O. Immunopathogenesis of human lymphatic filariasis. Parasitol. Today, in press. [DOI] [PubMed]
- 14.Horgan K J, Shaw S. Immunologic studies in humans. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: Wiley Interscience; 1991. p. 7.4.1. [Google Scholar]
- 15.King C L, Kumaraswami V, Poindexter R W, Kumari S, Jayaraman K, Alling D W, Ottesen E A, Nutman T B. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King C L, Mahanty S, Kumaraswami V, Abrams J S, Regunathan J, Jayaraman K, Ottesen E A, Nutman T B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal R B, Kumaraswami V, Krishnan N, Nutman T B, Ottesen E A. Lymphocyte subpopulations in Bancroftian filariasis: activated (DR+) CD8+ T cells in patients with chronic lymphatic obstruction. Clin Exp Immunol. 1989;77:77–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Lal R B, Ramzy R M, Gad A A. Elevated levels of soluble CD8 molecule in patients with lymphatic filariasis. Immunol Lett. 1990;26:85–88. doi: 10.1016/0165-2478(90)90180-x. [DOI] [PubMed] [Google Scholar]
- 19.Lammie P J, Addiss D G, Leonard G, Hightower A W, Eberhard M L. Heterogeneity in filarial-specific immune responsiveness among patients with lymphatic obstruction. J Infect Dis. 1993;167:1178–1183. doi: 10.1093/infdis/167.5.1178. [DOI] [PubMed] [Google Scholar]
- 20.Mahanty S, Luke H E, Kumaraswami V, Narayanan P R, Vijayshekaran V, Nutman T B. Stage-specific induction of cytokines regulates the immune response in lymphatic filariasis. Exp Parasitol. 1996;84:282–290. doi: 10.1006/expr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 21.Maizels R M, Sartono E, Kurniawan A, Partono F, Selkirk M E, Yazdanbakhsh M. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11:50–56. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy J S, Guinea A, Weil G J, Ottesen E A. Clearance of circulating filarial antigen as a measure of macrofilaricidal activity of diethylcarbamazine in Wuchereria bancrofti infection. J Infect Dis. 1995;172:521–526. doi: 10.1093/infdis/172.2.521. [DOI] [PubMed] [Google Scholar]
- 23.Michael E, Bundy D A, Grenfell B T. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 24.Michael E, Grenfell B T, Bundy D A. The association between microfilaraemia and disease in lymphatic filariasis. Proc R Soc Lond B. 1994;256:33–40. doi: 10.1098/rspb.1994.0045. [DOI] [PubMed] [Google Scholar]
- 25.Miller R A. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 26.More S J, Copeman D B. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop Med Parasitol. 1990;41:403–406. [PubMed] [Google Scholar]
- 27.Norões J, Addiss D, Santos A, Medeiros Z, Coutinho A, Dreyer G. Ultrasonographic evidence of abnormal lymphatic vessels in young men with adult Wuchereria bancrofti infection in the scrotal area. J Urol. 1996;156:409–412. doi: 10.1097/00005392-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Nutman T B, Kumaraswami V, Pao L, Narayanan P R, Ottesen E A. An analysis of in vitro B cell immune responsiveness in human lymphatic filariasis. J Immunol. 1987;138:3954–3959. [PubMed] [Google Scholar]
- 29.Ottesen E A. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans R Soc Trop Med Hyg. 1984;78:9–18. doi: 10.1016/0035-9203(84)90309-2. [DOI] [PubMed] [Google Scholar]
- 30.Ottesen E A, Weller P F, Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977;33:413–421. [PMC free article] [PubMed] [Google Scholar]
- 31.Pearlman E, Lass J H, Bardenstein D S, Hazlett F E J, Diaconu E, Kazura J W. Interleukin 4 and T helper 2 cells are required for development of experimental onchocercal keratitis (river blindness) J Exp Med. 1995;182:931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piessens W F, McGreevy P B, Ratiwayanto S, McGreevy M, Piessens P W, Koiman I, Saroso J S, Dennis D T. Immune responses in human infections with Brugia malayi: correlation of cellular and humoral reactions to microfilarial antigens with clinical status. Am J Trop Med Hyg. 1980;29:563–570. doi: 10.4269/ajtmh.1980.29.563. [DOI] [PubMed] [Google Scholar]
- 33.Swain S L, Bradley L M, Croft M, Tonkonogy S, Atkins G, Weinberg A D, Duncan D D, Hedrick S M, Dutton R W, Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 34.van Besouw N M, van der Meide P H, Bakker N P M. The mitogen-induced generation of interferon-gamma producing cells in cultures of rhesus monkey peripheral blood mononuclear cells is age-dependent. J Med Primatol. 1994;23:42–48. doi: 10.1111/j.1600-0684.1994.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. WHO Technical Report Series 821. Geneva, Switzerland: World Health Organization; 1992. [PubMed] [Google Scholar]
- 36.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 37.Yazdanbakhsh M, Paxton W A, Kruize Y C, Sartono E, Kurniawan A, van het Wout A, Selkirk M E, Partono F, Maizels R M. T cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J Infect Dis. 1993;167:925–931. doi: 10.1093/infdis/167.4.925. [DOI] [PubMed] [Google Scholar]
- 38.Yazdanbakhsh M, Sartono E, Kruize Y C, Kurniawan A, van der Pouw-Kraan T, van der Meide P H, Selkirk M E, Partono F, Hintzen R Q, van Lier R A, Maizels R M. Elevated levels of T cell activation antigen CD27 and increased interleukin-4 production in human lymphatic filariasis. Eur J Immunol. 1993;23:3312–3317. doi: 10.1002/eji.1830231238. [DOI] [PubMed] [Google Scholar]