Abstract
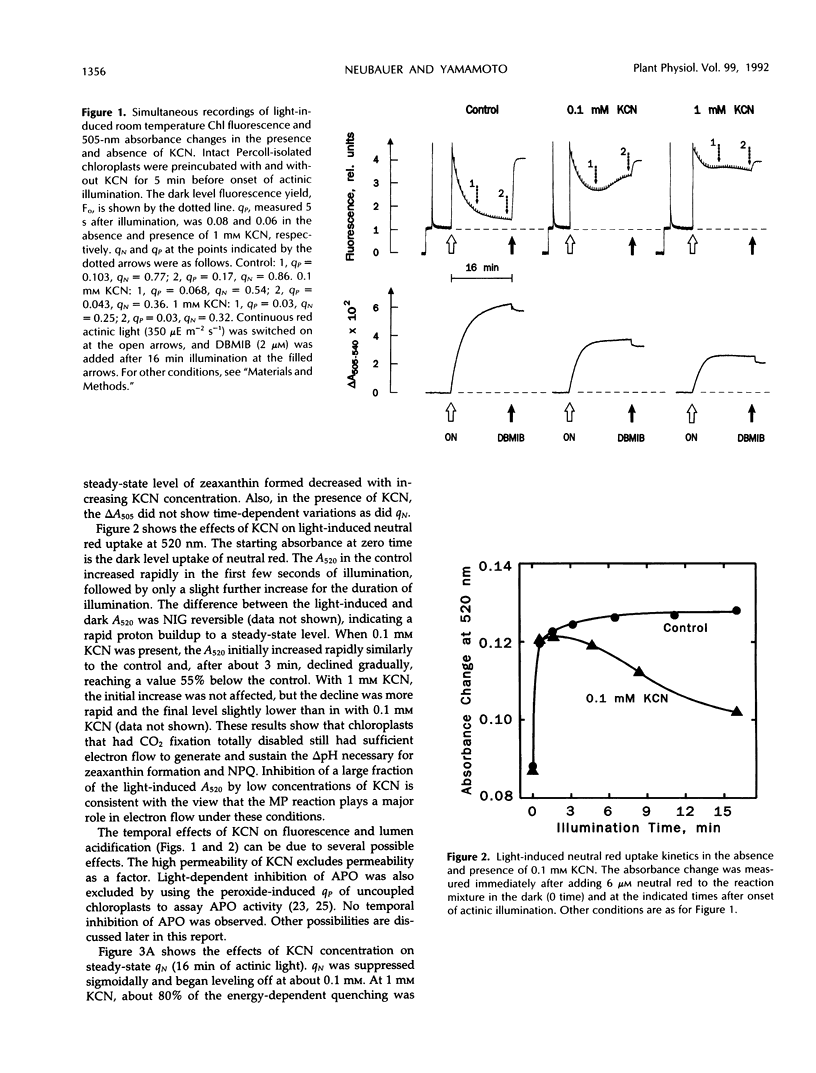
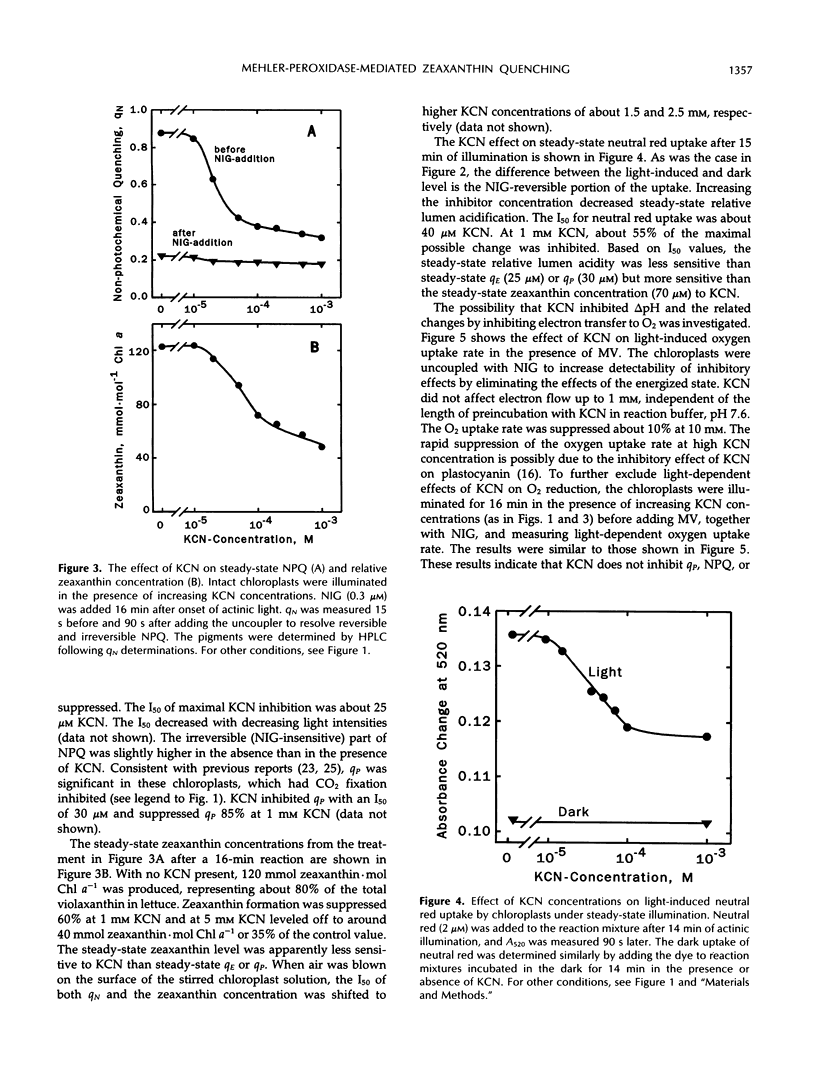
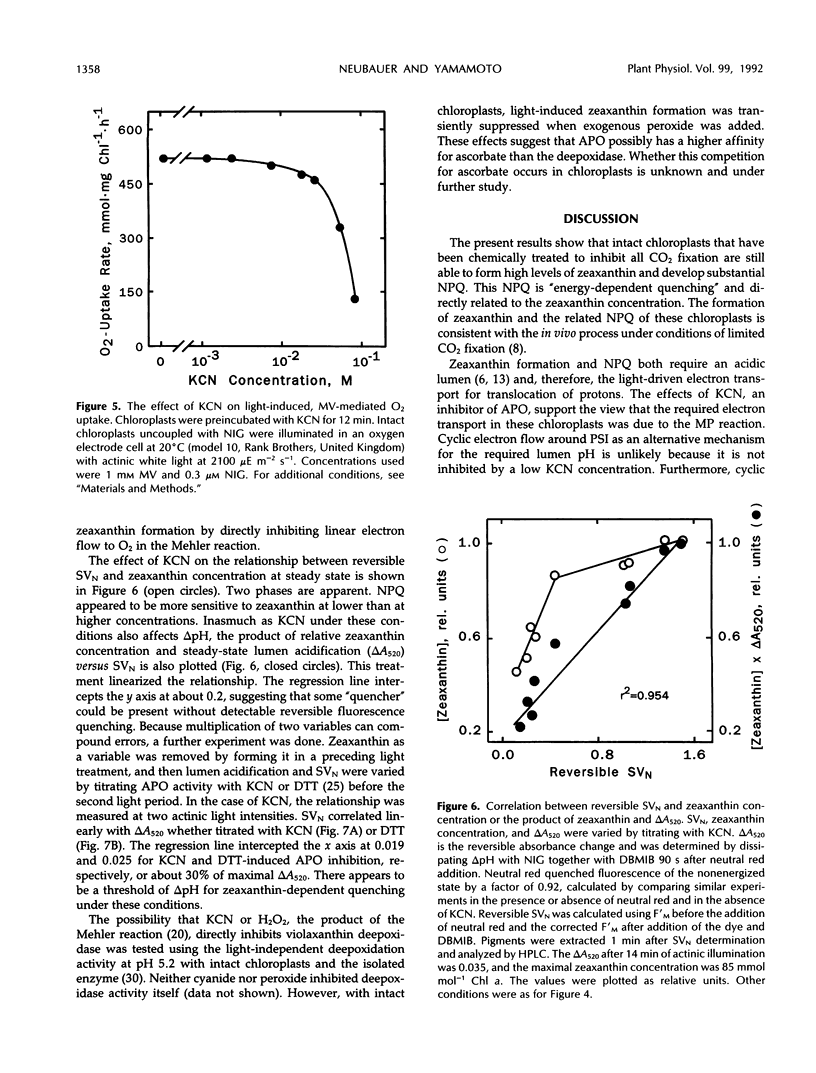
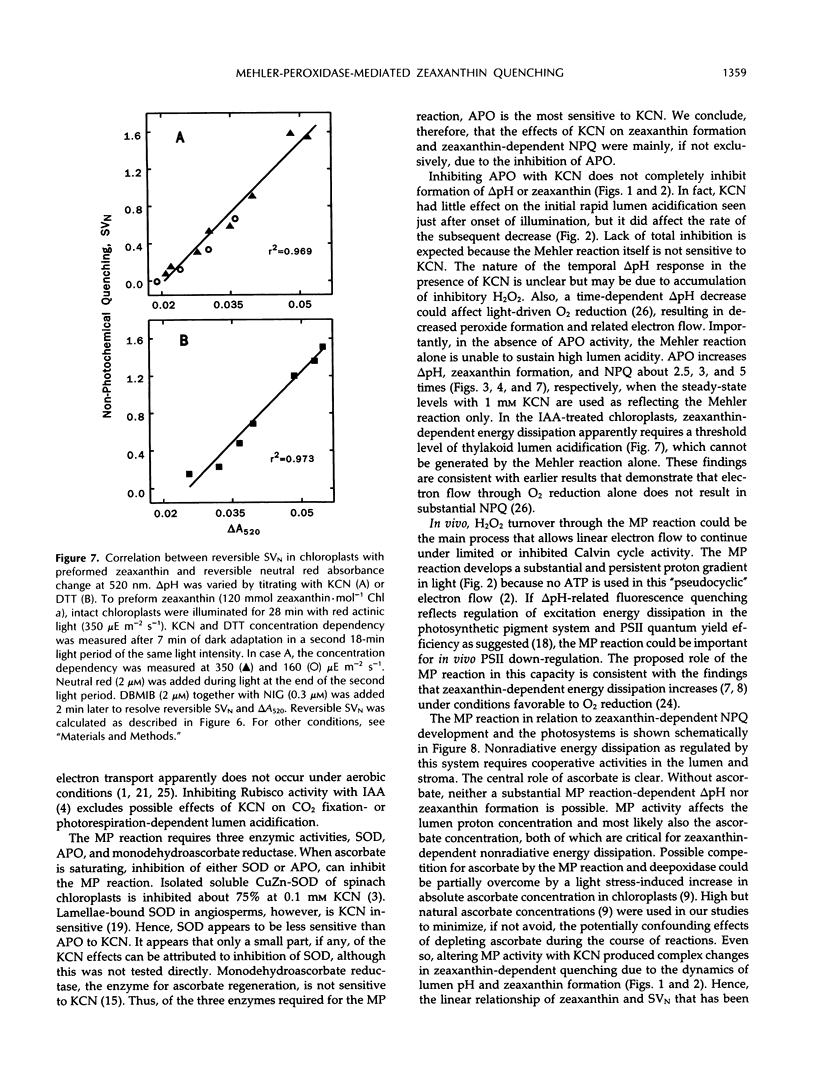
Induction of zeaxanthin formation and the associated nonphotochemical quenching in iodoacetamide-treated, non-CO2-fixing intact chloroplasts of Lactuca sativa L. cv Romaine is reported. The electron transport needed to generate the required ΔpH for zeaxanthin formation and nonphotochemical quenching are ascribed to the Mehler-ascorbate peroxidase reaction. KCN, an inhibitor of ascorbate peroxidase, significantly affected these activities without affecting linear electron transport to methyl viologen or violaxanthin deepoxidase activity. At 1 millimolar KCN, zeaxanthin formation and ΔpH were inhibited 60 and 55%, respectively, whereas ascorbate peroxidase activity was inhibited almost totally. The KCN-resistant activity, which apparently was due to electron transport mediated by the Mehler reaction alone, however, was insufficient to support a high level of nonphotochemical quenching. We suggest that in vivo, as CO2 fixation becomes limiting, the Mehler-peroxidase reaction protects photosystem II against the excess light by supporting the electron transport needed for zeaxanthin-dependent nonphotochemical quenching and concomitantly scavenging H2O2. Ascorbate is essential for this process to occur.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1979 Oct 10;548(1):128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. M., Yamamoto H. Y. Zeaxanthin Formation and Energy-Dependent Fluorescence Quenching in Pea Chloroplasts under Artificially Mediated Linear and Cyclic Electron Transport. Plant Physiol. 1991 Jun;96(2):635–643. doi: 10.1104/pp.96.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Izawa S., Kraayenhof R., Ruuge E. K., Devault D. The site of KCN inhibition in the photosynthetic electron transport pathway. Biochim Biophys Acta. 1973 Sep 26;314(3):328–339. doi: 10.1016/0005-2728(73)90117-5. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Hall D. O. Soluble & membrane-bound superoxide dismutases in a blue-green algae (Spirulina)and spinach. Biochem Biophys Res Commun. 1974 May 7;58(1):35–41. doi: 10.1016/0006-291x(74)90887-0. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D. The accumulation of neutral red in illuminated thylakoids. Biochim Biophys Acta. 1978 Nov 9;504(2):265–277. doi: 10.1016/0005-2728(78)90175-5. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO H. Y., NAKAYAMA T. O., CHICHESTER C. O. Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys. 1962 Apr;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L., Wang Y. Y. An Ascorbate-induced Absorbance Change in Chloroplasts from Violaxanthin De-epoxidation. Plant Physiol. 1972 Feb;49(2):224–228. doi: 10.1104/pp.49.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]


