Abstract
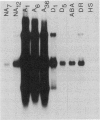
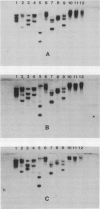
We have isolated, sequenced, and expressed a cold-specific cDNA clone, Wcs120, that specifically hybridizes to a major mRNA species of approximately 1650 nucleotides from cold-acclimated wheat (Triticum aestivum L.). The accumulation of this mRNA was induced in less than 24 hours of cold treatment, and remained at a high steady-state level during the entire period of cold acclimation in the two freezing-tolerant genotypes of wheat tested. The expression of Wcs120 was transient in a less-tolerant genotype even though the genomic organization of the Wcs120 and the relative copy number were the same in the three genotypes. The mRNA level decreased rapidly during deacclimation and was not induced by heat shock, drought, or abscisic acid. The Wcs120 cDNA contains a long open reading frame encoding a protein of 390 amino acids. The encoded protein is boiling stable, highly hydrophilic, and has a compositional bias for glycine (26.7%), threonine (16.7%), and histidine (10.8%), although cysteine, phenylalanine, and tryptophan were absent. The WCS120 protein contains two repeated domains. Domain A has the consensus amino acid sequence GEKKGVMENIKEKLPGGHGDHQQ, which is repeated 6 times, whereas domain B has the sequence TGGTYGQQGHTGTT, which is repeated 11 times. The two domains were also found in barley dehydrins and rice abscisic acid-induced protein families. The expression of this cDNA in Escherichia coli, using the T7 RNA polymerase promoter, produced a protein of 50 kilodaltons with an isoelectric point of 7.3, and this product comigrated with a major protein synthesized in vivo and in vitro during cold acclimation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cattivelli L., Bartels D. Molecular cloning and characterization of cold-regulated genes in barley. Plant Physiol. 1990 Aug;93(4):1504–1510. doi: 10.1104/pp.93.4.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Hahn M., Walbot V. Low-temperature accumulation of alcohol dehydrogenase-1 mRNA and protein activity in maize and rice seedlings. Plant Physiol. 1991 Mar;95(3):699–706. doi: 10.1104/pp.95.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Kortt A. A., Chandler P. M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989 Jul;13(1):95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Danyluk J., Rassart E., Sarhan F. Gene expression during cold and heat shock in wheat. Biochem Cell Biol. 1991 May-Jun;69(5-6):383–391. doi: 10.1139/o91-058. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Shaw D. C. Heat-stable proteins and abscisic Acid action in barley aleurone cells. Plant Physiol. 1989 Dec;91(4):1520–1526. doi: 10.1104/pp.91.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela S., Franck M. Cloning and characterization of a cold- and ABA-inducible Arabidopsis gene. Plant Mol Biol. 1990 Jul;15(1):137–144. doi: 10.1007/BF00017731. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C., Guo W. W., Everson E., Thomashow M. F. Cold acclimation in Arabidopsis and wheat : a response associated with expression of related genes encoding ;boiling-stable' polypeptides. Plant Physiol. 1990 Nov;94(3):1078–1083. doi: 10.1104/pp.94.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Abscisic Acid-regulated gene expression in relation to freezing tolerance in alfalfa. Plant Physiol. 1988 Jun;87(2):468–473. doi: 10.1104/pp.87.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Wolfraim L., Poole R. J., Dhindsa R. S. Molecular cloning and relationship to freezing tolerance of cold-acclimation-specific genes of alfalfa. Plant Physiol. 1989 Jan;89(1):375–380. doi: 10.1104/pp.89.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas O., Laliberté J. F. The use of PCR for cloning of large cDNA fragments of turnip mosaic potyvirus. J Virol Methods. 1991 Apr;32(1):57–66. doi: 10.1016/0166-0934(91)90185-3. [DOI] [PubMed] [Google Scholar]
- Perras M., Sarhan F. Synthesis of Freezing Tolerance Proteins in Leaves, Crown, and Roots during Cold Acclimation of Wheat. Plant Physiol. 1989 Feb;89(2):577–585. doi: 10.1104/pp.89.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney M. J., Gusta L. V. Factors Influencing the Induction of Freezing Tolerance by Abscisic Acid in Cell Suspension Cultures of Bromus inermis Leyss and Medicago sativa L. Plant Physiol. 1987 Feb;83(2):423–427. doi: 10.1104/pp.83.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher-Chambonnet C., Berreur P., Houde M., Tiveron M. C., Lepesant J. A., Brégégère F. Cloning and partial characterization of the xanthine dehydrogenase gene of Calliphora vicina, a distant relative of Drosophila melanogaster. Gene. 1987;59(2-3):201–212. doi: 10.1016/0378-1119(87)90328-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus P. L., Lynch D. V. Freeze/thaw-induced destabilization of the plasma membrane and the effects of cold acclimation. J Bioenerg Biomembr. 1989 Feb;21(1):21–41. doi: 10.1007/BF00762210. [DOI] [PubMed] [Google Scholar]
- Weretilnyk E. A., Hanson A. D. Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2745–2749. doi: 10.1073/pnas.87.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zachariassen K. E. Physiology of cold tolerance in insects. Physiol Rev. 1985 Oct;65(4):799–832. doi: 10.1152/physrev.1985.65.4.799. [DOI] [PubMed] [Google Scholar]