Abstract
With changes in modern lifestyles, type 2 diabetes mellitus (T2DM) has become a global epidemic metabolic disease, and hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide. T2DM is a complex metabolic disorder and has been considered an independent risk factor for HCC. Growing evidence supports that T2DM-related risk factors facilitate hepatocarcinogenesis via abundant mechanisms. With the wide implementation of microbiomics, transcriptomics, and immunotherapy, the understanding of the complex mechanisms of intestinal flora and immune cell subsets have advanced tremendously in T2DM-related HCC, uncovering new findings in T2DM-related HCC patients. In addition, reports have indicated the different effects of anti-DM drugs on the progression of HCC. In this review, we summarize the effects of major T2DM-related risk factors (including hyperglycemia, hyperinsulinemia, insulin, chronic inflammation, obesity, nonalcoholic fatty liver disease, gut microbiota and immunomodulation), and anti-DM drugs on the carcinogensis and progression of HCC, as well as their potential molecular mechanisms. In addition, other factors (miRNAs, genes, and lifestyle) related to T2DM-related HCC are discussed. We propose a refined concept by which T2DM-related risk factors and anti-DM drugs contribute to HCC and discuss research directions prompted by such evidence worth pursuing in the coming years. Finally, we put forward novel therapeutic approaches to improve the prognosis of T2DM-related HCC, including exploiting novel diagnostic biomarkers, combination therapy with immunocheckpoint inhibitors, and enhancement of the standardized management of T2DM patients.
Keywords: type 2 diabetes mellitus, hepatocellular carcinoma, NAFLD, gut microbiota, inflammatory, immunotherapy
Introduction
China has a high incidence of liver cancer, and liver cancer mortality ranks third among all cancer-related deaths.1 Hepatocellular carcinoma (HCC) accounts for 75%–85% of liver cancer cases, and approximately 45% of HCC deaths occur in China.2 Chronic inflammations caused by viral hepatitis, especially hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, contribute majorly to HCC via changing the hepatic immune system, while the etiology cause by alcohol abuse shows an increasing trend. Despite great efforts put into medical technologies, the survival likelihood of HCC patients is still gloomy due to the lack of early diagnosis and drug resistance.3 Therefore, there is an urgent need to develop novel treatment strategies to improve the prognosis of HCC patients. Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease characterized by insulin-secretion deficiency or insensitive insulation of target cells, resulting in insulin resistance (IR) and liver damage. The increasing incidence of T2DM has become a global public health concern.4 Increasing evidence suggests that T2DM is an important risk factor for HCC;5–7 however, studies on how DM contributes to the occurrence and progression of HCC are scarce. It is necessary to explore the relationship between T2DM and HCC, particularly the role of T2DM in HCC.
This paper discusses the mechanisms that T2DM contribute to the progression of HCC that are currently known, mainly including the effects of hyperglycemia, hyperinsulinemia, IR, chronic inflammation, nonalcoholic fatty liver disease (NAFLD), obesity, and altered gut microbiota. Meanwhile, the effects of hypoglycemic drugs on HCC will be analyzed, with the aim of revealing the connection between T2DM and HCC and the potential mechanisms. This review broadens the understanding of HCC risk factors and contributes toward the development of better treatment strategies for HCC by utilizing T2DM related factors.
Epidemiology
HCC is the sixth–most common human malignancy worldwide and the third-leading cause of cancer-related deaths after lung and colorectal cancers. The global incidence of HCC is 9.3 per 100,000 individuals, with a corresponding mortality rate of 8.5%.8 The International Diabetes Federation reported 463 million patients with 2019 worldwide, and this number is estimated to reach 700 million by 2045.9 In Chinese adults, DM and high blood glucose levels were positively associated with liver cancer and major chronic liver diseases in a prospective study of 500,000 people. Studies of different populations have also shown that DM is a driving force of HCC, which increases the occurrence of HCC by 1.5- to 3-fold, especially in males.10–14 In addition, a population-based cohort study enrolled 674,178 DM subjects demonstrated that glucose variability was an independent predictor of HCC and contributed to the increasing prevalence of HCC.15,16 Table 1 summarizes clinical trials on the relationship between DM and HCC.
Table 1.
Clinical trials on the relationship between diabetes mellitus and HCC
| Reference | Study characteristics | Diabetes diagnosis | Covariate adjustments considered | Main findings |
|---|---|---|---|---|
| [5] | Meta-analysis: a total of 27 studies (23 articles, 85.2% were from Asia), inclusive of approximately 144,566 individuals | Self-report, medical records | Geographic location, alcohol intake, history of cirrhosis, or HBV and HCV infections | Strong evidence that T2DM unfavorably affects HCC progression, recurrence, and patient survival after treatment, irrespective of the approach used. |
| [6] | Prospective cohort study: 239 patients with T2DM who were diagnosed with nonviral HCC, and 3277 non-HCC T2DM patients from a prospective cohort study as controls. Mean follow-up 4.7 years | Medical records | Vital status, age, HBV, HCV | T2DM was associated with increased risk of incident HCC. FIB4 index in diabetes clinics can be the first step toward surveillance of HCC with a nonviral etiology. |
| [7] | Hospital-based retrospective case–control study: 1568 participants, of whom 716 were diagnosed with benign liver diseases and 852 diagnosed with HCC. | Fasting glucose ≥126 mg/dL or 2- hour postload glucose ≥200 mg/dL, HbA1c ≥6.5% | Age, sex, HBV and HCV infections, cirrhosis, gallstone disease, cholinesterase, alkaline phosphatase | DM patients with HBV infection represent a very high HCC risk population and should be considered for HCC close surveillance program. |
| [12] | Meta-analysis: 17 case–control studies (a total of nearly 6000 HCC cases and 74,000 controls) and 32 cohort studies (a total of nearly 6,500,000 individuals) | Self-report, medical records | BMI, prior hepatitis, cirrhosis, alcohol intake, smoking, treatment, duration of diabetes | DM is associated with moderately increased risk of HCC. Meta-analysis of 7 cohort studies found a significant increased risk of HCC mortality for individuals with DM compared to those without. |
| [13] | Meta-analysis: a total of 25 cohort studies, enrolling 1,283,112 persons. Mean follow-up 8.8 years | Self-report, medical records | BMI, prior hepatitis, cirrhosis, alcohol intake, smoking, treatment, duration of diabetes | 18 studies showed that DM was associated with an increased incidence of HCC. In addition, meta-analysis of 7 cohort studies found a significant increased risk of HCC mortality for individuals with DM. |
| [14] | Community-based cohort study: 63,257 middle-aged and older individuals. Prevalence of DM was 8.6%, 499 cohort participants developed HCC. Mean follow-up 14 years | Self-report | Age, sex, BMI, recruitment year, education level, smoking, alcohol intake, consumption of coffee and tea | A history of diabetes at baseline is highly associated with nonviral HCC. DM was associated with an increased risk of incident nonviral HCC. |
| [17] | Hospital-based case–control study: 224 HCC patients and 389 controls | Self-report | Age, sex, smoking, alcohol intake | This study confirms a doubled HCC risk in diabetics, with a stronger excess risk in diabetic subjects who are also tobacco smokers. Metformin may decrease the risk of HCC, whereas insulin may increase the risk. |
Abbreviations: T2DM, type 2 diabetes mellitus; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus.
Major Risk Factors of T2DM Contributing to HCC
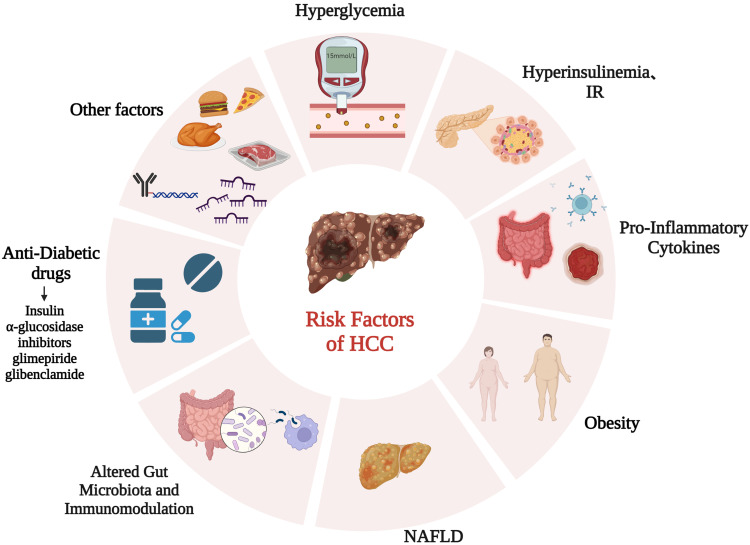
Previous reports have implied a close association between T2DM and HCC; however, the mechanisms underlying the involvement of T2DM in HCC have not been completely elucidated. The following section reviews risk factors associated with T2DM and HCC (Figure 1).
Figure 1.
Risk factors for HCC in patients with T2DM.
Hyperglycemia, Hyperinsulinemia, and Insulin Resistance
Existing evidence shows that hyperglycemia is persistently an independent key factor for HCC.18 High glucose levels provide abundant nutrients and induce abnormal energy metabolism, facilitating tumor cell growth and metastasis. Under the stress of high glucose, HCC cells exhibit mesenchymal phenotype plasticity and metabolic reprogramming by activation of c-Met, promoting the growth and aggressiveness of HCC.19 High glucose stimulation induces the production of reactive oxygen species (ROS),20 which can cause mitochondrial dysfunction and liver inflammation, eventually lead to liver injury and hepatocarcinogenesis.21 Overcoming excessive ROS production has been proved to facilitate tumor progression in sorafenib-resistant HCC cells.22 In addition, hyperglycemia stimulation leads to the accumulation of advanced glycolytic end products, which can form a feedback loop with ROS and favor the occurrence of HCC by transcriptionally activating angiogenesis and proliferation.23,24
Chronic hyperglycemia progressively precipitates inadequate peripheral tissue response to circulatory insulin, resulting in IR and subsequent compensatory hyperinsulinemia in T2DM patients.25 A nationwide prospective study that enrolled 119,316 participants of two large cohorts revealed that hyperinsulinemia and IR were associated with increased risk of developing HCC after an average follow-up of 25.6 years.26,27 Mechanistically, hyperinsulinemia/IR played a key role in the pathophysiology of HCC by activating IRS1 to regulate multiple cytokine pathways, such as JNK.28 Moreover, the existence of hyperinsulinemia/IR, along with proinflammatory factors, such as IL6, TNFα, and leptin, jointly constructed a liver microenvironment prone to HCC in patients with DM. In addition, hyperinsulinemia and IR upregulated the expression of IGF1, which has been proved to be a key mechanism in DM-related hepatocarcinogenesis, contributing to the invasion and sorafenib resistance by regulating the cell cycle, apoptosis, EMT, and autophagy.29–31
Proinflammatory Cytokines
Many studies have supported the hypothesis that T2DM patients undergo a chronic low-grade systemic inflammatory state. Significant differences in proinflammatory cytokines, such as IL6 and TNFα, have been observed in the healthy population and pre-DM and T2DM patients, and contribute crucially to the development of HCC.32,33 IL6 was found to be elevated in HCC patients and acted as an independent predictor of survival in HCC patients.34 In a mouse HCC model induced by diethyl nitrite, male mice who harbored higher IL6 levels developed more HCC through transcriptional repression by eARα in Kupffer cells. Meanwhile, IL6 upregulates the androgen receptor in HCC cells, reduces the expression of tumor suppressor p53, and increases ROS production, resulting in HCC development. By binding with the IL6 receptor, IL6 triggers JAK–STAT3 and downstream signals to regulate multiple processes, such as apoptosis, angiogenesis, metastasis, drug resistance, and immunoescape of HCC.35 Besides IL6, TNFα has also been found to be required for chronic liver diseases and hepatocarcinogenesis.36 Anti-TNFα treatment decreases the expression of proinflammatory cytokines, including IL1β, IL6, and IL17, to suppress the cell viability of HCC and prolong the survival time of xenograft mice, providing a novel therapy for HCC.37 Inflammatory alterations in T2DM patients play a pivotal role in HCC progression and might be a therapeutic option for HCC patients.38
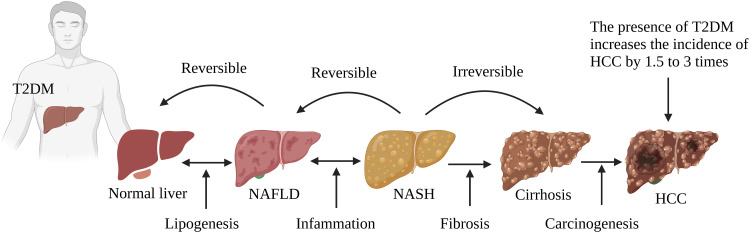
Obesity and Nonalcoholic Fatty Liver Disease (Figure 2)
Figure 2.
T2DM and obesity aggravate the progression of NAFL/NASHI to HCC. T2DM coexists with NAFLD, and it aggravates NAFLD to more severe forms of NASH, cirrhosis, and HCC.
Obesity is known to be a common promoter of T2DM, and has also been found to exert a significant protumor effect on HCC.39 A long-term prospective study that enrolled 15,280 T2DM patients lasting at least 10 years found that obesity increased the hazard ratio of liver cancer compared to nonobese patients.40 Being obese, a high-BMI population was at higher risk of HCC by 86%–119%.41,42 With an increasing of 5 cm of the waist, the risk of liver cancer increases 11%.43 Elevated FABP4 synthesized and secreted by hepatocytes was found to be a crucial connection between T2DM patients with obesity and HCC. With IR and dyslipidemia in T2DM patients with obesity, FABP4 facilitated the proliferation and migration of HCC cells.44,45 In addition, increases in gut microbial metabolite and STAT3 signaling have been reported in obesity-related HCC, which might be a driving pathogenesis of HCC.46,47 T2DM has been reported to be an independent risk factor for NAFLD, one of the most frequent liver metabolic diseases and exhibiting a histological spectrum from bland steatosis to nonalcoholic steatohepatitis (NASH) and eventually liver cirrhosis.48 Emerging evidence reports that NAFLD doubles the risk of HCC in T2DM patients.49,50 The pathogenesis of NAFLD involvement in T2DM-related HCC is complex, and the inflammatory response might be the main culprit.51 Inflammation is involved in the entire spectrum of NAFLD and is characterized by “metabolic inflammation” triggered by excessive lipid storage, IR, and metabolic pathways. Elevated cytokines, such as CCL5, have been observed in cirrhosis patients, and overexpression of CCL5 in NAFLD mouse models activates a wide range of immune cells that facilitate the development of steatosis and HCC.52 Moreover, there is evidence that the application of CCL5-neutralizing antibody treatment protects against the occurrence of new HCC nodules and inhibits the growth of HCC tumors.53 In addition, the accumulation of hepatic cholesterol in liver promotes the development of NAFLD-related HCC via selectively killing natural killer T cells or CD4+ T-cell apoptosis.54,55
Altered Gut Microbiota and Immunomodulation
The gut microbiota is a key regulator of the immune system and metabolism.56 Emerging evidence indicates that the gut microbiome plays an essential role in the T2DM, NAFLD, and obesity.57 In vivo animal experiments and epidemiological studies have shown that the intestinal microbiota of DM mice or patients with liver fibrosis changes, eg, increased phyla firmicutes and brevibacteria.58 Through gut microbiota dysbiosis, high cholesterol in an NAFLD mouse model resulted in hepatic steatosis and inflammation during hepatocarcinogenesis progression. Moreover, restoring the dysbiosis of gut microbiota prevented the development of NAFLD-related HCC completely.59 The effects of gut microbiota contributing to HCC has been attributed to the modulation of peripheral immunoresponse with the suppression of the T-cell immunophenotype, characterized by expansion of regulatory T cells and attenuation of CD8+ T cells.60 The serum level of lipopolysaccharide (LPS) has also been found to be elevated in T2DM patients with dysbiosis of the gut microbiome.58 Also, elevated circulating LPS has been found in both HCC patients and animal models, and this pathogenesis, contributed to by accumulation of LPS, increased intestinal permeability and bacterial translocation.61 Mechanistically, LPS modifies MIR155HG lncRNA by m6A methylation to upregulate the expression of PDL1 and promote the immunoescape of HCC cells.62
Alteration of immunostatus is a common pathological process in T2DM patients, usually occurs in cirrhosis-related HCC, and functions crucially in HCC progression. Persistently high blood sugar stimulates the transient activation of neutrophils, which drives the progression of HCC by mediating the immunosuppressive environment.63,64 Targeting neutrophils might be a novel therapeutic choice through strengthening the response to cancer immunotherapy. Different subtypes of T cells have been shown to benefit NAFLD/NASH-related HCC progression. Loss of CD4+ T and hepatic lipid accumulation have been found in an NAFLD mouse model, promoting hepatocarcinogenesis.65 Furthermore, PD1+ CD8+ T cells are increased in NASH mouse liver, facilitating HCC progression.17 An imbalance in Th17 cells has also been found to regulate adipogenesis, glucose homeostasis, and insulin sensitivity, which are involved in the development of HCC by secreting IL17A.66 The frequency and absolute number of circulating NK cells have been found to be significantly lower in T2DM patients and HCC patients with impaired IFNγ and cytotoxic function of NK cells, while Treg cells were elevated.67,68
Antidiabetic Drugs
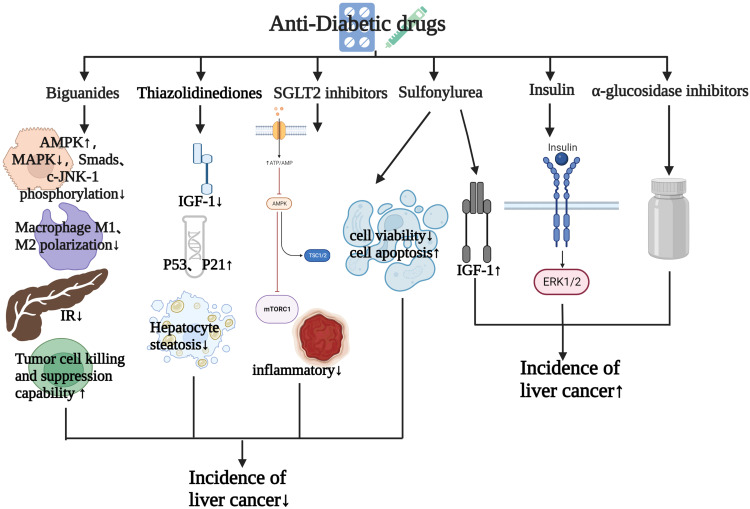
Studies have shown that anti-DM drugs play a crucial role in the development of HCC. In this section, we review the effects of anti-DM drugs on HCC (Figure 3). Table 2 summarizes the effects of anti-DM drugs on the occurrence and development of HCC.
Figure 3.
Effects of antidiabetic drugs on the occurrence and development of HCC.
Table 2.
Effects of antidiabetic drugs on the occurrence and development of HCC
| Reference | Study characteristics | Diabetes diagnosis | Covariate adjustments considered | Main findings |
|---|---|---|---|---|
| [69] | Hospital-based case–control study: 224 HCC patients and 389 controls | Self-report | Age, sex, smoking, alcohol intake | More than doubled HCC risk in diabetics, with stronger excess risk in diabetic subjects who are also tobacco smokers. Metformin may decrease the risk of HCC, whereas insulin may increase the risk. |
| [70] | Hospital-based retrospective case–control study: 85,963 patients with NAFLD and DM, and 852 patients were diagnosed with HCC, mean follow-up 10.3 years | Self-report, medical records | Age, sex, cirrhosis, alcoholic liver damage, viral hepatitis, hereditary hemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, α1 antitrypsin disease, or autoimmune hepatitis | In this large cohort of patients with NAFLD and DM, use of metformin was associated with a reduced risk of HCC, whereas use of combination therapy was associated with increased risk. |
| [71] | Retrospective multicenter study: among 5093 patients with HCC, 1917 (37.6%) were diagnosed with T2DM, of which 338 (17.6%) received treatment with metformin | Medical records | Geographic location, alcohol intake, history of cirrhosis, or HBV and HCV infections | Treatment with metformin was associated with improved survival in patients with T2DM and HCC. |
| [72] | Retrospective cohort study: 2499 elderly diabetic HCC patients | Medical records | Age, sex, and pre- and postdiagnosis medication use | Prediagnosis use of metformin ≤1500 mg/day may improve overall survival of elderly diabetic HCC patients. |
| [73] | Population-based case–control study: 76,349 newly diagnosed DM patients were identified from claims; among diabetics, 3026 and 12,104 patients, respectively, received or did not receive TZDs | Self-report, medical records | Age, sex, comorbidities of diabetes, HBV, HCV, cirrhosis, alcoholic liver disease, NAFLD, end-stage renal disease, hypertension, and hyperlipidemia | The use of TZDs may reduce the risk of developing HCC among DM patients. |
| [74] | Population-based cohort study: 19,349 newly diagnosed DM patients and 77,396 control subjects without DM, nean follow-up 5 years | Electronic register | Age, sex, cirrhosis, alcoholic liver damage, viral hepatitis | The use of metformin or thiazolidinediones may reduce the risk of developing HCC. |
| [75] | Retrospective case–control study: 47,738, participants of whom 241 were diagnosed with HCC | Hospital discharge diagnosis | Age, sex, area of residence, education, alcohol intake, BMI, smoking, history of chronic hepatitis and cirrhosis, family history of liver cancer | Compared to patients never treated with a sulfonylurea, those treated with a sulfonylurea had a 1.7-fold increased risk of HCC development. |
| [76] | Community-based cohort study: 363,426 participants, after excluding those with cancer at baseline, mean follow-up 8.5 years, 176 HCC cases identified | Self-report | Age, sex, center, education, smoking, alcohol intake, BMI, waist:height ratio | DM was independently associated with higher risk of incident HCC and biliary tract cancer. The risk of HCC was particularly higher in participants treated with insulin. |
| [77] | Population-based retrospective cohort study, 3185 HCC patients, mean follow-up 3.5 years | Self-report, medical records | Cirrhosis, HBV, HCV, and alcohol-related diseases | HCC patients with preexisting T2DM treated with SGLT2 inhibitors had significantly lower risk of mortality, especially among those treated >12 months. |
Abbreviations: T2DM, type 2 diabetes mellitus; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus.
Biguanides
Metformin is a first-line guanidine anti-DM drug to treat T2DM in most guidelines.78 Recent studies have shown that T2DM patients treated with metformin have a low risk of cancer and mortality.69,79,80 A retrospective study enrolled 1061 T2DM patients and reported that metformin exposure decreased the incidence of HCC and prolonged their survival.70 Moreover, another report of meta-analysis also revealed that treatment with metformin significantly extended overall survival (OS) and progression-free survival compared with other antihyperglycemic agents in HCC patients with T2DM after curative HCC treatment.71,81 Prediagnostic use of metformin ≤1500 mg/day has also been shown to be associated with longer OS in elderly DM HCC patients.72
The beneficial effects of metformin for anti-HCC can be exerted by plentiful molecular mechanisms. Long‐term exposure to metformin can lead to metabolic adaptation of HCC cells exhibiting a characteristic EMT phenotype and compensatory elevation of oxidative phosphorylation.82 Hu et al found that metformin exerted an antiproliferation effect on HCC cells by targeting AMPK via sonic hedgehog.83 Furthermore, metformin decreased the production of glycolysis and induced mitochondrial damage to alter the bioenergetics of HCC cells.84 In addition, cyclin D1, greatly elevated in human liver cancers with DM, was reported to be suppressed by metformin in hepatocytes, protecting against the occurrence of DM HCC.85
Immunity regulation is a plausible crucial mechanism by which metformin is involved in HCC progression. It has been found that diet aggravated tumorigenesis in a NAFLD/NASH animal model, while metformin benefited therapy efficiency for HCC by modulating macrophage polarization and T-cell infiltration.86 Hepatic CD8+ T cells have been changed into a proinflammatory phenotype and impaired the efficacy of anti-PD1 therapy in a primary liver cancer model. Metformin improves the response of immunocheckpoint inhibitors by regulating CD8+ T-cell metabolism.87 Both in vitro and in vivo studies have shown that metformin reduced hepatocarcinogenesis by reducing protumor CD8+ T cells.88 As aforementioned, metformin has great potential for anti-HCC therapy, and further studies may continue to broaden its application prospects.
Thiazolidinediones
Thiazolidinediones (TZDs) are multitarget insulin sensitizers by virtue of activating PPARγ and have been reported to reduce incident HCC risk in T2DM patients significantly.73,74,89 A meta-analysis revealed that T2DM patients treated with TZDs had an 8% lower risk of HCC incidence than those without TZD treatment and a 10% lower risk of HCC incidence in Asian TZD users.90 Preclinical studies have unveiled that TZDs exert tumor-inhibitory effects in terms of ROS production, proliferation, and inflammation via the mTOR and NFκB pathways.91 Rosiglitazone and pioglitazone are common TZD drugs.92 A low-dose rosiglitazone metformin adduct (RZM) has been found to enhance the antiproliferative effect of HCC by upregulating p21 expression in an AMPK-dependent manner.93 Pioglitazone was reported to be an effective chemoprophylaxis drug retarding liver fibrosis and hepatocarcinogenesis in both T2DM patients and a DM mouse model. These effects of pioglitazone might be ascribed to inactivation of the MAPK pathway and the upregulation of circulate adiponectin to trigger the AMPK pathway.94,95 Moreover, pioglitazone suppresses the growth and invasion of HCC cells by specifically blocking RAGE signaling to induce apoptosis and cell-cycle arrest accompanied by the downregulation of Ki67 and cyclin D1.96
Sulfonylureas
The effects of sulfonylureas, insulin secretagogues, on liver cancer tumorigenesis are complex based on the current evidence and depends on the type of sulfonylurea. A meta-analysis of 281,180 participants and 19,466 HCC cases from eight studies reported that there was no significant relationship between sulfonylureas and HCC incidence overall; however, a slight increase of HCC incident risk observed in patients with established liver disease was noted.90 A cohort study demonstrated that sulfonylurea treatment increased by 1.7-fold the risk of HCC incidence compared to those never treated with sulfonylureas. In that study, they further analyzed the effects of different sulfonylureas, showing a higher risk of HCC in patients using glimepiride or glibenclamide alone, but no association for gliclazide alone.75 The oncogenic effect of sulfonylurea was consistent with Bosetti’s study.97 The specific mechanism of sulfonylurea-promoted tumorigenesis might be related to increased IGF1 activity, leading to abnormal stimulation of multiple cell-signaling cascades to enhance growth factor–dependent cell proliferation.98 Inconsistently, another retrospective study reported that gliclazide resulted in lower HCC incidence than glimepiride, especially in those patients with chronic liver disease.99 In a word, the exact roles of sulfonylureas in the incidence of HCC remain controversial and need to be further elucidated.
Insulin
Insulin has irreplaceable value in the treatment of T2DM patients. Evidence in the last few decades has revealed the hepatocarcinogenesis effect of insulin on HCC.13 Insulin treatment has been found to be an independent prognostic factor for disease-free survival and OS in HCC patients with non-HBV and non-HCV infection.100 A prospective analysis enrolled 363,426 participants and investigated risk factors for HCC during a follow-up of 8.5 years, and found that treatment with insulin conferred the highest risk of HCC, while no relationship was found in participants who received no insulin treatment.76 Moreover, a meta-analysis of 10 studies showed that insulin increased the risk of HCC incidence by 161% for insulin use in T2DM patients. This carcinogenic effect was more notable in Asian populations than Western populations.101 Mechanistically, insulin exerts HCC tumorigenesis effects through modulating the ERK1/2 pathway, which was examined in a DM HCC mouse model.102 In addition, insulin promotes the proliferation of HCC via modulating the abundance of 5-carboxylcytosine of the SREBP1 promoter.103 In summary, the specific mechanisms responsible for the oncogenic role of insulin need more exploration and validation.
SGLT2 Inhibitors and α-Glucosidase Inhibitors
SGLT2 inhibitors are novel anti-DM drugs that reduce glucose reabsorption in the proximal tubule to control glucose levels. Experiments from different animal models have shown that SGLT2 inhibitors protected the liver from damage and attenuated the progression of hepatic fibrosis and hepatocarcinogenesis through modulating glutathione metabolism and oxidative stress.104,105 Experimental data in vitro have also validated that SGLT2 inhibitors suppressed cell growth of HCC by inducing cell-cycle arrest, apoptosis, and metabolic reprogramming.77,106 A SEER–Medicare linked data study indicated that SGLT2-inhibitor initiation was associated with improvement in OS in HCC patients with preexisting T2DM compared to no use of an SGLT2 inhibitor.107 However, there is no current clinical evidence on the direct effects of SGLT2 inhibitors on HCC in T2DM patients. Evidence of the effects of α-glucosidase inhibitors on HCC patients with T2DM remains sparse. One large meta-analysis suggested that α-glucosidase inhibitors were related to 8% higher incidence in HCC with T2DM.100 Further research is needed to elucidate the role and adverse effects of α-glucosidase inhibitors in HCC patients with T2DM.
Other Factors
A key player in HCC and T2DM is miRNAs, which may be hidden culprits in DM-associated HCC. Recent studies have documented that miR34a and miR221 were significantly upregulated and miR16, miR23-3p, miR122-5p, miR198, and miR199a-3p were significantly downregulated in HCC-positive T2DM patients compared to liver cirrhosis–positive T2DM patients.108 Gene expression is also an important risk factor for DM patients with HCC. A recent bioinformatic analysis of key genes associated with DM-associated HCC identified nine central genes that were the key drivers of HCC progression.109 Another bioinformatic analysis indicated that the DM-related genes ST3GAL2 and ZNF613 were highly methylated and positively correlated with HCC stage.110 In addition, diet and exercise have been showed to be related to metabolically related HCC, and positive modulation of the immunoresponse might contribute to this effect.111
Summary and Perspectives
In conclusion, there is growing evidence that T2DM contributes to HCC occurrence and progression. This review aimed to elucidate the mechanisms linking HCC and T2DM. Overall, addresssing hyperglycemia, IR, and obesity in T2DM patients and reduction of inflammation in NAFLD may reduce the incidence of T2DM-related HCC and postpone the progression of HCC.
Unexpectedly, different hypoglycemic agents have different effects on the risk of HCC development. Metformin and TZDs are generally associated with a lower incidence of HCC, whereas insulin increases the incidence of HCC. For example, gliclazide may be more favorable than glimepiride when applying sulfonylurea. Moreover, the evidence from clinical trials on how novel types of anti-DM drugs, such as SGLT2 inhibitors, affect HCC is barren. Therefore, the validity and relevance of anti-DM drugs in HCC still need to be confirmed further by laboratory-based mechanistic studies, as well as extensive human clinical trials. Studies elucidating the potential effects of different dosages of anti-DM drugs on hepatocarcinogenesis are needed. The relationship between T2DM and the etiologies of HCC is worthy of attention. NAFLD contributes crucially to the occurrence and development of HCC. However, there is no proper approved pharmaceutical treatment for NAFLD currently. Numerous trials investigating the effects of anti-DM drugs on NAFLD are under development, and new effective agents are promising. We hope that in the future, large clinical trials can evaluate and approve new agents to treat NAFLD patients with T2DM to retard the development of HCC. In conclusion, we believe that due to the rapid changes in the etiologies of HCC and the high prevalence of HCC in T2DM patients, we will witness the potential modulation effects of anti-DM drugs on HCC, and how to use anti-DM drugs may help us to focus on intervention and improve the prognosis of T2DM patients with HCC.
With the advancement of high-throughput sequencing technologies, altered gut microbiota is noted as an emerging etiology of T2DM related HCC. Further investigations for selecting appropriate bacterial strains are necessary to gain the possibility of using probiotics as an alternative therapeutic method for T2DM-related HCC. Immunodysfunction is commonly found in T2DM patients, suggesting inferior immunotherapy effects for HCC patients with T2DM. Therefore, developing a novel treatment strategy, such as a combination of anti-DM drugs and immunotherapy, to favor immunity efficiency by activating immunity response and reducing immunity impairment in HCC patients with T2DM is worth pursuing. It is expected that novel biomarkers for early diagnosis of HCC will be developed to help better surveillance in T2DM patients. Meanwhile, subgroup and stratification analysis of differences in immunotherapy response and various characteristics in T2DM patients with HCC should be emphasized to achieve the goal of personalized treatment. These investigations will be essential to alleviate the disease and improve the prognosis of T2DM patients with HCC in the future. Moreover, lifestyle changes, including diet and increased exercise, have indispensable roles in improving the prognosis of T2DM patients with HCC. Adjuvant exercise after HCC surgery and healthy food intake should be encouraged. Through publicity, education, and medical care, strengthening the standardized management of T2DM patients will greatly alleviate the occurrence of HCC.
Funding Statement
This work was supported by the National Nature Science Foundation of China (81902500), the China Postdoctoral Science Foundation (2022MD723764), the Innovation Project of Guangxi Graduate Education (YCSW2023229), the Guangxi Natural Science Foundation (2023GXNSFBA026108) and the Guangxi Natural Science Foundation(No.2017JJA10238).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou H, Li M, Lei Q, et al. Economic burden and quality of life of hepatocellular carcinoma in greater China: a systematic review. Front Public Health. 2022;10:801981. doi: 10.3389/fpubh.2022.801981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Li Q, Xu S, et al. Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: from modulation to combination therapy targeting the microenvironment. Cancer Cell Int. 2022;22(1):73. doi: 10.1186/s12935-021-02435-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uuh-Narvaez JJ, Segura-Campos MR. Cabbage (Brassica oleracea var. capitata): a food with functional properties aimed to type 2 diabetes prevention and management. J Food Sci. 2021;86(11):4775–4798. doi: 10.1111/1750-3841.15939 [DOI] [PubMed] [Google Scholar]
- 5.Mrzljak A, Cigrovski Berković M, Giovanardi F, Lai Q. The prognostic role of diabetes mellitus type 2 in the setting of hepatocellular carcinoma: a systematic review and meta-analysis. Croat Med J. 2022;63(2):176–186. doi: 10.3325/cmj.2022.63.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tateishi R, Matsumura T, Okanoue T, et al. Hepatocellular carcinoma development in diabetic patients: a nationwide survey in Japan. J Gastroenterol. 2021;56(3):261–273. doi: 10.1007/s00535-020-01754-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Z, Zhang C, Yan J, et al. Diabetes mellitus is associated with hepatocellular carcinoma: a retrospective case-control study in hepatitis endemic area. PLoS One. 2013;8(12):e84776. doi: 10.1371/journal.pone.0084776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabet Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 10.Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61(10):2140–2154. doi: 10.1007/s00125-018-4664-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang Y, Kartsonaki C, Turnbull I, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68(4):1308–1318. doi: 10.1002/hep.30083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int, J, Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165 [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(2):109–122. doi: 10.1002/dmrr.1291 [DOI] [PubMed] [Google Scholar]
- 14.Koh WP, Wang R, Jin A, Yu MC, Yuan JM. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108(5):1182–1188. doi: 10.1038/bjc.2013.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Y, Yang B, Qiu W, et al. ER-residential Nogo-B accelerates NAFLD-associated HCC mediated by metabolic reprogramming of oxLDL lipophagy. Nat Commun. 2019;10(1):3391. doi: 10.1038/s41467-019-11274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo -J-J, Cho EJ, Han K, et al. Glucose variability and risk of hepatocellular carcinoma in patients with diabetes: a nationwide population-based study. Cancer Epidemiol Biomarkers Prev. 2021;30(5):974–981. doi: 10.1158/1055-9965.EPI-20-1654 [DOI] [PubMed] [Google Scholar]
- 17.Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:7854):450–456. doi: 10.1038/s41586-021-03362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Supabphol S, Seubwai W, Wongkham S, Saengboonmee C. High glucose: an emerging association between diabetes mellitus and cancer progression. J Mol Med. 2021;99(9):1175–1193. doi: 10.1007/s00109-021-02096-w [DOI] [PubMed] [Google Scholar]
- 19.Topel H, Bağırsakçı E, Yılmaz Y, et al. High glucose induced c-Met activation promotes aggressive phenotype and regulates expression of glucose metabolism genes in HCC cells. Sci Rep. 2021;11(1):11376. doi: 10.1038/s41598-021-89765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizwan H, Pal S, Sabnam S, Pal A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020;241:117148. doi: 10.1016/j.lfs.2019.117148 [DOI] [PubMed] [Google Scholar]
- 21.G Bardallo R, Panisello-Roselló A, Sanchez-Nuno S, Alva N, Roselló-Catafau J, Carbonell T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2022;289(18):5463–5479. doi: 10.1111/febs.16336 [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Ji L, Ruan Y, et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1β in hepatocellular carcinoma. Signal Transduct Target Ther. 2021;6(1):190. doi: 10.1038/s41392-021-00594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon J-M, Thomas F, Czernichow S, et al. Hyperglycaemia is associated with cancer-related but not non-cancer-related deaths: evidence from the IPC cohort. Diabetologia. 2018;61(5):1089–1097. doi: 10.1007/s00125-017-4540-8 [DOI] [PubMed] [Google Scholar]
- 24.Ichimura-Shimizu M, Kageyama T, Oya T, et al. Verification of the impact of blood glucose level on liver carcinogenesis and the efficacy of a dietary intervention in a spontaneous metabolic syndrome model. Int J Mol Sci. 2021;22(23):12844. doi: 10.3390/ijms222312844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rachdaoui N. Insulin: the Friend and the Foe in the Development of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21(5):1770. doi: 10.3390/ijms21051770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Sui J, Zhao L, et al. Association of Inflammatory and Insulinemic Potential of Diet and Lifestyle with Risk of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(4):789–796. doi: 10.1158/1055-9965.EPI-20-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatsuka T, Tateishi R. Development and prognosis of hepatocellular carcinoma in patients with diabetes. Clin Mol Hepatol. 2023;29(1):51–64. doi: 10.3350/cmh.2022.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5(13):270. doi: 10.21037/atm.2017.04.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Sun Y, Liu B, et al. Insulin-like growth factor-1 induces epithelial-mesenchymal transition in hepatocellular carcinoma by activating survivin. Oncol Rep. 2018;40(2):952–958. doi: 10.3892/or.2018.6516 [DOI] [PubMed] [Google Scholar]
- 30.Juratli MA, Zhou H, Oppermann E, et al. Integrin α2 and β1 cross-communication with mTOR/AKT and the CDK-cyclin axis in hepatocellular carcinoma cells. Cancers (Basel). 2022;14(10). doi: 10.3390/cancers14102430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo M-HT, Jeng H-Y, Kuo Y-C, et al. The Role of IGF/IGF-1R signaling in hepatocellular carcinomas: stemness-related properties and drug resistance. Int J Mol Sci. 2021;22(4):1931. doi: 10.3390/ijms22041931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang K, Liang Y, Ma Y, Wu J, Luo H, Yi B. The variation and correlation of serum adiponectin, nesfatin-1, IL-6, and TNF-α levels in prediabetes. Front Endocrinol. 2022;13:774272. doi: 10.3389/fendo.2022.774272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Liu S, Yang M. Hepatocellular carcinoma and obesity, type 2 diabetes mellitus, cardiovascular disease: causing factors, molecular links, and treatment options. Front Endocrinol. 2021;12:808526. doi: 10.3389/fendo.2021.808526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Mahony G, Petersen J, Ek M, et al. Discovery by virtual screening of an inhibitor of CDK5-Mediated PPARγ phosphorylation. ACS Med Chem Lett. 2022;13(4):681–686. doi: 10.1021/acsmedchemlett.1c00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol. 2021;11:760971. doi: 10.3389/fonc.2021.760971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omaru N, Watanabe T, Kamata K, Minaga K, Kudo M. Activation of NOD1 and NOD2 in the development of liver injury and cancer. Front Immunol. 2022;13:1004439. doi: 10.3389/fimmu.2022.1004439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Jian Y-B. Antitumor necrosis factor-α antibodies as a novel therapy for hepatocellular carcinoma. Exp Ther Med. 2018;16(2):529–536. doi: 10.3892/etm.2018.6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39(1):26–42. doi: 10.1055/s-0038-1676806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Wang Q, Zhang Y, et al. Multigenerational maternal obesity increases the incidence of HCC in offspring via miR-27a-3p. J Hepatol. 2020;73(3):603–615. doi: 10.1016/j.jhep.2020.03.050 [DOI] [PubMed] [Google Scholar]
- 40.Mao D, Lau ESH, Wu H, et al. Risk associations of glycemic burden and obesity with liver cancer-A 10-year analysis of 15,280 patients with type 2 diabetes. Hepatol Commun. 2022;6(6):1350–1360. doi: 10.1002/hep4.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagström H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population-based cohort study in 1.2 million men. Gut. 2018;67(8):1536–1542. doi: 10.1136/gutjnl-2016-313622 [DOI] [PubMed] [Google Scholar]
- 42.Yang B, Petrick JL, Kelly SP, Graubard BI, Freedman ND, McGlynn KA. Adiposity across the adult life course and incidence of primary liver cancer: the NIH-AARP cohort. Int, J, Cancer. 2017;141(2):271–278. doi: 10.1002/ijc.30737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Florio AA, Campbell PT, Zhang X, et al. Abdominal and gluteofemoral size and risk of liver cancer: the liver cancer pooling project. Int, J, Cancer. 2020;147(3):675–685. doi: 10.1002/ijc.32760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trojnar M, Patro-Małysza J, Kimber-Trojnar Ż, Leszczyńska-Gorzelak B, Mosiewicz J. Associations between fatty acid-binding protein 4⁻a proinflammatory adipokine and insulin resistance, gestational and type 2 diabetes mellitus. Cells. 2019;8(3):227. doi: 10.3390/cells8030227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson KJ, Austin RG, Nazari SS, Gersin KS, Iannitti DA, McKillop IH. Altered fatty acid-binding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma. Liver Int. 2018;38(6):1074–1083. doi: 10.1111/liv.13639 [DOI] [PubMed] [Google Scholar]
- 46.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347 [DOI] [PubMed] [Google Scholar]
- 47.Grohmann M, Wiede F, Dodd GT, et al. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell. 2018;175(5):1289–1306.e20. doi: 10.1016/j.cell.2018.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774. doi: 10.3390/ijms17050774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanase DM, Gosav EM, Costea CF, et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020;2020:3920196. doi: 10.1155/2020/3920196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484–495. doi: 10.1038/s41574-021-00507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77(4):1136–1160. doi: 10.1016/j.jhep.2022.06.012 [DOI] [PubMed] [Google Scholar]
- 52.Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69(1):107–120. doi: 10.1002/hep.30036 [DOI] [PubMed] [Google Scholar]
- 53.Koh MY, Gagea M, Sargis T, et al. A new HIF-1α/RANTES-driven pathway to hepatocellular carcinoma mediated by germline haploinsufficiency of SART1/HAF in mice. Hepatology. 2016;63(5):1576–1591. doi: 10.1002/hep.28468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang W, Zhou J, Yang W, et al. Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver. Cell Mol Immunol. 2022;19(7):834–847. doi: 10.1038/s41423-022-00872-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown ZJ, Fu Q, Ma C, et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4+ T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9(6):620. doi: 10.1038/s41419-018-0687-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C, Huang X, Li Y, Chen J, Lv Y, Dai S. Prognosis and personalized treatment prediction in TP53-mutant hepatocellular carcinoma: an in silico strategy towards precision oncology. Brief Bioinform. 2021;22:3. [DOI] [PubMed] [Google Scholar]
- 57.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z [DOI] [PubMed] [Google Scholar]
- 58.Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18(5):3461–3469. doi: 10.3892/etm.2019.7943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Coker OO, Chu ES, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–774. doi: 10.1136/gutjnl-2019-319664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behary J, Amorim N, Jiang X-T, et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12(1):187. doi: 10.1038/s41467-020-20422-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. 2018;7(1):11–20. doi: 10.21037/hbsn.2017.12.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng L, Pan B, Zhang X, et al. Lipopolysaccharide facilitates immune escape of hepatocellular carcinoma cells via m6A modification of lncRNA MIR155HG to upregulate PD-L1 expression. Cell Biol Toxicol. 2022;38(6):1159–1173. doi: 10.1007/s10565-022-09718-0 [DOI] [PubMed] [Google Scholar]
- 63.Kummer U, Zobeley J, Brasen JC, et al. Elevated glucose concentrations promote receptor-independent activation of adherent human neutrophils: an experimental and computational approach. Biophys J. 2007;92(7):2597–2607. doi: 10.1529/biophysj.106.086769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(4):257–273. doi: 10.1038/s41575-021-00568-5 [DOI] [PubMed] [Google Scholar]
- 65.Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:7593):253–257. doi: 10.1038/nature16969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi T, Kobara H, Oura K, Masaki T. Mechanisms underlying hepatocellular carcinoma progression in patients with type 2 diabetes. J Hepatocell Carcinoma. 2021;8:45–55. doi: 10.2147/JHC.S274933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang L, Wang H, Cao K, et al. Dysfunction of circulating CD3+CD56+ NKT-like cells in type 2 diabetes mellitus. Int J Med Sci. 2023;20(5):652–662. doi: 10.7150/ijms.83317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koo S-Y, Park E-J, Lee C-W. Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma. Exp Mol Med. 2020;52(8):1209–1219. doi: 10.1038/s12276-020-0480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kramer JR, Natarajan Y, Dai J, et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75(6):1420–1428. doi: 10.1002/hep.32244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tangjarusritaratorn T, Tangjittipokin W, Kunavisarut T. Incidence and survival of hepatocellular carcinoma in type 2 diabetes patients with cirrhosis who were treated with and without metformin. Diabetes Metab Syndr Obes. 2021;14:1563–1574. doi: 10.2147/DMSO.S295753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulte L, Scheiner B, Voigtländer T, et al. Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int. 2019;39(4):714–726. doi: 10.1111/liv.14048 [DOI] [PubMed] [Google Scholar]
- 72.Antwi SO, Li Z, Mody K, Roberts LR, Patel T. Independent and joint use of statins and metformin by elderly patients with diabetes and overall survival following HCC diagnosis. J Clin Gastroenterol. 2020;54(5):468–476. doi: 10.1097/MCG.0000000000001182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang M-Y, Chung C-H, Chang W-K, et al. The role of thiazolidinediones in hepatocellular carcinoma risk reduction: a population-based cohort study in Taiwan. Am J Cancer Res. 2017;7(7):1606–1616. [PMC free article] [PubMed] [Google Scholar]
- 74.Lai S-W, Chen P-C, Liao K-F, Muo C-H, Lin -C-C, Sung F-C. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107(1):46–52. doi: 10.1038/ajg.2011.384 [DOI] [PubMed] [Google Scholar]
- 75.Lee J-Y, Jang S-Y, Nam CM, Kang ES. Incident hepatocellular carcinoma risk in patients treated with a sulfonylurea: a nationwide, nested, case-control study. Sci Rep. 2019;9(1):8532. doi: 10.1038/s41598-019-44447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlesinger S, Aleksandrova K, Pischon T, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. 2013;24(9):2449–2455. doi: 10.1093/annonc/mdt204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakano D, Kawaguchi T, Iwamoto H, Hayakawa M, Koga H, Torimura T. Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: multi-omics analysis of metabolomics and absolute quantification proteomics (iMPAQT). PLoS One. 2020;15(4):e0232283. doi: 10.1371/journal.pone.0232283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. 2023;19(8):460–476. doi: 10.1038/s41574-023-00833-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iranshahy M, Rezaee R, Karimi G. Hepatoprotective activity of metformin: a new mission for an old drug? Eur J Pharmacol. 2019;850:1–7. doi: 10.1016/j.ejphar.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 80.Miele L, Bosetti C, Turati F, et al. Diabetes and insulin therapy, but not metformin, are related to hepatocellular cancer risk. Gastroenterol Res Pract. 2015;2015:570356. doi: 10.1155/2015/570356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan B, Ma J, Wang J, Hao J. The effect of metformin usage on survival outcomes for hepatocellular carcinoma patients with type 2 diabetes mellitus after curative therapy. Front Endocrinol. 2022;13:1060768. doi: 10.3389/fendo.2022.1060768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin P, Jiang J, Zhou L, et al. Disrupting metformin adaptation of liver cancer cells by targeting the TOMM34/ATP5B axis. EMBO Mol Med. 2022;14(12):e16082. doi: 10.15252/emmm.202216082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu A, Hu Z, Ye J, et al. Metformin exerts anti-tumor effects via Sonic hedgehog signaling pathway by targeting AMPK in HepG2 cells. Biochem Cell Biol. 2022;100(2):142–151. doi: 10.1139/bcb-2021-0409 [DOI] [PubMed] [Google Scholar]
- 84.Tawfik SM, Abdollah MRA, Elmazar MM, El-Fawal HAN, Abdelnaser A. Effects of metformin combined with antifolates on hepG2 cell metabolism and cellular proliferation. Front Oncol. 2022;12:828988. doi: 10.3389/fonc.2022.828988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo C, Liang J, Sharabi K, et al. Obesity/type 2 diabetes-associated liver tumors are sensitive to cyclin D1 deficiency. Cancer Res. 2020;80(16):3215–3221. doi: 10.1158/0008-5472.CAN-20-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Oliveira S, Houseright RA, Graves AL, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70(4):710–721. doi: 10.1016/j.jhep.2018.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wabitsch S, McCallen JD, Kamenyeva O, et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77(3):748–760. doi: 10.1016/j.jhep.2022.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papadakos SP, Ferraro D, Carbone G, et al. The emerging role of metformin in the treatment of hepatocellular carcinoma: is there any value in repurposing metformin for HCC immunotherapy? Cancers (Basel). 2023;15(12):3161. doi: 10.3390/cancers15123161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yip TC-F, Wong VW-S, Chan HL-Y, et al. Thiazolidinediones reduce the risk of hepatocellular carcinoma and hepatic events in diabetic patients with chronic hepatitis B. J Viral Hepat. 2020;27(9):904–914. doi: 10.1111/jvh.13307 [DOI] [PubMed] [Google Scholar]
- 90.Arvind A, Memel ZN, Philpotts LL, Zheng H, Corey KE, Simon TG. Thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, sulfonylureas, and hepatocellular carcinoma risk: a meta-analysis. Metabolism. 2021;120:154780. doi: 10.1016/j.metabol.2021.154780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biondo LA, Teixeira AAS, de O S Ferreira KC, Neto JCR. Pharmacological strategies for insulin sensitivity in obesity and cancer: thiazolidinediones and metformin. Curr Pharm Des. 2020;26(9):932–945. doi: 10.2174/1381612826666200122124116 [DOI] [PubMed] [Google Scholar]
- 92.Qiu -Y-Y, Zhang J, Zeng F-Y, Zhu YZ. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol Res. 2023;192:106786. doi: 10.1016/j.phrs.2023.106786 [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Hu X, Shan X, Chen K, Tang H. Rosiglitazone metformin adduct inhibits hepatocellular carcinoma proliferation via activation of AMPK/p21 pathway. Cancer Cell Int. 2019;19:13. doi: 10.1186/s12935-019-0732-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bril F, Kalavalapalli S, Clark VC, et al. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clin Gastroenterol Hepatol. 2018;16(4):558–566.e2. doi: 10.1016/j.cgh.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 95.Li S, Ghoshal S, Sojoodi M, et al. Pioglitazone reduces hepatocellular carcinoma development in two rodent models of cirrhosis. J Gastrointest Surg. 2019;23(1):101–111. doi: 10.1007/s11605-018-4004-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y, Zhao L-H, Huang B, et al. Pioglitazone, a PPARγ agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol, Carcinog. 2015;54(12):1584–1595. doi: 10.1002/mc.22231 [DOI] [PubMed] [Google Scholar]
- 97.Bosetti C, Franchi M, Nicotra F, et al. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: a nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiol Drug Saf. 2015;24(7):771–778. doi: 10.1002/pds.3801 [DOI] [PubMed] [Google Scholar]
- 98.Plaz Torres MC, Jaffe A, Perry R, Marabotto E, Strazzabosco M, Giannini EG. Diabetes medications and risk of HCC. Hepatology. 2022;76(6):1880–1897. doi: 10.1002/hep.32439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JY, Kim G, Lee YH, et al. Comparison of hepatocellular carcinoma risk between patients treated with glimepiride and gliclazide. Diabetes Metab. 2019;45(1):83–85. doi: 10.1016/j.diabet.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 100.Amemiya H, Matsuda M, Saito R, et al. Impact of insulin treatment on prognosis of non-B non-C hepatocellular carcinoma after hepatectomy. Anticancer Res. 2021;41(1):317–326. doi: 10.21873/anticanres.14778 [DOI] [PubMed] [Google Scholar]
- 101.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:6. [DOI] [PubMed] [Google Scholar]
- 102.Baba H, Kurano M, Nishida T, Hatta H, Hokao R, Tsuneyama K. Facilitatory effect of insulin treatment on hepatocellular carcinoma development in diabetes. BMC Res Notes. 2017;10(1):478. doi: 10.1186/s13104-017-2783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan J-B, Lai -C-C, Jhu J-W, et al. Insulin and metformin control cell proliferation by regulating TDG-Mediated DNA demethylation in liver and breast cancer cells. Mol Ther Oncolytics. 2020;18:282–294. doi: 10.1016/j.omto.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meng Z, Liu X, Li T, et al. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int Immunopharmacol. 2021;94:107492. doi: 10.1016/j.intimp.2021.107492 [DOI] [PubMed] [Google Scholar]
- 105.Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, et al. Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in High Fat Diet Fed ApoE(-/-) Mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 2021;22(2):818. doi: 10.3390/ijms22020818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jojima T, Wakamatsu S, Kase M, et al. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci. 2019;20(20):5237. doi: 10.3390/ijms20205237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hendryx M, Dong Y, Ndeke JM, Luo J. Sodium-glucose cotransporter 2 (SGLT2) inhibitor initiation and hepatocellular carcinoma prognosis. PLoS One. 2022;17(9):e0274519. doi: 10.1371/journal.pone.0274519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elemeery MN, Mohamed MA, Madkour MA, et al. MicroRNA signature in patients with hepatocellular carcinoma associated with type 2 diabetes. World J Gastroenterol. 2019;25(42):6322–6341. doi: 10.3748/wjg.v25.i42.6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu G-M, Zeng H-D, Zhang C-Y, J-W X. Key genes associated with diabetes mellitus and hepatocellular carcinoma. Pathol Res Pract. 2019;215(11):152510. doi: 10.1016/j.prp.2019.152510 [DOI] [PubMed] [Google Scholar]
- 110.Wei H, Wang J, Li W, et al. The underlying pathophysiology association between the Type 2-diabetic and hepatocellular carcinoma. J Cell Physiol. 2019;234(7):10835–10841. doi: 10.1002/jcp.27919 [DOI] [PubMed] [Google Scholar]
- 111.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16(7):411–428. doi: 10.1038/s41575-019-0145-7 [DOI] [PubMed] [Google Scholar]