Abstract
Initiating alcohol use in adolescence significantly increases the likelihood of developing adult alcohol use disorder (AUD). However, it has been difficult to replicate adolescent alcohol exposure leading to increased adult alcohol intake across differing preclinical models. In the present study, differentially housed male rats (group vs. single cages) were used to determine the effects of voluntary intermittent exposure of saccharin-sweetened ethanol (EtOH) during adolescence on adult intake of unsweetened 20% EtOH. Adolescent male rats were assigned to group or isolated housing conditions and underwent an intermittent 2-bottle choice in adolescence (water only or water vs. 0.2% saccharin/20% EtOH), and again in adulthood (water vs. 20% EtOH). Intermittent 2-bottle choice sessions lasted for 24 h, and occurred three days per week, for 5 weeks. Rats were moved from group or isolated housing to single housing cages for 2-bottle choice tests and returned to their original housing condition on off days. During adolescence, rats raised in isolated housing conditions consumed significantly more sweetened EtOH than rats raised in group housing conditions, an effect that was enhanced across repeated exposures. In adulthood, rats raised in isolated housing conditions and exposed to sweetened EtOH during adolescence also consumed significantly higher levels of unsweetened 20% EtOH compared to group housed rats. The effect that was most pronounced over the first 5 re-exposure sessions. Housing conditions alone had little effect on adult EtOH intake. These preclinical results suggest that social isolation stress, combined with adolescent EtOH exposure, may play a key role in adult AUD risk.
Keywords: Adolescence, 2-Bottle Choice, Alcohol, Social Isolation, Housing, Rat
Introduction
A key variable that predicts the propensity to develop an alcohol use disorder (AUD) is the age at which alcohol use is first initiated. Adolescents that begin alcohol use between the ages of 11 and 14 display a four-fold greater risk for an AUD diagnosis compared to individuals that first consume alcohol after the age of 20 (DeWit, Adlaf, Offord, & Ogborne, 2000; Grant & Dawson, 1997). Between the ages of 11 and 14, the adolescent brain is completing final maturation of limbic structures that are key integrators of alcohol reward (e.g. the nucleus accumbens, amygdala, and hippocampus) (De Bellis et al., 2000; Nixon, Morris, Liput, & Kelso, 2010; Spear, 2018). Concurrently, brain regions responsible for executive function and impulse control are just beginning to mature (e.g. prefrontal and orbitofrontal cortices), with full “adult- like” top-down control over limbic structures still a decade away (Crews & Boettiger, 2009; De Bellis et al., 2005; Romer, Reyna, & Satterthwaite, 2017; Spear, 2015, 2018).
Due to the ontogenetic state of the brain in adolescence, adolescents are more susceptible to the stimulatory effects of alcohol, and less susceptive to alcohol’s sedative effects (Ristuccia & Spear, 2008). Therefore, adolescents often consume alcohol in quantities comparable to adults, despite differences in body size and metabolism (Deas, Riggs, Langenbucher, Goldman, & Brown, 2000). Heavy alcohol consumption during adolescence can have particularly devastating effects, as alcohol has the potential to both disrupt the brain’s developmental trajectory, as well as cause significant damage to more mature brain regions (Crews, Braun, Hoplight, Switzer, & Knapp, 2000; Spear, 2018). However, the precise mechanisms for how adolescent alcohol exposure increases the vulnerability for AUD in adulthood are poorly understood.
Preclinical models with face validity are needed to develop novel treatment strategies that target the behavioral and neurobiological consequences unique to initiating alcohol use in adolescence. However, a disconnect often exists between studies assessing the neurobiological versus the behavioral consequences of adolescent alcohol use. For instance, rats are commonly used to assess the effects of adolescent alcohol exposure in preclinical neurobiological studies (Spear, 2018). However, outbred rat strains do not naturally consume high enough levels of ethanol (EtOH) that would induce significant neurobiological alterations; therefore, methods of forced delivery to EtOH are often used in these studies to deliver consistent and physiologically relevant doses (Tunstall, Vendruscolo, & Allen-Worthington, 2020). Alternatively, researchers have employed methods to increase voluntary intake in rats such as the selective breeding of high alcohol drinking strains, intermittent access schedules, sucrose-fading procedures, or simply sweetening the EtOH (McBride, Rodd, Bell, Lumeng, & Li, 2014; Samson, 1986; Samson, Files, & Denning, 1999). The use of sweeteners to alter the taste of EtOH may offer improved face validity over forced EtOH delivery particularly in adolescent models, as adolescents often consume alcohol containing a non-caloric sweetener (Stamates, Linden-Carmichael, & Lau-Barraco, 2016).
Several studies using a rat model have investigated the effects of adolescent EtOH use on adult EtOH consumption, but the outcomes have been mixed. In rats, forced exposure to EtOH in adolescence via IP injections has led to increases in adult EtOH intake in some cases (Alaux- Cantin et al., 2013; Pandey, Sakharkar, Tang, & Zhang, 2015; Pascual, Boix, Felipo, & Guerri, 2009), while others have found no effect, or decreased adult EtOH intake following adolescent EtOH injections (Chandler, Shaykin, Nixon, & Bardo, 2021; Gilpin, Karanikas, & Richardson, 2012). Similarly, forced exposure to EtOH via oral gavage during adolescence resulted in increased voluntary EtOH consumption in some studies (Acevedo, Nizhnikov, Molina, & Pautassi, 2014; Fabio, Nizhnikov, Spear, & Pautassi, 2014), but the adolescent EtOH gavage can also have opposing effects, resulting in a reduction in adult EtOH intake (Chandler et al., 2022). The adolescent intermittent vapor paradigm is another method of forced EtOH exposure that induces moderate to high levels of intoxication, and although adult rats exposed intermittently to vapor during adolescence do not display increased adult EtOH self-administration (Slawecki & Betancourt, 2002), they do demonstrate enhanced resistance to the extinction of EtOH seeking (Gass et al., 2014). A study that utilized voluntary binge exposure to sweetened-EtOH during adolescence found no effect of the binge on baseline adult unsweetened-EtOH self- administration; however, relapse-like drinking was augmented in adolescent binged rats during withdrawal from intermittent EtOH vapor inhalation (Gilpin et al., 2012). Voluntary access to EtOH during adolescence has also been reported to increase drinking in adult rats (Amodeo, Kneiber, Wills, & Ehlers, 2017; Toalston et al., 2015), but again, negative findings have also been reported (Tolliver & Samson, 1991; Vetter, Doremus-Fitzwater, & Spear, 2007).
While there are multiple procedural differences across studies that may underlie the various discrepancies in the literature, one neglected difference has been the home cage environment, with housing conditions unclear in some studies, or using either single- pair- and/or group-housing conditions (Chandler et al., 2022; Doremus, Brunell, Rajendran, & Spear, 2005). In the case of voluntary intake, adult rats housed in isolation consume more EtOH than rats housed in pair or group housing conditions (Butler, Karkhanis, Jones, & Weiner, 2016; Vazquez- Leon, Martinez-Mota, Quevedo-Corona, & Miranda-Paez, 2017). The state of the home environment during adolescence is also known to be a mediator of adult vulnerability for substance misuse (de Almeida Magalhaes, Correia, de Carvalho, Damasceno, & Brunialti Godard, 2018; DeWit, MacDonald, & Offord, 1999), with numerous studies in rats showing that adolescent social isolation housing increases drug taking, including EtOH consumption (Bardo, Hammerslag, & Malone, 2021; Butler, Ariwodola, & Weiner, 2014; Hofford, Chow, Beckmann, & Bardo, 2017; Lesscher et al., 2015; Lopez, Doremus-Fitzwater, & Becker, 2011; McCool & Chappell, 2009). This isolation-induced increase in vulnerability is thought to involve sensitization of the hypothalamic-pituitary-adrenal (HPA) stress axis, as social isolation during adolescence has lasting effects on HPA axis function that can result in increased adult EtOH consumption in male rats (Butler, Ariwodola, et al., 2014). In addition, other stressors such as cold swim elicit enhanced adult alcohol intake when combined with adolescent EtOH exposure (Gamble & Diaz, 2020). Despite these findings, relatively little is known about the effects of social isolation combined with exposure to alcohol in adolescence on subsequent adult EtOH intake.
In one study using male Long-Evans rats, social isolation led to increased 20% EtOH intake during voluntary adolescent exposure; however, no effects of housing were found on subsequent operant EtOH self-administration (Wukitsch, Reinhardt, Kiefer, & Cain, 2019). Similarly, male Wistar rats housed in isolation with a period of intermittent exposure to 8% EtOH as the only source of liquid in the home cage drank more than social housed rats as adolescents, but the isolated housing condition had no effect on adult 8% EtOH intake in a free- access 2-bottle choice (Juarez & Vazquez-Cortes, 2003). The inability of adolescent alcohol exposure and isolated housing to increase adult alcohol drinking in these previous studies may be due to utilization of unsweetened EtOH in adolescence and adulthood, which led to low overall levels of consumption. A prior study from our laboratory also indicated that exposure to 20% EtOH in an intermittent 2-bottle choice in adolescence resulted in decreased adult 20% EtOH, but that sweetening the EtOH during adolescence circumvented this decrease (Chandler et al., 2021). Thus, in the present study, we tested the effects of adolescent exposure to a saccharin-sweetened 20% EtOH solution in an intermittent 2-bottle choice in rats raised in isolated or group housing on adult intake of an unsweetened 20% EtOH solution.
Materials and Methods
Animals
Adolescent male Sprague Dawley rats arrived on postnatal day (PND) 22 (n=24, Charles River, Wilmington, MA) and were assigned to isolated or group housing. Males were used because, in our previous experiments testing the effects of forced exposure to EtOH during adolescence on adult EtOH intake, significant effects were found in male rats only (Chandler et al., 2022; Chandler et al., 2021). Rats were kept in a temperature-controlled colony room under a 12:12 h light/dark cycle. Water was available ad libitum and food was available ad libitum except on test days when all rats were restricted to 20 g. All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011), and approved by the IACUC at the University of Kentucky.
Apparatus
Isolated rats were housed singly in small stainless-steel hanging cages (17 × 24 × 20 cm) with metal grid floors without bedding and solid metal walls except for the metal grid front panel that allowed access to food and water. Group rats were housed 6 per cage, in a large custom-built stainless-steel cage (122 × 61 × 45.5 cm) with bedding. For the 2-bottle choice procedures, all rats were moved to single housing in standard polypropylene cages (48 × 27 × 20 cm) equipped with locking wire tops, micro-isolator lids, and bedding. Two 250 mL bottles with a standard drinking spout (Allentown, Allentown, NJ), were fixed to the wire top, under the micro-isolator lids, so that the spouts could be accessed from inside the cage for 2-bottle choice tests.
Solutions
In the adolescent 2-bottle choice, a 0.2% saccharin/20% EtOH solution (Sacc/EtOH) was obtained by diluting 95% EtOH (190 proof, EtOH Pharmaco-AAPER, Shelbyville, KY) to a concentration of 20% (v/v) in distilled water, and adding saccharin sodium salt hydrate (SIGMA- Aldrich, St. Louis, MO) to the solution at a 0.2% (w/v) concentration. For the adult 2-bottle choice, the 95% EtOH (v/v) was diluted to a 20% (v/v) EtOH concentration in distilled water.
Procedures
Adolescent Intermittent 2-Bottle Choice
From PND 28–61, every other day, 3 days per week (MWF), adolescent rats were moved from their group or isolated housing condition to single housing in standard polypropylene cages with bedding for the 2-bottle choice procedure. For each housing condition (group or isolated), rats were further segregated into adolescent exposure groups, receiving either H2O vs. H2O or H2O vs. Sacc/EtOH on 2-bottle choice days, thus making up a 2 × 2 experimental design. Two- bottle choice sessions lasted 24 hr and rats were food restricted to 20 g during those 24 hr. Rats were returned to group or isolated housing at the end of each 2-bottle choice session (4 days per week), where food and water were available ad libitum. The adolescent 2-bottle choice consisted of 15 sessions that lasted for 5 weeks (Amodeo et al., 2017). Bottle placement was counterbalanced daily for side, and the bottles were weighed after each session to determine intakes. Additionally, two empty cages were set up with one H2O bottle and one Sacc/EtOH bottle and were placed on the shelf that housed the single cages during 2-bottle choice tests to determine the rate of bottle leaking. A correction factor was introduced to account for bottle leakage. The amount of fluid lost from each leak bottle was averaged over the course of each 2-bottle choice test (adolescent, adult, and relapse-like drinking) and for intakes was divided by the average weight of all rats over the course of each timepoint and represented as average lost in g/kg. All daily intakes were adjusted by subtracting this correction factor from each rat’s daily intake value (g/kg), while average volume lost (mL) from the respective H2O or Sacc/EtOH bottle was subtracted from intake volumes (mL) before preference was calculated.
Adult Intermittent 2-Bottle Choice
For two weeks (PND 62–76), rats were maintained in their group or isolated housing conditions. Beginning on PND 77, rats were again moved to single housing in standard polypropylene cages, as described above, for the adult 2-bottle choice. Rats in each treatment group (2 × 2; housing condition x adolescent exposure) underwent an intermittent 2-bottle choice between H2O and 20% EtOH (v/v) in the same 3 days per week pattern described above. During off days, the rats were returned to their original group or isolated housing assignments. Bottles were rotated for side and weighed daily to determine intakes. Bottle leakage was accounted for as described above; however, one leak bottle contained H2O and the other 20% EtOH.
Adult Relapse-Like Drinking
From PND 110–114 rats were abstinent from EtOH and were maintained in their group or isolated housing conditions. On PND 115, 116, and 117, as a model of relapse-like drinking following a period of abstinence, all rats were allowed 90 min access to a 2-bottle choice (H2O vs. 20% EtOH) in the single standard polypropylene cages, before being returned to group or isolated housing between sessions. Bottle leakage was accounted for as described above. Immediately after the final 90 min re-exposure session ended, rats were deeply anesthetized with isoflurane and decapitated for the collection of trunk blood for analysis of blood EtOH concentration (BEC) with an AM1 Alcohol Analyzer (Analox, London, UK).
Statistical Analysis
All statistical tests were run using Graphpad Prism (version 9.1.0; GraphPad Software, La Jolla, CA), the alpha level was set at p ≤ 0 .05, and all data are presented as mean ± SEM. Adolescent Sacc/EtOH intake and preference were analyzed with 2-way repeated measures ANOVAs, with housing as the between-subjects variable and session as the within-subjects variable. As a follow-up analysis, significant effects were analyzed with unpaired t-tests between group and isolated rats for intake and preference data collapsed across the first 5, middle 5, and last 5 sessions.
Adult intake and preference data were analyzed with a 3-way mixed effects analysis, with adolescent exposure and housing condition as between-subjects variables, and session as the within-subjects variable. Significant effects were followed with 2-way ANOVAs with intake and preference collapsed across the first 5, middle 5, and last 5 sessions. The 2-way ANOVAs were followed by Tukey’s multiple comparisons tests.
Results
Adolescent Sacc/EtOH Intake
The effects of housing condition (group vs. isolated) on adolescent alcohol intake were assessed in a 2-bottle choice between H2O and Sacc/EtOH across 15 intermittent exposure sessions (Fig. 1). For the adolescent 2-bottle choice, a 2-way repeated measures ANOVA revealed a significant session x housing interaction, F(14, 140) = 2.23, p = 0.01. For a follow-up analysis, the average intake of the Sacc/EtOH solution was collapsed across sessions 1–5, 6–10 and 11–15, and is shown in Figs 1b, 1c, and 1d, respectively. Average Sacc/EtOH intake was higher for adolescent isolated rats throughout the 2 bottle choice; however, isolated rats diverged significantly from group housed rats across the sessions, with isolated rats drinking more during the last 5 sessions, t(10) = 2.53, p = 0.03 (Fig. 1d).
Fig. 1.
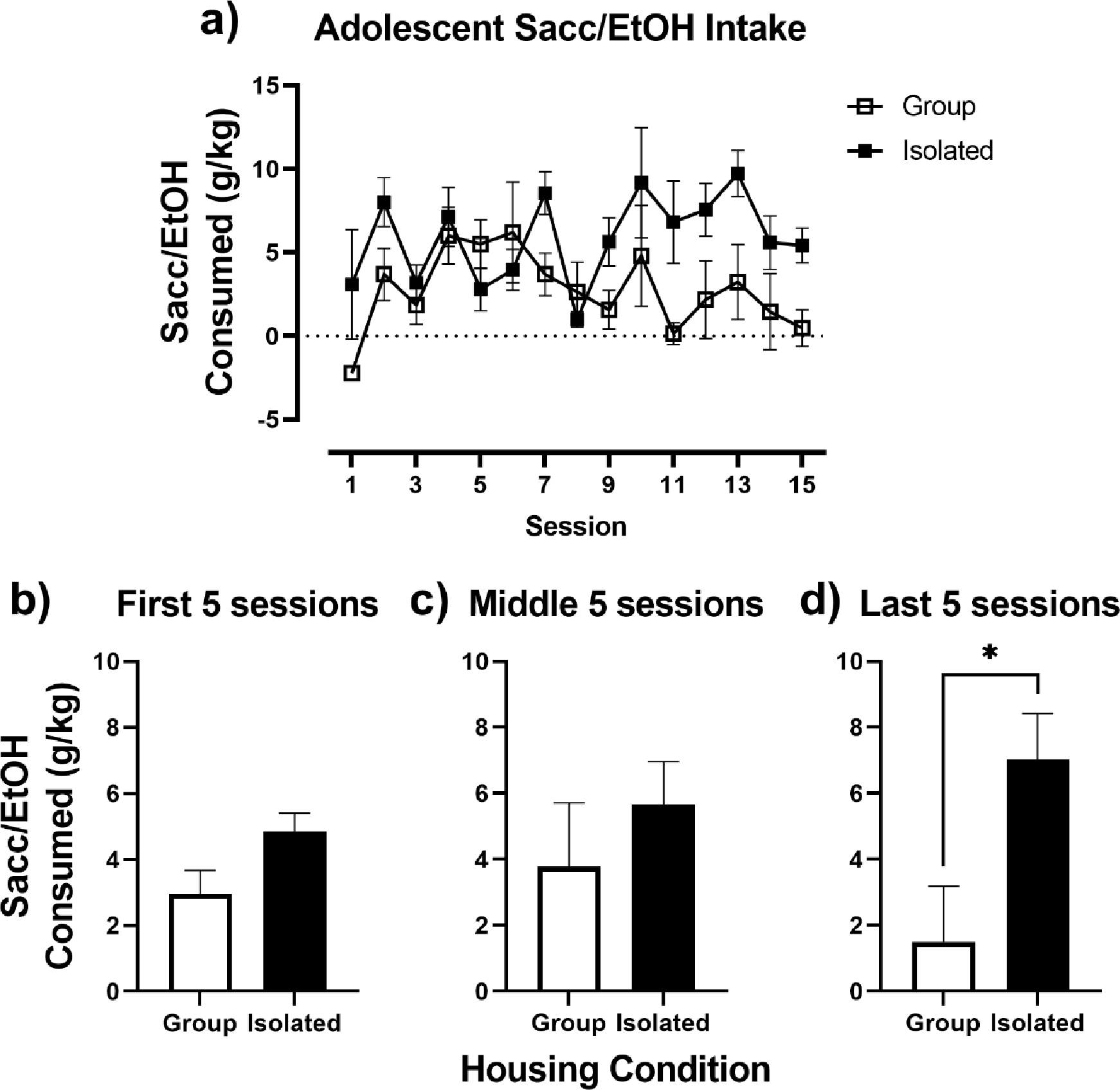
Adolescent Sacc/EtOH intake. A) Adolescent Sacc/EtOH intake (g/kg) in Group (open squares) and Isolated (closed squares) housed rats over the 15 intermittent 2-bottle choice sessions. (B-D) Adolescent Sacc/EtOH intake collapsed across the first five, middle five, and last five intermittent 2-bottle choice sessions. * p < 0.05 vs. Group.
Percent preference for the Sacc/EtOH solution was calculated to assess Sacc/EtOH intake in relation to H2O consumption in group vs. isolated adolescent rats and is shown in Fig. 2. For adolescent Sacc/EtOH preference, a 2-way repeated measures ANOVA indicated a significant session x housing interaction, F(14, 140) = 2.49, p = 0.003 (Fig. 2a). When Sacc/EtOH preference was collapsed across the first 5, middle 5, and last 5 sessions, isolated rats demonstrated increased preference for the Sacc/EtOH solution over time, whereas group housed rats did not. Compared to group housed rats, isolated rats displayed significantly greater Sacc/EtOH preference during the first 5 sessions, t(10) = 2.25, p = 0.048 (Fig. 2b), and the last 5 sessions, t(10) = 3.19, p = 0.01 (Fig. 2d).
Fig. 2.
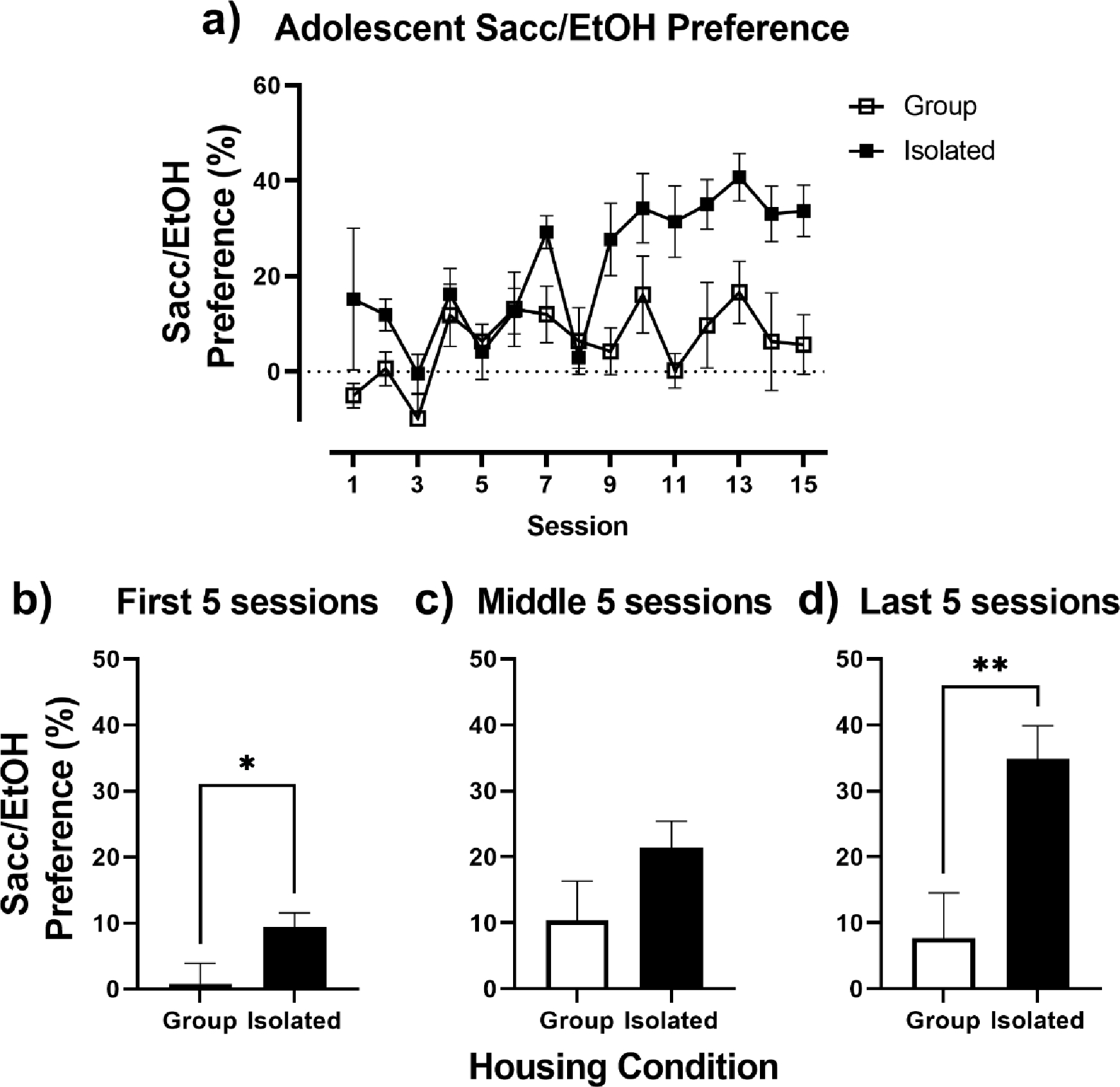
Adolescent Sacc/EtOH Preference. A) Adolescent Sacc/EtOH Preference (%) in relation to water, in Group (open squares) and Isolated (closed squares) housed rats over the 15 intermittent 2-bottle choice sessions. (B-D) Adolescent Sacc/EtOH Preference collapsed across the first five, middle five, and last five intermittent 2-bottle choice sessions. * p < 0.05 vs. Group.
Adult 20% EtOH Intake
The impact of adolescent exposure to H2O or Sacc/EtOH in group vs. isolated housed rats was assessed on adult alcohol intake in an intermittent 2-bottle choice between H20 and 20% EtOH (Fig. 3). For adult intake in the 2-bottle choice, a 3-way mixed effects analysis revealed a significant session x adolescent exposure x housing interaction, F(14, 277) = 2.57, p = 0.002 (Fig. 3a). To conduct follow-up 2-way ANOVAs, data were collapsed across the first 5 (Fig. 3b), middle 5 (Fig. 3c), and last 5 sessions (Fig. 3d). The 2-way ANOVAs indicated the initial significant 3-way interaction was driven by group differences across the first 5 sessions, as a significant housing x treatment group interaction was revealed, F(1, 20) = 7.79, p = 0.01. A post- hoc Tukey’s test indicated a significant difference in adult 20% EtOH intake for rats exposed to Sacc/EtOH in adolescence based on housing condition, with isolated, Sacc/EtOH exposed rats drinking significantly more 20% EtOH than group housed, Sacc/EtOH exposed rats (p=0.01). For the last 5 sessions, a 2-way ANOVA revealed a main effect of adolescent exposure, F(1,20) = 4.71, p = 0.04, that was largely driven by higher 20% EtOH intake in the isolated, Sacc/EtOH exposed rats; however, pairwise comparisons between Group H2O vs. Isolated Sacc/EtOH rats and Group Sacc/EtOH vs. Isolated Sacc/EtOH rats just failed to reach significance with the follow-up Tukey’s test (p = 0.056 and p = 0.069 respectively).
Fig. 3.
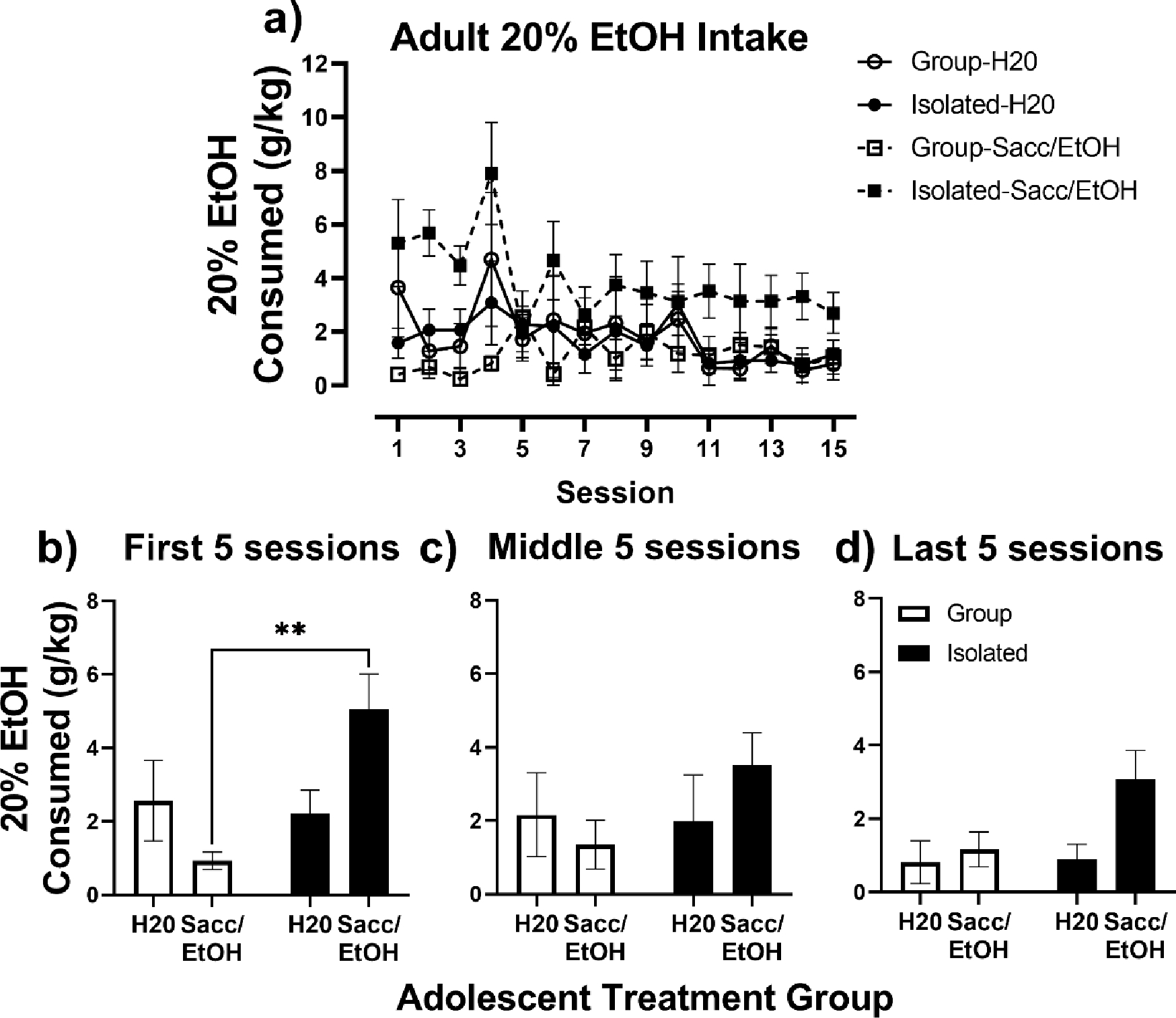
Adult 20% EtOH intake. A) Adult 20% EtOH intake (g/kg) in Group (open symbols) and Isolated (closed symbols) housed rats exposed to water (circles) or Sacc/EtOH (squares) as adolescents over the 15 intermittent 2-bottle choice sessions. (B-D) Adult 20% EtOH intake collapsed across the first five, middle five, and last five intermittent 2-bottle choice sessions. * p < 0.05 relative to Tukey’s test.
Preference for the 20% EtOH solution relative to H2O in the adult 2-bottle choice is shown in Fig. 4. When session was included as a factor, the 3-way mixed effects analysis revealed a significant session x adolescent exposure x housing interaction, F(14, 272) = 2.29, p = 0.01 (Fig. 4a). When adult 20% EtOH preference was collapsed across the first 5 sessions, a 2- way ANOVA indicated a significant adolescent exposure x housing interaction, F(1, 20) = 5.77, p = 0.03 (Fig 4b). A post-hoc Tukey’s test revealed a significant difference in adult 20% EtOH preference in group vs. isolated rats exposed to Sacc/EtOH in adolescence (p = 0.01).
Fig. 4.
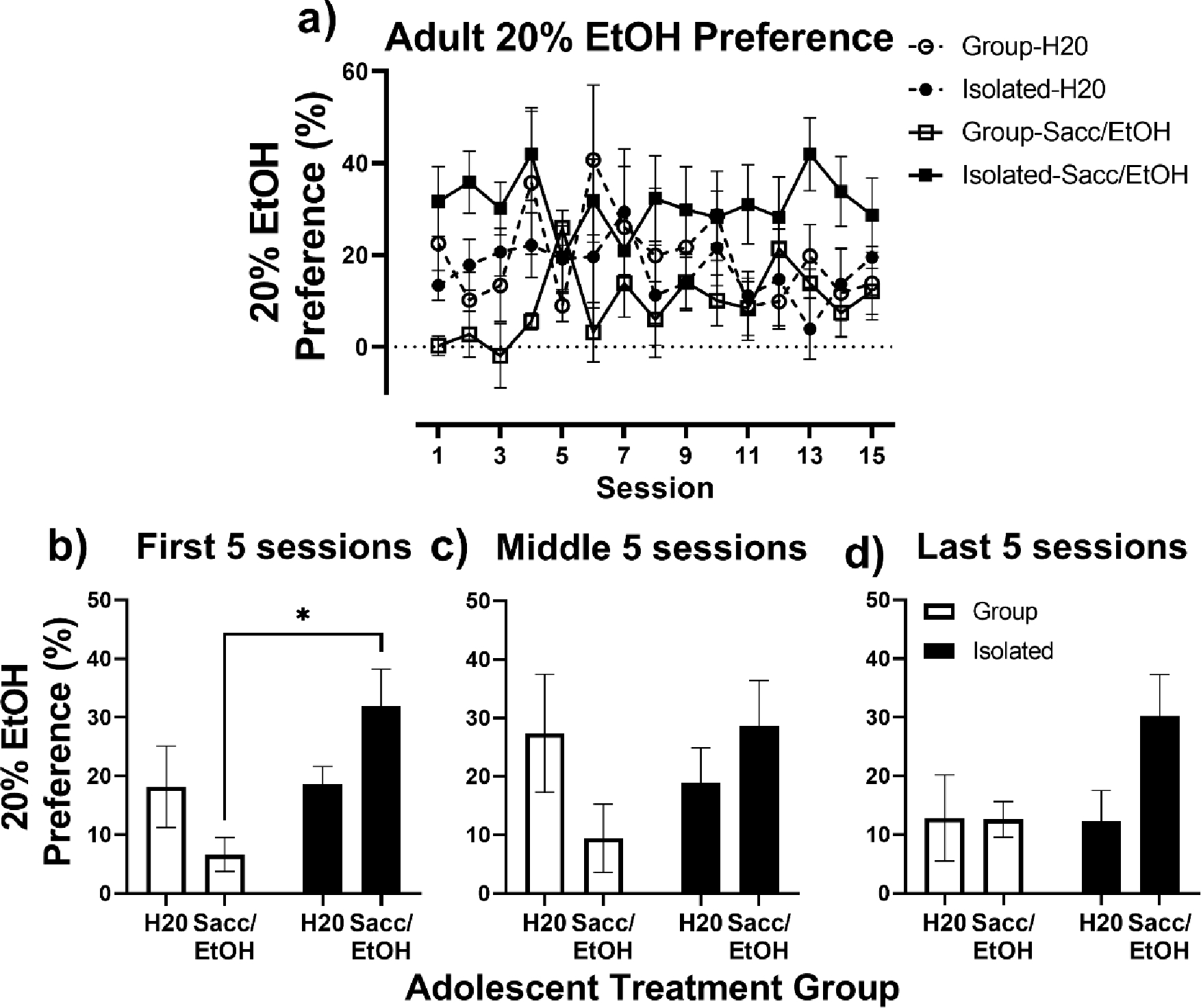
Adult 20% EtOH Preference. A) Adult 20% EtOH Preference (%) in relation to water, in Group (open symbols) and Isolated (closed symbols) housed rats exposed to water (circles) or Sacc/EtOH (squares) as adolescents over the 15 intermittent 2-bottle choice sessions. (B-D) Adult 20% EtOH Preference collapsed across the first five, middle five, and last five intermittent 2-bottle choice sessions. * p < 0.05 relative to Tukey’s test.
The effects of housing condition and the adolescent 2-bottle choice on relapse-like drinking following a 5-day abstinence period are shown in Fig. 5. No significant differences were found between groups for 20% EtOH intake across the three re-exposure sessions (Fig. 5a). No significant differences were found in relapse-like drinking when the data were collapsed across the three sessions (Fig 5b); however, there was a trend for Isolated Sacc/EtOH rats to drink more 20% EtOH than Group Housed Sacc/EtOH rats based on a near-significant treatment x housing interaction (p = 0.059). BECs obtained immediately after the final re-exposure session were minimal, likely below the limit of detection (Group H2O: 0.87 ± 0.28 mg/dL, Group Sacc/EtOH: 0.43 ± 0.12 mg/dL, Isolated H20: 0.76 ± 0.32 mg/dL, Isolated Sacc/EtOH: 1.82 ± 0.61 mg/dL). A 2-way ANOVA found no significant effects of housing or adolescent exposure on BECs; however, the adolescent exposure x housing interaction just failed to reach significance (p = 0.06; data not shown).
Fig. 5.
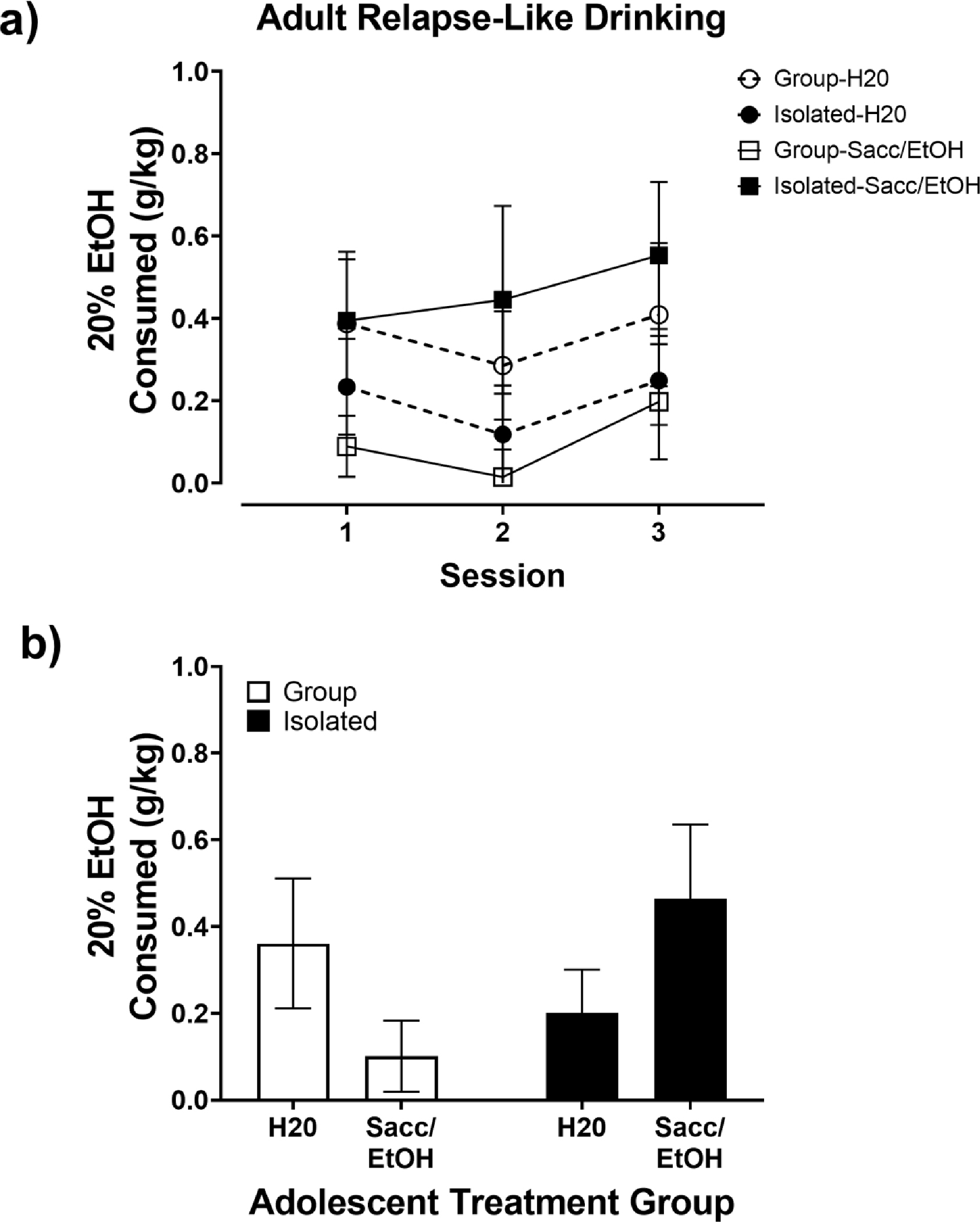
Adult Relapse-Like Drinking. A) Adult 20% EtOH intake (g/kg) over the 3, 90-minute re- exposure sessions in Group (open symbols) and Isolated (closed symbols) housed rats exposed to water (circles) or Sacc/EtOH (squares) as adolescents. (B) Average Adult 20% EtOH intake (g/kg) collapsed across the 3 re-exposure sessions.
Discussion
Rat studies examining the effects of adolescent alcohol exposure on adult alcohol intake often neglect the impact of critical procedural variables such as housing condition. In the present study we found isolated housing combined with adolescent Sacc/EtOH exposure resulted in higher levels of adult 20% EtOH intake and preference, while housing condition alone had little influence on adult intake. A few previous studies have investigated the effects of social isolation and EtOH exposure during adolescence in male rats; however, none of the studies found these variables to impact subsequent adult EtOH exposure, as was found here.
In one previous study, when male Long-Evans rats were isolated for the duration of the study in hanging wire isolation cages similar to those used here, adolescents consumed more 20% EtOH than pair-housed controls in an intermittent 2-bottle choice; however, housing condition had no impact on adult operant responding for 20% EtOH (Wukitsch et al., 2019). We also found that isolated rats consumed more Sacc/EtOH than group housed controls during the adolescent 2-bottle choice. However, isolated rats exposed to Sacc/EtOH in adolescence also consumed more 20% EtOH than group housed controls in adulthood. Aside from an innate strain difference between Long-Evans and Sprague Dawley rats, one explanation for the discrepancy between studies may be that we utilized Sacc/EtOH for the adolescent exposure and unsweetened 20% EtOH for the adult 2-bottle choice. In a prior study from our laboratory, single housed rats (standard cage with bedding) exposed to a 0.2% saccharin solution or a 20% EtOH solution in the adolescent intermittent 2-bottle choice decreased adult intake of 20% EtOH in the intermittent 2-bottle choice (Chandler et al., 2021). In that same study, we found rats given Sacc/EtOH in the adolescent 2-bottle choice consumed similar levels of 20% EtOH in adulthood as control rats. That finding is similar to another study where past juvenile exposure to sweetened 30% EtOH prevented a later aversion to unsweetened EtOH among rats housed in pairs (Truxell, Molina, & Spear, 2007). These results indicate that altering the taste of EtOH between adolescent and adult exposures may prevent taste aversions upon re-exposure, regardless of housing condition. Moreover, altering or not altering the taste of EtOH between adolescent and adult exposure may contribute to the different findings across studies.
Another variable that may contribute to the different outcomes across studies is whether isolated rats were compared to pair, group, or enriched controls. For instance, a study in male Sprague Dawley rats demonstrated that isolate housing reduced intake of a 0.1% saccharin/ 10% EtOH solution in both adolescent rats and EtOH naïve adult rats compared to pair-house controls (Doremus et al., 2005). However, prior to the assignment of housing condition, rats in that study were housed in same sex pairs in hanging wire cages, whereas rats housed in isolation immediately after weaning, and before EtOH exposure consumed more EtOH than rats isolated at the start of the 2-bottle choice. This latter finding suggests that the discrepancy between their study and ours could be attributed to differences in acclimation to isolation housing before the 2-bottle choice was introduced.
Another variable that may contribute to disagreements between studies is whether the rats were shipped from a commercial vendor or were bred in-colony (e.g., Doremus et al., 2005). In mice, the stress of shipping from a commercial vendor during the adolescent period has been shown to alter HPA axis function (Laroche, Gasbarro, Herman, & Blaustein, 2009). Thus, it is possible that the more extreme isolation housing conditioned used in the current study combined with early-life shipment stress may have influenced HPA axis activity resulting in an elevated adult EtOH drinking phenotype.
In a study similar to ours, adult intake of an EtOH/sucrose solution was no different among male Sprague Dawley rats placed in isolated, pair, or enriched housing conditions as adolescents; however, rats treated with methylphenidate and isolated during adolescence increased EtOH/sucrose intake as adults (Gill, Chappell, Beveridge, Porrino, & Weiner, 2014). This finding parallels the current result that adolescent isolation alone had no impact on adult 20% EtOH intake, but isolation combined with Sacc/EtOH exposure in adolescence elevated adult 20% EtOH intake. One procedural difference between our study and that by Gill et al. (2014) is they moved rats permanently to individual housing for adult EtOH exposure, while rats in the current study were moved between group/isolated housing to single housing for 2-bottle choice tests. The isolation housing condition used here represents a more extreme stress condition than standard single housing that is often described as isolation housing in rat studies (e.g., Doremus et al. 2005, Pisu et al. 2011). In a past report from our laboratory, male Sprague Dawley rats housed in hanging wire isolation cages during adolescence (PND 21–51) demonstrated elevated basal levels of corticosterone compared to rats housed in groups with and without environmental enrichment (Stairs, Prendergast, & Bardo, 2011). We also previously demonstrated that rats placed in isolated housing beginning in adolescence exhibit elevated operant responding for i.v. delivery of amphetamine, remifentanil, and fentanyl (Bardo et al., 2022; Hofford et al., 2017; Stairs et al., 2011). Together, these studies suggest Sprague Dawley rats isolated in hanging metal wire-bottom cages during adolescence and through the duration of the study display sensitized HPA axis responsivity to stress that results in elevated substance intake.
Converging evidence suggests that 2-hit models of adolescent stress, perhaps including adolescent EtOH exposure as a stressor, are needed to impact adult AUD relevant behaviors. For example, in male Wistar rats, free access to sweetened-EtOH in adolescence had no impact on baseline adult alcohol drinking, but after vapor exposure in adulthood, adolescent EtOH exposed rats drank more EtOH voluntarily and displayed anxiogenic behavior in the elevated plus maze (Gilpin et al., 2012). This result suggests the vapor exposure served as the second hit leading to increased EtOH intake. Similarly, a study in Sprague Dawley rats exposed to EtOH vapor in adolescence found male rats that underwent two days of forced swim stress displayed increased voluntary adult EtOH intake compared to rats exposed to air in adolescence (Gamble & Diaz, 2020). Together, these results suggest the isolation model combined with intermittent Sacc/EtOH exposure during adolescence may serve as a 2-hit model for chronic stress in adolescence that elevates alcohol drinking in adulthood.
One caveat to the present study is that no group had a 0.2% saccharin solution in the adolescent 2-bottle choice. Therefore, we cannot rule out the contribution of saccharin in the Sacc/EtOH solution offered during adolescence on subsequent adult 20% EtOH intake. However, adolescent saccharin exposure has been shown to decrease adult 20% EtOH in the 2-bottle choice in single housed Sprague Dawley rats (Chandler, Shaykin, Nixon, & Bardo, 2021), and saccharin exposure in isolated peri-adolescent alcohol preferring rats had no effect on adult alcohol drinking (Toalston et al., 2015). Thus, it is unlikely that the increased 20% EtOH intake in adulthood following adolescent Sacc/EtOH exposure was due to the saccharin additive.
The alcohol deprivation effect (ADE) is a phenomenon that occurs in several animal species and is thought to model the excessive alcohol drinking associated with relapse in human with cyclical AUD (Sinclair, 1971; Vengeliene, Bilbao, & Spanagel, 2014). The ADE is characterized by a transient increase in alcohol drinking over baseline levels following a period of abstinence before intake levels return to baseline (Vengeliene et al., 2014). The pattern of adult 20% EtOH intake in the isolated Sacc/EtOH exposed rats in the current study is characteristic of an ADE, in that intake was elevated over the course of the first 5 adult 2-bottle choice sessions, before stabilizing at a consistent yet lower level in subsequent sessions. However, one ADE criterion not met by the adult isolated Sacc/EtOH exposed rats is that they did not drink significantly more 20% EtOH on the first day of exposure than their last day of Sacc/EtOH exposure during the adolescent 2-bottle choice. Still, the adult rats consumed near adolescent levels of the unsweetened 20% EtOH, ~5.3 g/kg on day 1, while on the last day of the adolescent 2-bottle choice they consumed ~5.4 g/kg of the Sacc/EtOH solution. Adolescent and adult rats display differences in EtOH pharmacokinetics, and adolescent rats require higher EtOH doses than adults to reach equivalent BECs to adult rats (Morris, Kelso, Liput, Marshall, & Nixon, 2010). Thus, differences in adolescent vs. adult EtOH metabolism, as well as the impact of the switch to unsweetened EtOH in adulthood on palatability, should be considered as relevant factors for why the model failed to meet all criteria for an ADE.
In the current study, we also assessed relapse-like drinking in a 90-minute 2-bottle choice occurring across a 3-day period after a 5-day period of abstinence. Across the 3 re-exposure sessions, only the isolated adolescent Sacc/EtOH exposed rats increased their intake of 20% EtOH. However, likely due to the short access period, the effect of Sacc/EtOH exposure and isolation housing on relapse-like drinking was not significant. Thus, length of access to EtOH in adulthood is another critical variable when developing a preclinical model for adolescent AUD vulnerability.
A limitation of the present study is that social isolation and adolescent EtOH exposure were tested only in male Sprague Dawley rats, and these results may not generalize to female rats. Several studies, including ones from our laboratory, have demonstrated sex differences in the effects of housing and adolescent EtOH exposure on subsequent EtOH intake, and the outcomes found in males do not always translate to females (Butler, Carter, & Weiner, 2014; C. Chandler et al., 2021; Chandler et al., 2022; Gamble & Diaz, 2020). A second limitation is that blood EtOH concentrations (BECs) obtained immediately after the final relapse-like drinking session were low (ranging from 0.2–4.23 mg/dL), perhaps reflecting pre-peak BECs. Although isolated Sacc/EtOH rats displayed the highest average BECs overall, which corresponds with measured 20% EtOH intakes, the values were likely below the limit of detection for the Analox system. Despite these limitations, the current study indicates that adolescent exposure to sweetened EtOH, when combined with social isolation stress, may serve as a useful preclinical model of increased risk for AUD in adulthood.
Highlights.
Housing condition modulates adult alcohol drinking after adolescent alcohol exposure.
Social isolation increased voluntary alcohol intake in adolescent rats.
Social isolation or adolescent alcohol exposure individually had no adult impact.
Social isolation and adolescent alcohol exposure elevated adult alcohol drinking.
Funding
This work was supported by the National Institutes of Health [grant numbers R01 AA025591; T32 DA035200].
Footnotes
CRediT Author Statement
Cassie M. Chandler: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft, Visualization, Supervision. Jacob D. Shaykin: Methodology, Investigation, Writing – Review & Editing. Hui Peng: Investigation, Writing – Review & Editing. James R. Pauly: Supervision, Writing – Review & Editing. Kimberly Nixon: Conceptualization, Methodology, Writing- Review & Editing, Supervision, Funding acquisition. Michael T. Bardo: Conceptualization, Methodology, Writing- Review & Editing, Supervision, Funding acquisition.
References
- Acevedo MB, Nizhnikov ME, Molina JC, & Pautassi RM (2014). Relationship between ethanol- induced activity and anxiolysis in the open field, elevated plus maze, light-dark box, and ethanol intake in adolescent rats. Behav Brain Res, 265, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–531. [DOI] [PubMed] [Google Scholar]
- Amodeo LR, Kneiber D, Wills DN, & Ehlers CL (2017). Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol, 59, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Chandler CM, Denehy ED, Carper BA, Prendergast MA, & Nolen TL (2022). Effect of the glucocorticoid receptor antagonist PT150 on acquisition and escalation of fentanyl self-administration following early-life stress. Exp Clin Psychopharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Hammerslag LR, & Malone SG (2021). Effect of early life social adversity on drug abuse vulnerability: Focus on corticotropin-releasing factor and oxytocin. Neuropharmacology, 191, 108567–108567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, & Weiner JL (2014). The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front Integr Neurosci, 7, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Carter E, & Weiner JL (2014). Adolescent social isolation does not lead to persistent increases in anxiety- like behavior or ethanol intake in female long-evans rats. Alcohol Clin Exp Res, 38(8), 2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Karkhanis AN, Jones SR, & Weiner JL (2016). Adolescent Social Isolation as a Model of Heightened Vulnerability to Comorbid Alcoholism and Anxiety Disorders. Alcohol Clin Exp Res, 40(6), 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CM, Shaykin K, Nixon K, & Bardo MT (2021). The effects of adolescent intermittent alcohol exposure on voluntary adult alcohol intake and alcohol and nicotine co-use. The College on the Problems of Drug Dependence. [Google Scholar]
- Chandler CM, Hamid U, Maggio SE, Peng H, Pauly JR, Beckmann J, et al. (2022). Effects of adolescent alcohol exposure via oral gavage on adult alcohol drinking and co-use of alcohol and nicotine in Sprague Dawley rats. Drug Alcohol Depend, 232, 109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, & Boettiger CA (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav, 93(3), 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC 3rd, & Knapp DJ (2000). Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res, 24(11), 1712–1723. [PubMed] [Google Scholar]
- de Almeida Magalhaes T, Correia D, de Carvalho LM, Damasceno S, & Brunialti Godard AL (2018). Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain Behav, 8(1), e00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. (2000). Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry, 157(5), 737–744. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, & Clark DB (2005). Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res, 29(9), 1590–1600. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, & Brown S (2000). Adolescents are not adults: developmental considerations in alcohol users. Alcohol Clin Exp Res, 24(2), 232–237. [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, & Ogborne AC (2000). Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry, 157(5), 745–750. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, MacDonald K, & Offord DR (1999). Childhood stress and symptoms of drug dependence in adolescence and early adulthood: social phobia as a mediator. Am J Orthopsychiatry, 69(1), 61–72. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, & Spear LP (2005). Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res, 29(10), 1796–1808. [DOI] [PubMed] [Google Scholar]
- Fabio MC, Nizhnikov ME, Spear NE, & Pautassi RM (2014). Binge ethanol intoxication heightens subsequent ethanol intake in adolescent, but not adult, rats. Dev Psychobiol, 56(3), 574–583. [DOI] [PubMed] [Google Scholar]
- Gamble ME, & Diaz MR (2020). Moderate Adolescent Ethanol Vapor Exposure and Acute Stress in Adulthood: Sex-Dependent Effects on Social Behavior and Ethanol Intake in Sprague-Dawley Rats. Brain Sci, 10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology, 39(11), 2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Chappell AM, Beveridge TJ, Porrino LJ, & Weiner JL (2014). Chronic methylphenidate treatment during early life is associated with greater ethanol intake in socially isolated rats. Alcohol Clin Exp Res, 38(8), 2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, & Richardson HN (2012). Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One, 7(2), e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, & Dawson DA (1997). Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse, 9, 103–110. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Chow JJ, Beckmann JS, & Bardo MT (2017). Effects of environmental enrichment on self-administration of the short-acting opioid remifentanil in male rats. Psychopharmacology (Berl), 234(23–24), 3499–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez J, & Vazquez-Cortes C (2003). Alcohol intake in social housing and in isolation before puberty and its effects on voluntary alcohol consumption in adulthood. Dev Psychobiol, 43(3), 200–207. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, & Blaustein JD (2009). Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology, 150(5), 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Spoelder M, Rotte MD, Janssen MJ, Hesseling P, Lozeman-van’t Klooster JG, et al. (2015). Early social isolation augments alcohol consumption in rats. Behav Pharmacol, 26(7 Spec No), 673–680. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, & Becker HC (2011). Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol, 45(4), 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, & Li TK (2014). The alcohol-preferring (P) and high- alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol, 48(3), 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, & Chappell AM (2009). Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res, 33(2), 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, & Nixon K (2010). Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol, 44(1), 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, & Kelso ML (2010). Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol, 44(1), 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, & Zhang H (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis, 82, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, & Guerri C (2009). Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem, 108(4), 920–931. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, & Spear LP (2008). Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin Exp Res, 32(10), 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Reyna VF, & Satterthwaite TD (2017). Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev Cogn Neurosci, 27, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH (1986). Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res, 10(4), 436–442. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, & Denning C (1999). Chronic ethanol self-administration in a continuous- access operant situation: the use of a sucrose/ethanol solution to increase daily ethanol intake. Alcohol, 19(2), 151–155. [DOI] [PubMed] [Google Scholar]
- Sinclair JD (1971). The alcohol-deprivation effect in monkeys. Psychonomic Science, 25, 21–22. [Google Scholar]
- Slawecki CJ, & Betancourt M (2002). Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol, 26(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Spear LP (2015). Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav, 148, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci, 19(4), 197–214. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Prendergast MA, & Bardo MT (2011). Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology (Berl), 218(1), 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamates AL, Linden-Carmichael AN, & Lau-Barraco C (2016). Mixing alcohol with artificially sweetened beverages: Prevalence and correlates among college students. Addict Behav, 62, 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toalston JE, Deehan GA Jr., Hauser SR, Engleman EA, Bell RL, Murphy JM, et al. (2015). The reinforcing properties of ethanol are quantitatively enhanced in adulthood by peri- adolescent ethanol, but not saccharin, consumption in female alcohol-preferring (P) rats. Alcohol, 49(5), 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver GA, & Samson HH (1991). The influence of early postweaning ethanol exposure on oral self- administration behavior in the rat. Pharmacol Biochem Behav, 38(3), 575–580. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, & Spear NE (2007). Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res, 31(5), 755–765. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Vendruscolo LF, & Allen-Worthington K (2020). Rat Models of Alcohol Use Disorder. In Suckow MA, Hankenson FC, Wilson RP & Foley PL (Eds.), The Laboratory Rat (pp. 967–986): Academic Press. [Google Scholar]
- Vazquez-Leon P, Martinez-Mota L, Quevedo-Corona L, & Miranda-Paez A (2017). Isolation stress and chronic mild stress induced immobility in the defensive burying behavior and a transient increased ethanol intake in Wistar rats. Alcohol, 63, 43–51. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, & Spanagel R (2014). The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol, 48(3), 313–320. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, & Spear LP (2007). Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res, 31(7), 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wukitsch TJ, Reinhardt EK, Kiefer SW, & Cain ME (2019). Voluntary ethanol consumption during early social isolation and responding for ethanol in adulthood. Alcohol, 77, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


