Pneumonia is one of the most common infectious reasons for hospital admission, but current standard-of-care pneumonia diagnostics leave much to be desired. In a population-based survey of community-acquired pneumonia in hospitalized adults in the United States, only 38% had a pathogen identified despite exhaustive clinical testing with culture, multiplex PCR, and urinary antigens (1). This diagnostic gap leads to the overuse of broad-spectrum antibiotic agents, contributing to the ever-increasing global burden of antimicrobial resistance, which is outpacing novel antimicrobial agent development and cited by the World Health Organization as one of the top 10 global threats facing humanity (2). Furthermore, as the number of immunocompromised patients steadily increases (3), so does the risk of infection with unusual pathogens often missed by standard microbiologic testing, resulting in delayed or missed diagnoses in our most vulnerable patients (4). There is an urgent need for new respiratory diagnostics that are less biased and more sensitive and provide rapid results.
A sequencing-based approach can overcome many of the limitations of existing pneumonia diagnostics. Metagenomic sequencing permits unbiased assessment of all nucleic acid in biological samples, enabling the detection of potential pathogens, the wider microbiome, and the human host response in a single assay (5). Presently, Clinical Laboratory Improvement Amendments (CLIA) certified metagenomic tests are clinically available for plasma and cerebrospinal fluid (6, 7), but technical and bioinformatic complexity delays turnaround time, and high sensitivity makes it challenging to distinguish signal from noise (8). Respiratory metagenomics (RMg) presents an even greater challenge because the respiratory tract is a nonsterile environment with a well-described microbiome, further complicating analysis and clinical interpretation.
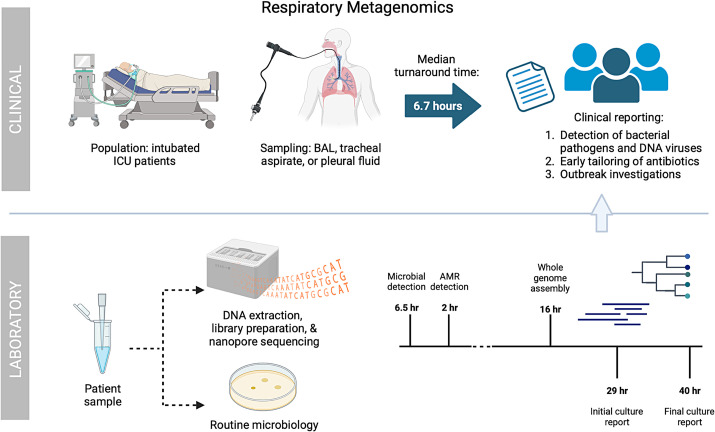
In this issue of the Journal, Charalampous and colleagues (pp. 164–174) conducted a prospective clinical pilot study (9) implementing a previously established RMg workflow (10) in the ICU of an academic hospital (Figure 1). Although others have explored metagenomics for the diagnosis of lower respiratory tract infections (11–15), this study is notably the first to truly implement RMg in a clinical care setting and assess its impact. Generation and analysis of high-complexity metagenomic data involves multiple steps, including nucleic acid extraction, library preparation, sequencing, removal of human reads, taxonomic alignment, and modeling to distinguish pathogens from the background microbiome (5). Here, the authors report a median turnaround time from sample acquisition to result of just 6.7 hours, which is impressive and much faster than traditional culture-based diagnostics. This pilot study had the capacity to perform RMg on three samples per day, so follow-up studies regarding scale-up and cost effectiveness will be important.
Figure 1.
Clinical implementation of respiratory metagenomics. Nanopore respiratory metagenomics were performed on BAL fluid, tracheal aspirates, and pleural fluid samples from intubated patients in the ICU with suspected respiratory infection. Routine clinical testing with culture was simultaneously performed. Sequencing reports were generated after 30 minutes and 2 hours for microbes and resistance mutations, respectively. Median turnaround time from sample acquisition to the reporting of results to clinicians was 6.7 hours. In comparison, preliminary culture results were reported after an average of 29 hours and were finalized in 40 hours. The impacts on clinical care, antibiotic agent prescribing, and infection control involvement were evaluated. AMR = antimicrobial resistance; BAL = bronchoalveolar lavage; ICU = intensive care unit.
Beyond the rapid turnaround time, this study demonstrated that RMg had a compelling impact on clinical management. When clinical testing results were negative, RMg detected a clinically relevant pathogen 19% of the time, often with organisms not initially suspected by clinicians, such as Legionella or Cytomegalovirus, and resulted in a management change. Conversely, RMg had a high level of agreement (93%) when standard culture results were positive. The high sensitivity gave clinicians confidence to discontinue antibiotic therapy when RMg returned negative results and, in some cases, even start early immunosuppression for suspected autoimmune inflammatory pulmonary conditions. RMg had the added benefit of early detection of resistance genes for many organisms, allowing clinicians to appropriately tailor antimicrobial agents more expediently. In total, the authors estimate that RMg contributed to prescribing decisions in a noteworthy 80% of cases.
Compared with standard culture, sequencing-based approaches pair nicely with infection control and public health efforts. With usual practice, in a suspected outbreak, cultured organisms from the microbiology laboratory are sent for whole-genome sequencing to assess relatedness with other isolates. However, with metagenomics, if sequencing depth is adequate and genome coverage is high enough, the sequencing data can be used for proactive and timely infection control interventions. The authors demonstrate this impact with a case of Legionella pneumophila that originated from a bedside water faucet and a confirmed patient-to-patient Klebsiella variicola transmission.
As with any study, there are some limitations. Because the RMg assay studied was exclusively DNA-based, the workflow is unable to detect RNA viruses, which are the most common cause of lower respiratory tract infection in adults and children (1, 16). This could potentially hamper antimicrobial stewardship efforts because a positive viral test result in the setting of negative bacterial testing results can provide the confidence to stop antimicrobial therapy. Ideally, the RMg workflow could be modified to include DNA and RNA sequencing to better capture the most common causes of respiratory infections and enable the detection of emerging viral pathogens that may not be detectable by standard PCR assays. This could also enable profiling of host gene expression, which could inform whether the detected microbes are matched to an immune response consistent with infection. Another consideration for future studies is the inclusion of a noninfectious control group to more rigorously define specificity, optimize the differentiation of pathogens from commensal organisms, and understand the impact on antibiotic agent use when incidental microbiota are detected.
All said, the authors have performed an impressive clinical pilot study of RMg and have successfully moved the needle toward future implementation of this technology in routine clinical practice, in which current methods often fall short. Although some challenges and limitations persist, this study opens the door for future research in assay optimization, launching of a randomized controlled trial, and cost-effectiveness analyses to propel RMg to prime time.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202311-2039ED on November 29, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med . 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. EClinicalMedicine. Antimicrobial resistance: a top ten global public health threat. EClinicalMedicine . 2021;41:101221. doi: 10.1016/j.eclinm.2021.101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA . 2016;316:2547–2548. doi: 10.1001/jama.2016.16477. [DOI] [PubMed] [Google Scholar]
- 4. Di Pasquale MF, Sotgiu G, Gramegna A, Radovanovic D, Terraneo S, Reyes LF, et al. GLIMP Investigators Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis . 2019;68:1482–1493. doi: 10.1093/cid/ciy723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet . 2019;20:341–355. doi: 10.1038/s41576-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol . 2019;4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 7. Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med . 2019;380:2327–2340. doi: 10.1056/NEJMoa1803396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dulanto Chiang A, Dekker JP. From the pipeline to the bedside: advances and challenges in clinical metagenomics. J Infect Dis . 2020;221:S331–S340. doi: 10.1093/infdis/jiz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charalampous T, Aloclea-Medina A, Snell LB, Alder C, Tan M, Williams TGS, et al. Routine metagenomics service for intensive care unit patients with respiratory infection. Am J Respir Crit Care Med . 2024;209:164–174. doi: 10.1164/rccm.202305-0901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charalampous T, Kay GL, Richardson H, Aydin A, Baldan R, Jeanes C, et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat Biotechnol . 2019;37:783–792. doi: 10.1038/s41587-019-0156-5. [DOI] [PubMed] [Google Scholar]
- 11. Langelier C, Kalantar KL, Moazed F, Wilson MR, Crawford ED, Deiss T, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci USA . 2018;115:E12353–E12362. doi: 10.1073/pnas.1809700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Yin Y, Gao H, Guo Y, Dong Z, Wang X, et al. Clinical utility of in-house metagenomic next-generation sequencing for the diagnosis of lower respiratory tract infections and analysis of the host immune response. Clin Infect Dis . 2020;71:S416–S426. doi: 10.1093/cid/ciaa1516. [DOI] [PubMed] [Google Scholar]
- 13. Zinter MS, Dvorak CC, Mayday MY, Iwanaga K, Ly NP, McGarry ME, et al. Pulmonary metagenomic sequencing suggests missed infections in immunocompromised children. Clin Infect Dis . 2019;68:1847–1855. doi: 10.1093/cid/ciy802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mick E, Tsitsiklis A, Kamm J, Kalantar KL, Caldera S, Lyden A, et al. Integrated host/microbe metagenomics enables accurate lower respiratory tract infection diagnosis in critically ill children. J Clin Invest . 2023;133:e165904. doi: 10.1172/JCI165904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlaberg R, Queen K, Simmon K, Tardif K, Stockmann C, Flygare S, et al. Viral pathogen detection by metagenomics and pan-viral group polymerase chain reaction in children with pneumonia lacking identifiable etiology. J Infect Dis . 2017;215:1407–1415. doi: 10.1093/infdis/jix148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med . 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]