Abstract
Background
Interstitial lung disease (ILD) is a significant cause of morbidity and mortality in patients with systemic sclerosis (SSc). To date, clinical practice guidelines regarding treatment for patients with SSc-ILD are primarily consensus based.
Methods
An international expert guideline committee composed of 24 individuals with expertise in rheumatology, SSc, pulmonology, ILD, or methodology, and with personal experience with SSc-ILD, discussed systematic reviews of the published evidence assessed using the Grading of Recommendations, Assessment, Development, and Evaluation approach. Predetermined conflict-of-interest management strategies were applied, and recommendations were made for or against specific treatment interventions exclusively by the nonconflicted panelists. The confidence in effect estimates, importance of outcomes studied, balance of desirable and undesirable consequences of treatment, cost, feasibility, acceptability of the intervention, and implications for health equity were all considered in making the recommendations. This was in accordance with the American Thoracic Society guideline development process, which is in compliance with the Institute of Medicine standards for trustworthy guidelines.
Results
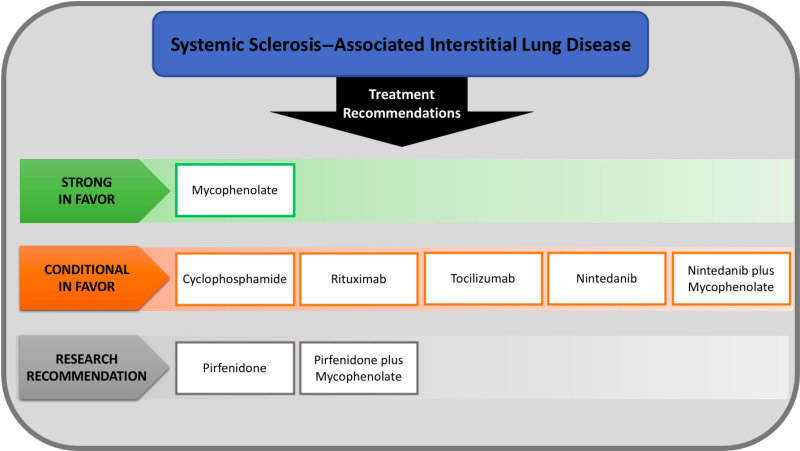
For treatment of patients with SSc-ILD, the committee: 1) recommends the use of mycophenolate; 2) recommends further research into the safety and efficacy of (a) pirfenidone and (b) the combination of pirfenidone plus mycophenolate; and 3) suggests the use of (a) cyclophosphamide, (b) rituximab, (c) tocilizumab, (d) nintedanib, and (e) the combination of nintedanib plus mycophenolate.
Conclusions
The recommendations herein provide an evidence-based clinical practice guideline for the treatment of patients with SSc-ILD and are intended to serve as the basis for informed and shared decision making by clinicians and patients.
Keywords: interstitial lung disease, systemic sclerosis, SSc-ILD
Contents
- Overview
- Use of the Guideline
- SSc-ILD Definition
- Therapies Assessed and Summary of Recommendations
Introduction
- Methods
- Committee Composition
- Confidentiality Agreement and Conflict-of-Interest Management
- Formulating Clinical Questions
- Literature Search
- Evidence Review and Development of Clinical Recommendations
- Manuscript Preparation
- Evidence-based Recommendations for the Treatment of SSc-ILD
- Question 1: Should patients with SSc-ILD be treated with cyclophosphamide?
- Question 2: Should patients with SSc-ILD be treated with mycophenolate?
- Question 3: Should patients with SSc-ILD be treated with rituximab?
- Question 4: Should patients with SSc-ILD be treated with tocilizumab?
- Question 5: Should patients with SSc-ILD be treated with nintedanib?
- Question 6: Should patients with SSc-ILD be treated with nintedanib plus mycophenolate?
- Question 7: Should patients with SSc-ILD be treated with pirfenidone?
- Question 8: Should patients with SSc-ILD be treated with pirfenidone plus mycophenolate?
Guideline Considerations and Future Directions
Conclusions
Overview
The purpose of this guideline is to provide clinicians with evidence-based treatment recommendations for patients with interstitial lung disease (ILD) associated with systemic sclerosis (SSc), or SSc-ILD, based on an analysis of the evidence available through October 2022. The recommendations are to be implemented within the context of individual patient values and preferences. They factor in confidence in effect estimates, importance of outcomes studied, balance of desirable and undesirable consequences of treatment, cost, feasibility, acceptability of the intervention, and implications for health equity. However, they should not be viewed as unequivocal recommendations and do not propose one treatment regimen over another or any particular sequence in therapy.
Use of the Guideline
This guideline provides the basis for rational decisions in the management of SSc-ILD and is not intended to impose a standard of care. Clinicians, patients, third-party payers, institutional review committees, the courts, and/or other stakeholders should not view the recommendations herein as dictates. No guidelines and recommendations can consider all the often compelling unique individual clinical circumstances. Therefore, no one charged with evaluating clinicians’ actions should attempt to apply the recommendations from these guidelines by rote or in a blanket fashion. Statements about the underlying values and preferences as well as qualifying remarks accompanying each recommendation are integral and serve to facilitate more accurate interpretation. They should never be omitted when quoting or translating recommendations from this guideline.
SSc-ILD Definition
We defined our patient population as having SSc-ILD if both SSc and ILD are present. SSc is defined using the former American Rheumatology Association 1980 criteria (1) or the 2013 American College of Rheumatology and European Alliance of Associations for Rheumatology (formerly the European League Against Rheumatism) classification criteria (2). ILD is defined as the radiologic presence of reticulation, traction bronchiectasis, traction bronchiolectasis, honeycomb cysts, ground-glass opacities or air space consolidation, other interstitial lung abnormalities, or any of the recognized patterns of interstitial pneumonias (usual interstitial pneumonia [UIP], probable UIP, indeterminate for UIP, nonspecific interstitial pneumonia, organizing pneumonia, lymphoid interstitial pneumonia, pleuroparenchymal fibroelastosis, or unclassifiable interstitial pneumonias) reported in the context of SSc.
After careful thought and consideration, the committee felt that it was clinically relevant to define subgroups of the disease state to help understand and appreciate the potential of differential treatment response to a particular treatment intervention. The committee members acknowledged that the evidence for subgroups may be limited but came to a consensus that it was an important point of consideration worth evaluation. When opinions and interpretation of the existence and the results of evidence differ among experts of the science, the matter needs to be resolved by consensus reached after discussion of all the points among the experts to try to come to a truth on matters, which is what was done in this guideline development process for the subgroups and their definitions. The guideline committee came to a consensus to evaluate three different subgroups: initial diagnosis of SSc-ILD, stable SSc-ILD, and progressive SSc-ILD. Those patients in whom the treatment intervention was initiated at the time of the initial diagnosis may respond differently from those who had a stable disease course and from those with progressive disease. Although the definitions of initial diagnosis and stable disease are relatively straightforward, the definition of progressive disease was less clear. Initial diagnosis refers to a new SSc-ILD diagnosis before treatment initiation. Patients with known SSc-ILD not meeting criteria for progressive SSc-ILD were deemed to have stable SSc-ILD.
To define progressive SSc-ILD, the committee came to a consensus to adapt the definition of progressive pulmonary fibrosis (PPF) as defined in the recently published 2022 American Thoracic Society (ATS) clinical practice guideline for a non–idiopathic pulmonary fibrosis (non-IPF) population (3). Although the criteria for PPF used in the 2022 guideline were extrapolated from the patient population of patients with IPF, the committee felt it appropriate to adapt the criteria to define progressive SSc-ILD after eliminating the timeline for disease progression. Therefore, progressive SSc-ILD was defined as manifesting at least two of the following three criteria during follow-up in patients with SSc-ILD: 1) worsening dyspnea or cough; 2) physiological evidence of disease progression (⩾5% absolute decline in FVC [forced vital capacity] or ⩾10% absolute decline in DlCO [diffusing capacity of the lung for carbon monoxide] adjusted for hemoglobin); or 3) radiological evidence of disease progression (radiological interpretation of increase in the extent or severity of ILD features on computed tomography [CT] assessed visually).
Therapies Assessed and Summary of Recommendations
Six individual therapies and two combination therapies were assessed in this guideline (Table 1). Each therapy in this guideline was compared with either placebo or standard of care. Standard of care for SSc was determined a priori by the committee to be cyclophosphamide or mycophenolate based on findings from the SLS I (Scleroderma Lung Study I) (4) and mycophenolate for all studies conducted after the completion of the SLS II (5). Following is the summary of recommendations for specific therapies, which were not assessed as a stepwise algorithm:
-
1.The recommendation for the use of the following agent for the treatment of SSc-ILD is strong:
-
a.Mycophenolate (•○○○, very low confidence in effect estimates)
-
a.
-
2.The recommendation for the use of the following agents for the treatment of SSc-ILD is conditional:
-
a.Cyclophosphamide (••○○, low confidence in effect estimates)
-
b.Rituximab (•○○○, very low confidence in effect estimates)
-
c.Tocilizumab (•○○○, very low confidence in effect estimates)
-
d.Nintedanib (•○○○, very low confidence in effect estimates)
-
e.Nintedanib plus mycophenolate (•○○○, very low confidence in effect estimates)
-
a.
-
3.The recommendation for further research because of insufficient evidence was made for the following agents for the treatment of SSc-ILD and is strong:
-
a.Pirfenidone (•○○○, very low confidence in effect estimates)
-
b.Pirfenidone plus mycophenolate (•○○○, very low confidence in effect estimates)
-
a.
Table 1.
PICO Questions for Systemic Sclerosis–associated Interstitial Lung Disease Therapies Assessed
| Question | Therapy |
|---|---|
| 1 | Should patients with SSc-ILD be treated with cyclophosphamide? |
| 2 | Should patients with SSc-ILD be treated with mycophenolate? |
| 3 | Should patients with SSc-ILD be treated with rituximab? |
| 4 | Should patients with SSc-ILD be treated with tocilizumab? |
| 5 | Should patients with SSc-ILD be treated with nintedanib? |
| 6 | Should patients with SSc-ILD be treated with nintedanib plus mycophenolate? |
| 7 | Should patients with SSc-ILD be treated with pirfenidone? |
| 8 | Should patients with SSc-ILD be treated with pirfenidone plus mycophenolate? |
Definition of abbreviation: PICO = population, intervention, comparison, outcome; SSc-ILD = systemic sclerosis–associated interstitial lung disease.
Introduction
Interstitial lung disease (ILD) is a significant cause of morbidity and mortality in patients with systemic sclerosis (SSc) (6). ILD has emerged as the leading cause of SSc-related death in patients with SSc, contributing to 35% of deaths (7). As such, optimal treatment recommendations for the care of patients with SSc-ILD is imperative. Several randomized controlled trials (RCTs) have been performed demonstrating the efficacy of specific treatments for SSc-ILD (8), and clinical practice guidelines regarding the treatment approach for patients with SSc-ILD are primarily consensus based (9, 10). There is practice variation in terms of 1) when ILD-directed treatment is initiated; 2) what the initial therapeutic agent of choice is; and 3) when add-on therapy is needed. To improve the quality of care delivered to patients with SSc-ILD, we provide the following evidence-based recommendations to assist clinicians in the treatment of patients with SSc-ILD.
Other well-known manifestations of SSc, including pulmonary hypertension, esophageal dysmotility, and gastroesophageal reflux disease, which may affect outcomes in patients with SSc-ILD, were not addressed in this guideline, which is focused on treatment of SSc-ILD. The standard of care for patients with SSc includes lifestyle modification measures to minimize risks for overt and/or occult microaspiration, with or without treatment with proton pump inhibitors, H2-blockers, and/or prokinetic agents. Because the question of whether to treat patients with SSc-ILD with targeted treatment for symptomatic or asymptomatic gastroesophageal reflux was not addressed in this guideline, we refer providers to existing guidelines for treatment of this clinical manifestation of SSc (11). Similarly, we refer providers to existing guidelines for treatment of pulmonary hypertension (12).
The guideline committee found it important to note that renal crisis in patients with SSc has been associated with systemic corticosteroid therapy in some cases, particularly in those with early diffuse cutaneous SSc (13). The likelihood of developing renal crisis is less if the daily dose of corticosteroids does not exceed the equivalent of 15 mg prednisone (14). Therefore, the committee issued the following best clinical practice statement:
❖ Caution must be taken in using systemic corticosteroids in patients with SSc with or without SSc-ILD. Whenever possible, the daily dose should not exceed the equivalent of 15 mg prednisone.
Methods
This guideline follows the ATS guideline development process and is in compliance with the National Academy of Medicine (formerly Institute of Medicine) standards for trustworthy guidelines (15). Recommendations were informed by systematic reviews of the published evidence assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach (16).
Committee Composition
This guideline was developed by an international multidisciplinary committee that consisted of pulmonologists with recognized ILD expertise (n = 7: G.R., S.B.M., A. Adegunsoye, A. Azuma, R.J.K., M.K., and M.B.S.), rheumatologists with recognized SSc expertise (n = 7: R.M.S., L.C., G.C.G., M.H., V.S., E.R.V., and F.M.W.), a general pulmonologist (C.C.T.), a pulmonologist/rheumatologist (K.B.H.) with recognized expertise in SSc and ILD, an information scientist (S.L.K.), and two patients with SSc-ILD (D.B. and K.A.K.) who were recommended for participation by the National Scleroderma Foundation and were not under the care of any of the clinical experts on the committee. The committee was chaired by G.R. and co-chaired by M.G., S.B.M., and R.M.S.
The clinical experts worked closely with five health research methodologists (M.G., T.H., M.M., D.H., and H.B.) with expertise in evidence synthesis and guideline development. The methodologists conducted systematic reviews and prepared the systematic evidence summaries following the GRADE approach (16).
Confidentiality Agreement and Conflict-of-Interest Management
Committee members disclosed all potential conflicts of interest according to the ATS policies. All potential conflicts of interest of committee members were reviewed by the staff of the ATS conflict-of-interest and documents units.
Six members were considered to have disqualifying conflicts of interest based on disclosures, whereas an additional four had manageable conflicts of interest. All conflicted members were permitted to participate in the discussions of the evidence with the rest of the committee; however, those with disqualifying conflicts were instructed to abstain from voting on all recommendations, and those with manageable conflicts were instructed to abstain from voting for the question(s) for which they were considered conflicted. This approach was applied to all questions. Adherence to these rules was strict, with voting and discussions monitored by one of the co-chairs (M.G.) together with ATS staff members participating in the meetings. The nonconflicted committee members and the methodologists were allowed unrestricted participation (n = 14).
Formulating Clinical Questions
The committee met virtually to discuss the scope and objectives of the project before the literature search. Questions on population definition, interventions, comparisons, and outcomes were formulated, with six therapeutic areas prioritized based on consensus voting by the committee, and an additional two questions were added based on the availability of time. Of note, the committee did consider including azathioprine and autologous hematopoietic stem cell transplantation. Although the committee would have liked to have been able to include these therapeutic modalities, given limits in the number of clinical questions that could feasibly be addressed for the guidelines, the consensus of the committee was to exclude them because of limited data and, for autologous hematopoietic stem cell transplantation in particular, the fact that it is in general practice a salvage therapy for patients not responding to more conventional therapy.
Literature Search
In collaboration with the lead methodologist (M.G.), an information scientist (S.L.K.) designed a search strategy using medical subject heading keywords and text words limited to human studies inclusive of nonindexed citations and articles in English or in any language with English abstracts. The Ovid platform was used to search MEDLINE, EMBASE, Cochrane Registry of Controlled Trials, Health Technology Assessment, and the Database of Abstracts of Reviews of Affects.
Methodologists reviewed references for additional articles and contacted experts outside the committee for additional data when appropriate. After removal of duplicates, two methodologists screened titles, abstracts, and full texts for inclusion of studies on the basis of predefined eligibility criteria.
Evidence Review and Development of Clinical Recommendations
The methodologists extracted data and created a summary of the evidence following the GRADE approach (16). Data were pooled, and meta-analysis was performed where appropriate. The evidence summaries were disseminated to committee members and presented in virtual meetings. Committee members provided feedback on the evidence summaries, with corrections made when appropriate and additional data incorporated if critical studies were not identified by the search. When there was a paucity of randomized trials available, post hoc analyses and observational studies were included.
The quality of evidence was determined using the GRADE approach, with focus on risk of bias, precision, consistency, directness of the evidence, risk for publication bias, presence of dose–effect relationship, magnitude of effect, and assessment of the effect of plausible residual confounding or bias. The confidence in the effect estimates based on the quality of evidence was categorized as high, moderate, low, or very low. In developing recommendations, the quality of evidence played a critical role, together with the balance of desirable and undesirable effects, values and preferences, feasibility of implementation, and implications for resource use and health equity. Incorporating the above, the committee came to final recommendations based on consensus voting. All votes required at least 80% participation by nonconflicted voting members and at least 70% agreement for or against a proposed therapy. Recommendations were either “strong” or “conditional” (or “weak”) in favor of or against each therapy. Strong recommendations begin with the phrase “we recommend,” whereas conditional recommendations begin with the phrase “we suggest.” The implications of the strong and conditional recommendations are listed in Table 2.
Table 2.
Implications of the Guideline Recommendations for Patients with Systemic Sclerosis–associated Interstitial Lung Disease
| Stakeholder | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but some would not. |
| Clinicians | Most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with their values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making will require substantial debate and involvement of various stakeholders. |
Manuscript Preparation
The writing committee (Co-Chair M.G. and the methodologists T.H., M.M., D.H., and H.B.; the Chair G.R. and Co-Chairs S.B.M. and R.M.S.) drafted the guideline document. The manuscript was then reviewed by the entire committee. Feedback was provided via electronic communication. The entire committee (both conflicted and nonconflicted members) had the opportunity to correct factual errors, clarify the presentation of background information or evidence summaries, and suggest changes to the rationale sections to capture the discussion from the virtual meetings. However, only the nonconflicted voting members were permitted to comment on the recommendations. Conflicted committee members were not permitted to comment on the recommendations pertinent to the specific conflicted question and restricted their feedback to the presentation of the evidence and the identification of errors.
The wording of recommendations (including strength and direction) was not altered once finalized by the nonconflicted members during the virtual meetings. One of the nonconflicted co-chairs (M.G.) confirmed that the written version of the guideline reflected the recommendations made by the nonconflicted members. The same process was followed for each version of the document. The final approved version was submitted to ATS for peer review.
Evidence-based Recommendations for the Treatment of SSc-ILD
No studies were identified that addressed the committee’s predefined subgroups of interest for disease status (initial manifestation of SSc-ILD, stable SSc-ILD, and/or progressive SSc-ILD), and thus the treatment recommendations made in this guideline document are for a heterogenous population of patients with SSc-ILD regardless of their disease status. The systematic reviews informing the committee’s recommendations are being published separately (17–21).
Question 1: Should patients with SSc-ILD be treated with cyclophosphamide?
Background
Cyclophosphamide is a cytotoxic agent that has demonstrated efficacy in certain types of inflammation-driven ILD, including SSc-ILD (22). However, it is associated with serious side effects, including bone marrow suppression, infections, hemorrhagic cystitis, and subsequent risk for bladder cancer (23). The committee addressed the question “Should patients with SSc-ILD be treated with cyclophosphamide?” The guideline committee identified potential heterogeneity in the intervention, as cyclophosphamide may be administered intravenously or orally, with potential differences in efficacy and adverse events between routes of administration. Therefore, this led to dividing the overarching research question into: 1) “Should patients with SSc-ILD be treated with intravenous cyclophosphamide?” and 2) “Should patients with SSc-ILD be treated with oral cyclophosphamide?” Critical outcomes included disease progression (including FVC, DlCO, and the modified Rodnan Skin Score [mRSS], with the latter as an indirect measure for progression of disease used primarily for SSc) and mortality. Important outcomes included quality of life (including the Health Assessment Questionnaire– Disability Index [HAQ-DI]) and adverse events.
Summary of evidence
A systematic review of the evidence identified five studies (see Table E1 in the online supplement). Two RCTs compared cyclophosphamide to placebo. SLS I (Scleroderma Lung Study I) was a 24-month, multicenter U.S.-based, NIH-funded RCT that randomized patients to 12 months of cyclophosphamide or 12 months of placebo followed by 12 months off therapy. SLS I included participants with SSc and with active alveolitis on BAL or ground-glass opacity on high-resolution CT (HRCT) of the chest and at least moderate dyspnea (4). Hoyles and colleagues reported on a multicenter U.K.-based, charitable donation–funded RCT including participants with SSc and evidence of pulmonary fibrosis on HRCT or lung biopsy that compared placebo to a regimen of intravenous cyclophosphamide monthly for 6 months plus prednisolone 20 mg every other day followed by azathioprine (24). One RCT and two case–control studies compared the use of cyclophosphamide to mycophenolate. SLS II was a multicenter, U.S.-based, NIH-funded, double-blind RCT that compared oral cyclophosphamide for 12 months followed by placebo for 12 months to oral mycophenolate for 24 months (5). Shenoy and colleagues (25) and Panopoulos and colleagues (26) were both retrospective, single-center, unfunded case–control studies that identified patients with SSc-ILD who had been treated with intravenous or oral cyclophosphamide and compared outcomes with patients who were treated with oral mycophenolate for 12 or 24 months. There was not enough evidence to be able to separate cyclophosphamide therapy by route of administration.
Disease progression
When compared with placebo, the mean change in FVC % predicted at 12 months was 2.8%, favoring cyclophosphamide. Treatment with cyclophosphamide was associated with an improvement in FVC % predicted at 12 months in a greater proportion of participants compared with placebo (49.3% vs. 26.4%, respectively). At 24 months, the mean values of FVC % predicted were similar between treatment and placebo groups. There was no difference in DlCO % predicted between groups at 12 or 24 months. When cyclophosphamide was compared with mycophenolate, there was a difference in DlCO % predicted favoring mycophenolate at 6 months and 18 months, but not at 12 months or 24 months. When cyclophosphamide was compared with mycophenolate, both groups showed an improvement in FVC % predicted, but there was no difference between the two groups at any time point. The change from baseline at 12 months for the mRSS was 3.06 better in the subset of patients with diffuse SSc.
Mortality
When comparing placebo and cyclophosphamide, there was no difference in mortality at 12 or 24 months. When comparing cyclophosphamide to mycophenolate, there was no difference in mortality between groups at 24 months.
Quality of life
When compared with placebo, there was a significant improvement in the cyclophosphamide arm for breathlessness and disability according to the HAQ-DI. When comparing cyclophosphamide to mycophenolate, although both arms showed significant improvement in quality of life (QoL) outcomes such as breathlessness, cough, and disability, there was no difference between groups.
Adverse events
When compared with placebo, there was a 15-fold increased risk of hematologic adverse events using cyclophosphamide at 12 months, including leukopenia (requiring discontinuation in seven cyclophosphamide cases) and thrombocytopenia. There was also a fourfold increased risk of infections using cyclophosphamide at 12 months. At 24 months, there was an increased risk of constitutional symptoms using cyclophosphamide. There was no increased incidence of hematuria or hemorrhagic cystitis using cyclophosphamide compared with placebo (27). When compared with mycophenolate, participants were 1.7 times more likely to prematurely discontinue cyclophosphamide therapy. There was a sixfold increased risk of leukopenia using cyclophosphamide compared with mycophenolate, but there was no difference in any other reported adverse events.
Quality of evidence
The quality of evidence was rated as low for these outcomes, which means there was low confidence in the estimated effects. Therefore, the data should be interpreted with caution. Quality of evidence was reduced in cyclophosphamide compared with placebo because few trials studied this comparison, leading to imprecision, and the intervention in Hoyles and colleagues included azathioprine and prednisolone as well as cyclophosphamide (24). The quality of evidence was low for the cyclophosphamide versus mycophenolate comparison because of imprecision, study design (retrospective case–control studies), and indirectness of the comparator (multiple formulations of mycophenolate).
Recommendation 1: We suggest using cyclophosphamide to treat patients with SSc-ILD (conditional recommendation, low-quality evidence)
The committee vote was as follows: strongly in favor to use cyclophosphamide in people with SSc-ILD: 5 of 17 (29%); conditional recommendation to use cyclophosphamide in people with SSc-ILD: 12 of 17 (71%); conditional recommendation to not consider cyclophosphamide: 0 of 17 (0%); strong recommendation to not consider cyclophosphamide: 0 of 17 (0%). No guideline participants (0%) abstained from this vote.
Justification and implementation considerations
The committee discussed that although QoL measures were considered a priori to be important, rather than critical outcomes, the significant improvement in QoL measures as shown by the data would be important to clinicians and patients when considering this treatment. In addition, the committee discussed the clinical importance of the increased risk of adverse events. For example, although there was a 38-fold increased risk of leukopenia using cyclophosphamide compared with placebo, the confidence intervals were wide, reducing our confidence in the estimate of the true effect, and, ultimately, cyclophosphamide-related leukopenia resulted in an actual change in treatment for only seven patients. It is also worth noting that trial design impacts outcomes, as evidenced by the fact that the response to cyclophosphamide in SLS II was better than in SLS I. In addition, in a post hoc analysis of the SLS I and SLS II population, patients with radiologic progression of ILD, assessed by quantitative ILD (QILD) scores, had an increased risk for long-term mortality (28).
Future research opportunities
The committee noted that in clinical practice mycophenolate is used more frequently than cyclophosphamide because of a more favorable side effect profile with mycophenolate. The committee acknowledges that this applies to countries where both medications are accessible and may not be the case in countries where mycophenolate access is limited. In addition, it was noted that most beneficial effects of cyclophosphamide treatment waned 1 year after cessation of cyclophosphamide treatment (29). Further research is needed to determine treatment duration for disease-modifying therapies for SSc-ILD. Future research should also assess the use of cyclophosphamide in patients with an initial diagnosis of SSc-ILD, in stable SSc-ILD, and in progressive SSc-ILD, as well as by route of administration (oral vs. intravenous).
Question 2: Should patients with SSc-ILD be treated with mycophenolate?
Background
Given that mycophenolate is an inhibitor of inosine monophosphate dehydrogenase that impairs T and B cell proliferation (30) and is currently used as a standard-of-care treatment for SSc, the committee asked the question, “Should patients with SSc-ILD be treated with mycophenolate?” The committee was interested in the evidence base for the different formulations of mycophenolate, specifically mycophenolate mofetil and mycophenolic acid. Cyclophosphamide, which was the previous standard of care since the publication of SLS I (4), and placebo were both deemed appropriate comparators. Critical outcomes included mortality and disease progression (defined as changes in FVC, DlCO, radiologic disease, and the mRSS, with the latter an indirect measure for progression of disease used primarily for SSc). Important outcomes included quality of life indices (including the Transition Dyspnea Index [TDI]) and adverse events.
Summary of evidence
A systematic review of the evidence identified seven total studies (5, 25, 26, 31–34) (Table E2). Two were RCTs (5, 32): three were post hoc analyses of RCTs (31, 33, 34), and two were observational studies (25, 26). Two studies compared mycophenolate to placebo (32, 34), and five compared mycophenolate to cyclophosphamide (5, 25, 26, 31, 33). The predominance of data comparing mycophenolate to placebo was from a post hoc study that compared those who received mycophenolate in SLS II with those patients who received placebo in SLS I (34). The SLS II trial provided the majority of evidence for the comparison between mycophenolate and cyclophosphamide (5). All studies, except for one, used mycophenolate mofetil for the drug formulation.
Disease progression
When compared with placebo, the mean FVC % predicted significantly improved from baseline to 12 and 24 months for mycophenolate, with about a 5% difference between the two arms. In addition, the rate of overall improvement in FVC % predicted at 12 and 24 months was nearly 2.3-fold higher at both time points in the mycophenolate arm compared with placebo. Similarly, the mean change from baseline in DlCO % predicted was >4% less at both 12 and 24 months for the mycophenolate arm compared with placebo, favoring mycophenolate. There were no differences between mycophenolate and cyclophosphamide in mean change in FVC % predicted or DlCO % predicted at 12 or 24 months. There were also no differences between mycophenolate and cyclophosphamide in several measures of radiologic disease, given both treatments led to improvements in radiologic disease individually. In addition, between mycophenolate and placebo, changes in the mRSS favored mycophenolate.
Mortality
There was no significant difference in mortality at 24 months between mycophenolate and placebo or between mycophenolate and cyclophosphamide.
QoL
Significant differences in breathlessness (measured using the TDI score) at all time points, including 24 months, favored mycophenolate over placebo. There was no difference in any QoL measure between mycophenolate and cyclophosphamide, although both showed significant improvement separately.
Adverse events
There was a ninefold increased risk of anemia in patients treated with mycophenolate versus placebo, but there were no differences in premature discontinuation, serious adverse events, hematuria, leukopenia, neutropenia, or thrombocytopenia. Compared with patients receiving cyclophosphamide, the mycophenolate arm had a 41% lower risk of premature discontinuation of therapy for any reason and 86% lower risk of leukopenia.
Quality of evidence
The quality of evidence for all outcomes was rated very low, meaning the effect estimates should be interpreted with caution. The primary reasons were due to the majority of outcomes drawing data from indirect evidence. The main study comparing mycophenolate to placebo, for example, was post hoc in nature, with significant differences in baseline characteristics between the groups.
Recommendation 2: We recommend using mycophenolate to treat patients with SSc-ILD (strong recommendation, very low-quality evidence)
The committee vote was as follows: strongly in favor to use mycophenolate in people with SSc-ILD: 14 of 18 (78%); conditional recommendation to use mycophenolate in people with SSc-ILD: 4 of 18 (22%); conditional recommendation to not consider mycophenolate: 0 of 18 (0%); strong recommendation to not consider mycophenolate: 0 of 18 (0%). No guideline participants (0%) abstained from this vote.
Justification and implementation considerations
Although the quality of the evidence was poor by GRADE criteria, the committee decided to make a strong recommendation for mycophenolate in patients with SSc-ILD, given the significant reduction in disease progression and improvement in QoL measures with minimal adverse events. According to GRADE guidelines, strong recommendations can rarely be made despite low confidence in effect estimates when there is a possibility of appreciable gain with a low incidence of adverse effects (35).
Future research opportunities
Future research should assess the use of mycophenolate in patients with an initial diagnosis of SSc-ILD, in stable SSc-ILD, and in progressive SSc-ILD. In addition, specific focus should be placed on comparing mycophenolate mofetil to mycophenolic acid.
Question 3: Should patients with SSc-ILD be treated with rituximab?
Background
Rituximab is a monoclonal antibody that binds to cell surface proteins found on B cells to eliminate them. B cells are thought to play a key part in the pathogenesis of SSc (36). Therefore, the guideline committee addressed the question, “Should patients with SSc-ILD be treated with rituximab?” Critical outcomes included disease progression (including changes in FVC, DlCO, radiographic disease, and mRSS) and mortality. Important outcomes included QoL measures (including the Short Form 36 questionnaire and HAQ-DI) and adverse events.
Summary of evidence
A systematic review of the evidence identified three RCTs that enrolled patients with SSc and evaluated the effects of rituximab compared with placebo (37–39) (Table E3). However, two of the studies enrolled participants with SSc without a priori confirmation of ILD, thus providing only indirect data on the SSc-ILD population (37, 39). The sample sizes were small, ranging from a total of 14 to 54 patients, and two of the studies were underpowered for the studied outcomes (37, 38). Follow-up for these trials ranged from 24 to 96 weeks. Patients received rituximab infusion on Days 1 and 15 and at 6 months in one study (37), weekly for four doses at baseline and at 6 months in a second study (38), and only weekly for four doses at baseline in a third (39).
Disease progression
Meta-analysis revealed that at 24–48 weeks, rituximab attenuated the decline in FVC % predicted by 3.3% when compared with placebo. Individual study and pooled data analyses showed no differences in the mean change in the DlCO % predicted at 24, 24–48, or 96 weeks between the rituximab and placebo arms. Two studies found that rituximab reduced the decline in DlCO (improvement in DlCO, 0.7 to 9.7 ml/min/mm Hg), whereas one found rituximab increased the decline in DlCO (−3.5 ml/min/mm Hg). There were no significant differences in mean changes in several measures of radiographic disease at 24 or 48 weeks, but the estimates are based on small sample sizes. Patients with SSc-ILD who received rituximab had larger decline in the mRSS at 24–48 weeks by 7 points.
Mortality
There were no significant differences at 24 weeks between the rituximab and placebo arms for mortality.
QoL
Individual study and pooled data analyses showed no differences between the rituximab and placebo arms for the Short Form 36 bodily pain and general health question subsets or the HAQ-DI scores.
Adverse events
No significant differences in adverse events were noted between the rituximab and placebo arms at 24 weeks (diarrhea, enterocolitis, gastroesophageal reflux disease, mucositis, respiratory tract infection, arthralgia, decreased neutrophil count, dermatitis, increased C-reactive protein, skin ulcerations and pulmonary valve disease) or 96 weeks (blood and lymphatic disorders, infections and infestations, neoplasm, reproductive and breast, or vascular disorders). Similarly, no differences at 24 weeks were present for any adverse event, serious adverse event, or serious adverse event leading to treatment withdrawal.
Quality of evidence
The quality of evidence for study outcomes was very low as defined by the GRADE approach, because of risk of bias (premature closing in enrollment), imprecision (limited number of participants/studies contributing to the findings, different rituximab dosing between studies), and indirectness (ILD not determined a priori in the participants).
Recommendation 3: We suggest using rituximab to treat patients with SSc-ILD (conditional recommendation, very low-quality evidence)
The committee vote was as follows: strongly in favor to use rituximab in people with SSc-ILD: 1 of 18 (5.6%); conditional recommendation to use rituximab in people with SSc-ILD: 16 of 18 (88.9%); conditional recommendation to not consider rituximab: 0 of 18 (0%); strong recommendation to not consider rituximab: 0 of 18 (0%). One guideline participant (5.6%) abstained from this vote because of insufficient expertise to render a thoughtful judgment.
Justification and implementation considerations
The committee decided to make a conditional recommendation for rituximab in patients with SSc-ILD, balancing the significant reduction in disease progression with no difference in adverse events against the very low-quality evidence.
Future research opportunities
The committee noted from clinical experience that the use of rituximab for SSc-ILD is often as rescue therapy in individuals with evidence of SSc-ILD progression despite treatment with mycophenolate. However, the systematic review did not address the use of rituximab in this context. Further research is needed to determine the most optimal timing for the use of rituximab in the disease course of SSc-ILD (in patients with an initial diagnosis of SSc-ILD, in stable SSc-ILD, and in progressive SSc-ILD).
Question 4: Should patients with SSc-ILD be treated with tocilizumab?
Background
Elevated concentrations of IL-6 (interleukin-6) have been associated with skin fibrosis and development of SSc-ILD in patients with SSc (40). Tocilizumab is a monoclonal antibody that targets the IL-6 receptor. Therefore, the committee addressed the question “Should patients with SSc-ILD be treated with tocilizumab?” Critical outcomes included disease progression (including changes in FVC and DlCO, fibrotic changes on HRCT imaging, and, as an indirect measure, mRSS) and mortality. Important outcomes included QoL measures (including the 5-D Itch score, HAQ-DI, Functional Assessment of Chronic Illness Therapy [FACIT]-Fatigue, and the Patient Global Visual Analog Scale score) and adverse events.
Summary of evidence
A systematic review of the evidence identified five studies for inclusion: the faSScinate trial (41) and its open-label extension (42), the focuSSced trial (40) and its open-label extension (43), and a post hoc analysis of data from the focuSSced trial (44) (Table E4). The faSScinate trial was a phase 2 RCT that assigned 87 subjects with SSc across five countries to subcutaneous tocilizumab or placebo over 48 weeks. The open-label extension was extended to 96 weeks and gave tocilizumab to 30 subjects in the original tocilizumab arm and 31 subjects in the original placebo arm. The focuSSced trial was a phase 3 RCT that assigned 210 subjects with SSc across 20 countries to subcutaneous tocilizumab or placebo over 48 weeks. The open-label extension extended to 96 weeks and gave tocilizumab to 60 subjects in the original tocilizumab arm and 54 subjects in the original placebo arm. The post hoc analysis assessed QILD and quantitative lung fibrosis (QLF) scores on imaging with QILD categorized as mild (>5–10%), moderate (>10–20%), or severe (>20%). For both the faSScinate and focuSSced trials, the presence of ILD was not an inclusion criterion, and change in mRSS was the primary outcome. But in the focuSSced trial, 136 of the 210 participants (65%) were deemed to have SSc-ILD based on a visual read of HRCT by a thoracic radiologist.
Disease progression
The differences in mean absolute change from baseline in FVC between the tocilizumab and placebo arms were 118 ml less at 24 weeks, 241 ml less at 48 weeks, and 128.7 ml less at 96 weeks (the latter being the open-label period) in favor of tocilizumab. Similarly, the difference in mean change from baseline to 48 weeks in FVC % predicted was 6.5% less in the tocilizumab arm, with a median change of 3.4% less, but at 96 weeks (when the placebo arm was also given tocilizumab) there was no significant difference between the tocilizumab and placebo arms. The risk of FVC % predicted decrease >10% by 48 weeks was three times less in the tocilizumab arm, whereas the risk of any increase in the FVC % predicted at 48 weeks was nearly twice as much in the tocilizumab arm compared with placebo. By 96 weeks (when the placebo arm was also given tocilizumab) there were no significant differences in risk for these parameters. In contrast to the above trends, when evaluating data from 48 to 96 weeks in the open-label extension period, the mean change in the absolute FVC was 54.9 ml less and the mean change in FVC % predicted was 1.3% less in the placebo arm. The mean change in DlCO % predicted from baseline to 48 weeks was 1.5% less in the tocilizumab arm, but the difference was not significant at 96 weeks. During the interval from 48 to 96 weeks, the mean decrease in DlCO % predicted was 5.4% less in the tocilizumab arm. At 48 weeks, the change in QILD and QLF scores across all categories favored the tocilizumab group. The mRSS change from baseline at 72 weeks was 4.1 better in the tocilizumab arm when compared with placebo but was 0.8 better in the placebo arm compared with tocilizumab when looking at 48–96 weeks in the open-label extension period when the placebo arm was also given tocilizumab.
Mortality
There was no significant difference in mortality between the tocilizumab and placebo arms at 24, 48, or 96 weeks.
QoL
At 96 weeks in the open-label study, the mean change from baseline in the 5-D Itch score, HAQ-DI score, FACIT-Fatigue score, and the Patient Global Visual Analog Scale score all favored the placebo group that was transitioned to tocilizumab during the open-label period.
Adverse events
At 48 weeks, there were 3.8 fewer hypersensitivity events, 44 fewer overall adverse events, 7.6 fewer adverse events leading to treatment discontinuation, 9.1 fewer infectious serious adverse events, and 27.4 fewer overall serious adverse events, all per 100 patient-years, for tocilizumab compared with the placebo group. In the open-label extension from 48 to 96 weeks, the arm that received tocilizumab the full 96 weeks had 96.7 fewer overall adverse events, 5.6 fewer infectious serious adverse events, and 8.6 overall serious adverse events per 100 patient-years. The placebo arm, however, was found to have 10.2 fewer injection site reactions per 100 patient-years at 48 weeks and 6.8 fewer hypersensitivity events per 100 patient-years from 48 to 96 weeks.
Quality of evidence
The quality of evidence was rated as very low for all outcomes. Therefore, the effects summarized should be interpreted with caution, because the committee had low confidence in the estimated effects. The overall very low-quality rating is based on the lowest quality of evidence rating among the critical outcomes disease progression and mortality. The studies included did not a priori document ILD at enrollment and include post hoc and open-label extension studies, leading to indirectness of evidence and imprecision.
Recommendation 4: We suggest using tocilizumab to treat patients with SSc-ILD (conditional recommendation, very low-quality evidence)
The voting by the committee was as follows: strong recommendation for tocilizumab: 0 of 16 (0%); conditional recommendation for tocilizumab: 16 of 16 (100%); conditional recommendation against tocilizumab: 0 of 16 (0%); and strong recommendation against tocilizumab: 0 of 16 (0%). No participants (0%) abstained from voting.
Justification and implementation considerations
The committee decided to make a conditional recommendation for tocilizumab in patients with SSc-ILD, balancing the significant reduction in disease progression against the very low-quality evidence. Despite there being randomized trials with a true placebo arm in which participants were not exposed to any other medications, the data gathered were from two randomized studies, post hoc analyses, and open-label extensions with the patient population indirectly related to SSc-ILD because of a lack of a priori inclusion of patients with ILD as defined by this committee. In addition, the primary outcome of change in mRSS was not met in either the faSScinate or focuSSced trials.
Future research opportunities
The committee noted that the focuSSced trial included individuals with early diffuse cutaneous SSc with elevated levels of acute-phase reactants, who represent a small proportion of patients with SSc-ILD. As such, further research is needed into the efficacy and effectiveness of tocilizumab for individuals with SSc-ILD who would not have met the inclusion criteria for the focuSSced trial (later stage SSc, limited cutaneous SSc, patients with SSc without elevated levels of acute-phase reactants). Future research should assess the use of tocilizumab in patients at the initial diagnosis of SSc-ILD, in stable SSc-ILD, and in progressive SSc-ILD and also compare the use of tocilizumab to other SSc-ILD therapies, such as mycophenolate mofetil.
Question 5: Should patients with SSc-ILD be treated with nintedanib?
Background
Nintedanib is an oral intracellular tyrosine kinase inhibitor that blocks pathways involved in fibrogenesis. It has been recommended in previous guidelines for the treatment of IPF and PPF (3, 45). Therefore, the committee addressed the question “Should patients with SSc-ILD be treated with nintedanib?” Critical outcomes included disease progression (including changes in FVC, DlCO, and, indirectly, mRSS) and mortality, whereas important outcomes included QoL measures (including HAQ-DI, FACIT-Dyspnea, and the St. George’s Respiratory Questionnaire [SGRQ]) and adverse events.
Summary of evidence
A systematic review of the evidence identified three studies for inclusion: the safety and efficacy of nintedanib in systemic sclerosis (SENSCIS) trial (46), a post hoc analysis of the SENSCIS trial (47), and a post hoc analysis of the INBUILD trial (48) (Table E5). The SENSCIS trial was a phase 3 RCT that assigned 576 subjects with SSc-ILD across 32 countries to nintedanib or placebo over 52 weeks. Of note, background therapy with mycophenolate was allowed, with about half of the subjects receiving the therapy. The post hoc analysis examined changes in FVC % predicted at categorical ranges, including at 5%, 10%, and by the minimal clinically important difference (MCID) for improvement and worsening of FVC (49). The INBUILD trial was an RCT that assigned 663 subjects with progressive ILD across 15 countries to nintedanib or placebo over 52 weeks. The post hoc analysis assessed prespecified subgroups based on ILD diagnosis, from which 39 patients with SSc-ILD were extracted for data analysis.
Disease progression
The annual rate of decline in FVC was 44.5 ml less and the decline in FVC % predicted was 1.2% less in the nintedanib arm compared with placebo, based on data from the SENSCIS trial. The absolute change from baseline in FVC was 46.4 ml less for the nintedanib arm, with the risks of absolute decline from baseline in FVC of >5% predicted and relative decline in ml of >5%, both about 25% less in the nintedanib arm. When looking at the MCID (49), the nintedanib arm had >20% reduction in risk of FVC decrease ⩾3.3% predicted (the MCID threshold for worsening FVC) and had a 50% increase in risk of FVC increase of ⩾3.0% predicted (the MCID threshold for improvement in FVC). There was no significant difference in the mRSS.
Mortality
There was no significant difference between the nintedanib or placebo arms for all-cause mortality, fatal adverse events, or serious adverse events that included death. However, for composite outcomes of absolute decline in FVC ⩾10% predicted or death at 52 weeks and for absolute decline in FVC ⩾10% predicted or between 5% and 10% predicted with DlCO decline ⩾15% predicted or death at 52 weeks, the rate was approximately 40% less in the nintedanib arm compared with placebo.
QoL
There was no significant difference between the nintedanib or placebo arms for absolute change from baseline in the HAQ-DI, FACIT-Dyspnea, or SGRQ scores.
Adverse events
Nintedanib increased the risk of nausea (2.3 times), vomiting (2.4 times), diarrhea (2.4 times), weight loss (2.8 times), and adverse events leading to treatment discontinuation (1.8 times) but decreased the risk of cough as an adverse event by 35%.
Quality of evidence
The quality of evidence was rated as very low for all outcomes. Therefore, the effects summarized should be interpreted with caution, because the committee had low confidence in the estimated effects. The overall very low-quality rating is based on the lowest quality of evidence rating among the critical outcomes disease progression and mortality. Despite the SENSCIS trial being an RCT, the overall evidence quality was downgraded because the only other studies were post hoc analyses, leading to indirectness of evidence and imprecision. In addition, patients in the placebo arm of the SENSCIS trial were not true placebos, as many were receiving background immunosuppressive medications for treatment of SSc-ILD.
Recommendation 5: We suggest using nintedanib to treat patients with SSc-ILD (conditional recommendation, very low-quality evidence)
The voting by the committee was as follows: strong recommendation for nintedanib, 1 of 14 (7%); conditional recommendation for nintedanib, 11 of 14 (79%); conditional recommendation against nintedanib, 1 of 14 (7%); and strong recommendation against nintedanib, 0 of 14 (0%). One participant (7%) abstained from voting, citing insufficient expertise to render a thoughtful judgment.
Justification and implementation considerations
The committee decided to make a conditional recommendation for nintedanib in patients with SSc-ILD, balancing the significant reduction in disease progression against gastrointestinal adverse events, which can be managed with medication discontinuation, and low-quality evidence attributable to the limited number of randomized studies and primarily post hoc data analysis. It is worth noting that a recent guideline suggested the use of nintedanib for non-IPF PPF, including progressive SSc-ILD (3). Our recommendation broadens that suggestion to include all patients with SSc-ILD, regardless of whether their disease is progressive or stable. This reflects the observation that relevant trials found a benefit from nintedanib in heterogeneous populations of SSc-ILD. The trials did not distinguish those with progressive disease from those with stable disease; therefore, it is possible that 1) the modest benefit in all patients with SSc-ILD is the net effect of a large benefit in progressive SSc-ILD and no benefit in stable SSc-ILD; or 2) there is a benefit in patients with stable SSc-ILD through prevention of progression in those who are destined to eventually progress.
Future research opportunities
Future research should assess the use of nintedanib in patients with an initial diagnosis of SSc-ILD, in stable SSc-ILD, and in progressive SSc-ILD to discriminate its effect in stable versus progressive disease.
Question 6: Should patients with SSc-ILD be treated with nintedanib plus mycophenolate?
Background
For SSc-ILD, it is unknown if combining therapies with different mechanisms of action is preferable to individual agents and, if dual therapy is preferred, what is the combination of choice. The use of mycophenolate has demonstrated improvement in the absolute FVC % predicted over time, whereas nintedanib has been shown to reduce the rate of disease progression compared with placebo in patients with SSc-ILD (32, 34, 46, 47). Therefore, the committee addressed the question, “Should patients with SSc-ILD be treated with nintedanib plus mycophenolate?” The committee remained interested in the evidence base for the different formulations of mycophenolate, specifically mycophenolate mofetil and mycophenolic acid, but these data were not ascertainable. Appropriate comparators to combination therapy included placebo, mycophenolate only, or nintedanib only. Critical outcomes included disease progression (including changes in FVC, DlCO, or radiographic disease) and mortality. Important outcomes included quality of life (using the SGRQ) and adverse events.
Summary of evidence
A systematic review of the evidence identified three studies meeting inclusion criteria (46, 50, 51) (Table E6). One, the SENSCIS trial, was a study that randomized 576 patients with SSc-ILD to nintedanib or placebo (as noted above), but patients who had been on at least 6 months of therapy with mycophenolate at a stable dosage were permitted in the trial (46). The second study was a post hoc subgroup analysis of the SENSCIS trial that examined the efficacy and safety of patients treated with mycophenolate and nintedanib (50). This study reported results for four groups—combination therapy, mycophenolate plus placebo, nintedanib plus placebo, and placebo only—and provided the majority of data for the systematic review. The third trial was an open-label extension of the SENSCIS trial, in which all patients were offered 52 weeks of therapy with nintedanib to examine safety and efficacy (51).
Disease progression
Compared with placebo, there was nearly an 80 ml and 2.5% lower annual rate of decline in FVC and FVC % predicted, respectively, for combination therapy with nintedanib plus mycophenolate. Similarly, in the combination therapy arm, the risk of absolute decrease from baseline in FVC of >5% predicted and >10% predicted were 50% and 75% less than the placebo arm, respectively. These changes met established MCID thresholds (49). There were no significant differences in the annual rate of decline in FVC or FVC % predicted between combination therapy and mycophenolate or combination therapy and nintedanib, but the risk of FVC decrease from baseline by >5% was about one-third less in the combination therapy arm when compared with either mycophenolate alone or nintedanib alone. There were no differences identified in mRSS between combination therapy with nintedanib plus mycophenolate versus placebo, mycophenolate only, or nintedanib only.
Mortality
There were no differences in fatal adverse events comparing combination therapy with nintedanib plus mycophenolate to placebo, mycophenolate only, or nintedanib only.
QoL
There were no differences identified in SGRQ scores between combination therapy with nintedanib plus mycophenolate to placebo, mycophenolate only, or nintedanib only.
Adverse events
Combination therapy was associated with a sevenfold higher risk of decreased appetite, more than 2.5-fold higher risk of diarrhea, and about threefold higher risk of nausea, vomiting, and/or fatigue compared with placebo. Combination therapy was also associated with nearly twice the risk of diarrhea, nausea, and vomiting compared with mycophenolate only. Combination therapy was associated with a 1.65-fold increase in serious adverse events (defined as an event that resulted in death, was life-threatening, resulted in hospitalization or prolongation of hospitalization, resulted in persistent or clinically significant disability or incapacity, was a congenital anomaly or birth defect, or was deemed to be serious for any other reason) compared with mycophenolate only. Adverse event data could not be pooled for the comparison between combination therapy and nintedanib only, but, interestingly, combination therapy was associated with a 60% lower risk of liver test abnormalities compared with nintedanib only.
Quality of evidence
The quality of evidence for all outcomes was rated as very low, meaning that the committee had very low confidence in the estimated effects. As a result, the effect estimates should be interpreted with caution. There were multiple reasons for the very low quality of evidence. Each outcome was informed by only a single study, leading to imprecision. Furthermore, study design limitations downgraded evidence quality, as the majority of data were informed by a post hoc analysis of an RCT. Finally, although treatment with nintedanib was randomized, therapy with mycophenolate was not randomized, and those patients receiving background therapy with mycophenolate had several differences in demographics compared with patients not on background mycophenolate therapy (50).
Recommendation 6: We suggest using the combination of nintedanib plus mycophenolate to treat patients with SSc-ILD (conditional recommendation, very low-quality evidence)
The voting by the committee was as follows: strong recommendation for nintedanib plus mycophenolate, 1 of 14 (7%); conditional recommendation for nintedanib plus mycophenolate, 11 of 14 (79%); conditional recommendation against nintedanib plus mycophenolate, 0 of 14 (0%); and strong recommendation against nintedanib plus mycophenolate, 0 of 14 (0%). Two participants (14%) abstained from voting, citing insufficient expertise to render a thoughtful judgment.
Justification and implementation considerations
The committee decided to make a conditional recommendation for the combination of nintedanib plus mycophenolate in patients with SSc-ILD, balancing the significant reduction in disease progression against gastrointestinal adverse events and very low-quality evidence due to primarily post hoc data analysis. The decision to initiate combination therapy should be informed based on patient values and preferences in this situation, given the increased risk of gastrointestinal side effects.
Future research opportunities
Future research should assess the use of combination nintedanib plus mycophenolate in patients with an initial diagnosis of SSc-ILD, in stable SSc-ILD, and in progressive SSc-ILD.
Question 7: Should patients with SSc-ILD be treated with pirfenidone?
Background
Pirfenidone is an antifibrotic agent that has been recommended for use in IPF and evaluated in guidelines for PPF (3, 45). Therefore, the committee addressed the question, “Should patients with SSc-ILD be treated with pirfenidone?” Critical outcomes included disease progression (including changes in FVC, 6-minute-walk distance, and mRSS) and mortality, whereas important outcomes included quality of life measures (including TDI scores) and adverse events.
Summary of evidence
A systematic review of the evidence identified one RCT evaluating the use of pirfenidone in SSc-ILD (52) (Table E7). This study, however, was underpowered for the proposed outcomes, as it enrolled only 53% of the total planned participants (n = 34) because of limited availability of pirfenidone as a study drug. In addition, only 6% of the total participants received the pirfenidone target dose of 2,400 mg/d. A majority of participants were receiving background therapy, mostly mycophenolate mofetil, azathioprine, and prednisolone, which may have confounded the effect of pirfenidone on proposed outcomes. Although SSc-ILD was confirmed before enrollment, the extent of the ILD is not known. It is mentioned, however, that the majority of participants (61.7%) had nonspecific interstitial pneumonia, with the remaining (32.2%) having a UIP pattern on the HRCT of the chest.
Disease progression
There were no significant differences between pirfenidone and placebo for change from baseline in FVC % predicted, 6-minute-walk distance, or mRSS.
Mortality
Mortality was not reported in this study.
QoL
There was no difference at 24 weeks between pirfenidone and placebo in the median change from baseline in the TDI scores.
Adverse events
There was no difference at 24 weeks between pirfenidone and placebo for any adverse event (including nausea, vomiting, diarrhea, rashes, loss of appetite, constitutional symptoms, thrombocytopenia, or elevation of transaminases).
Quality of evidence
The quality of evidence for both critical and important outcomes was very low as defined by the GRADE approach, due primarily to study bias (low enrollment numbers owing to lack of pirfenidone availability as a study drug) and imprecision (limited number of participants/studies contributing to the findings, and lack of uniform distribution of pirfenidone dosing among the participants).
Recommendation 7: We recommend further research into the safety and efficacy of pirfenidone to treat patients with SSc-ILD
The voting by the committee was as follows: strong recommendation for pirfenidone, 0 of 13 (0%); conditional recommendation for pirfenidone, 0 of 13 (0%); conditional recommendation against pirfenidone, 2 of 13 (15%); and strong recommendation against pirfenidone, 0 of 13 (0%). Eleven participants (85%) abstained from voting, citing insufficient evidence to render a thoughtful judgment.
Justification and implementation considerations
Given the >20% abstention rate citing insufficient evidence to render a thoughtful judgment by the voting members of the committee, an insufficient quorum was reached. Instead, a research recommendation is made to further evaluate treatment with pirfenidone in SSc-ILD.
Future research opportunities
Because of the paucity of studies looking at pirfenidone for the treatment of patients with SSc-ILD, future research should focus on comparing the use of pirfenidone to placebo with larger sample sizes and evaluate administration by disease status (initial diagnosis of SSc-ILD, stable SSc-ILD, progressive SSc-ILD).
Question 8: Should patients with SSc-ILD be treated with pirfenidone plus mycophenolate?
Background
For SSc-ILD, it is unknown if combining therapeutic agents with different mechanisms of action is preferable to individual agents and, if dual therapy is preferred, what is the combination of choice. To evaluate combination therapy with pirfenidone and mycophenolate for the treatment of SSc-ILD, the guideline committee addressed the question, “Should patients with SSc-ILD be treated with pirfenidone plus mycophenolate?” Critical outcomes included disease progression (including changes in FVC, DlCO, radiographic disease, and mRSS) and mortality, whereas important outcomes included QoL measures (including TDI and HAQ-DI scores) and adverse events.
Summary of evidence
A systematic review of the evidence identified one published study, the LOTUSS trial (53), and one abstract from the SLS III trial (54) for inclusion (Table E8). The LOTUSS trial (53) was an open-label phase 2 study of 63 patients with SSc-ILD monitored over 16 weeks assessing safety and tolerability of pirfenidone. Patients were not randomized to mycophenolate, but 63.5% of patients were concomitantly on it, so the data analyzed was post hoc. The baseline mycophenolate dose varied between participants, and 20% of patients were on steroids and other antirheumatic medications. In addition, changes in lung function were exploratory outcomes, not primary. The abstract described the results of the SLS III RCT that compared the treatment with combined pirfenidone and mycophenolate to mycophenolate plus placebo, with the primary outcome being change in lung function at the end of 18 months. The study was aborted due to inability to enroll the intended sample size and had just enrolled 51 of the targeted 150 participants, so the results noted in the abstract were from a very small sample size and thus the study was underpowered (54). While the abstract of the SLS III study does not include many secondary outcomes that are anticipated to be published in the full report in the near future, the published primary outcomes in the abstract were also our critical outcomes of interest for decision-making.
Disease progression
No significant difference in FVC % predicted or DlCO % predicted was observed between the combination pirfenidone plus mycophenolate arm and pirfenidone alone. There were also no differences between the combination pirfenidone plus mycophenolate arm and the mycophenolate and placebo arms in FVC % predicted or time duration to >3% increase in FVC % predicted at 18 months.
Mortality
Mortality was not reported in either the LOTUSS trial or the SLS III abstract.
QoL
The LOTUSS trial found that compared with mycophenolate alone, the combination of pirfenidone plus mycophenolate showed a 2-point improvement in the TDI score at 16 weeks, but there was no significant difference in HAQ-DI scores.
Adverse events
The LOTUSS trial did not observe any significant differences in severe adverse events, withdrawal because of severe adverse events, or infections at 16 weeks between combination therapy and the pirfenidone-only arm.
Quality of evidence
The quality of evidence was very low by the GRADE approach because of bias (premature closure of enrollment), imprecision (limited number of participants/studies contributing to the findings, lack of uniform distribution of mycophenolate treatment in the pirfenidone and mycophenolate participants), and indirectness of evidence (post hoc analysis of data).
Recommendation 8: We recommend further research into the safety and efficacy of pirfenidone plus mycophenolate combination therapy to treat patients with SSc-ILD
The voting by the committee was as follows: strong recommendation for pirfenidone plus mycophenolate, 0 of 13 (0%); conditional recommendation for pirfenidone plus mycophenolate, 0 of 13 (0%); conditional recommendation against pirfenidone plus mycophenolate, 1 of 13 (8%); and strong recommendation against pirfenidone plus mycophenolate, 0 of 13 (0%). Twelve participants (92%) abstained from voting, citing insufficient evidence to render a thoughtful judgment.
Justification and implementation considerations
Given the >20% abstention rate citing insufficient evidence to render a thoughtful judgment by the voting members of the committee, an insufficient quorum was reached. Instead, a research recommendation is made for further evaluation of combination treatment with pirfenidone plus mycophenolate in SSc-ILD.
Future research opportunities
Because of the paucity of studies looking specifically at the combination of mycophenolate and pirfenidone for the treatment of patients with SSc-ILD, future research should focus on this combination against placebo and mycophenolate alone with larger sample sizes and by disease status (initial diagnosis of SSc-ILD, stable SSc-ILD, progressive SSc-ILD).
Guideline Considerations and Future Directions
The focus of this ATS clinical practice guideline on the treatment of SSc-ILD is to assess the use of separate therapies on their own or in combination for mycophenolate with nintedanib and pirfenidone. The summary of our recommendations are noted in Figure 1. The committee made a strong recommendation in favor of mycophenolate and conditional recommendations in favor of cyclophosphamide, rituximab, tocilizumab, nintedanib, and the combination of nintedanib plus mycophenolate. Research recommendations were made for the use of pirfenidone and the combination of pirfenidone plus mycophenolate. It is worth highlighting that among the conditional recommendations, there was a differentiation, with 29% of committee members voting strongly in favor of cyclophosphamide, whereas for nintedanib and the combination of nintedanib plus mycophenolate only 7% voted strongly in favor, for rituximab only 6% voted strongly in favor, and for tocilizumab 0% voted strongly in favor.
Figure 1.
Summary of treatment recommendations for patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD).
-
1)Recommends the use of mycophenolate to treat patients with SSc-ILD (18 votes: 14 strong recommendation for use, 4 conditional recommendations for use).
-
2)Suggests the use of cyclophosphamide to treat patients with SSc-ILD (17 votes: 5 strong recommendation for use, 12 conditional recommendations for use).
-
3)Suggests the use of rituximab to treat patients with SSc-ILD (18 votes: 1 strong recommendation for use, 16 conditional recommendation for use, 1 abstention due to insufficient expertise).
-
4)Suggests the use of tocilizumab to treat patients with SSc-ILD (16 votes: 16 conditional recommendation for use).
-
5)Suggests the use of nintedanib to treat patients with SSc-ILD (14 votes: 1 strong recommendation for use, 11 conditional recommendation for use, 1 conditional recommendation against use, 1 abstention due to insufficient expertise).
-
6)Suggests the use of nintedanib plus mycophenolate to treat patients with SSc-ILD (14 votes: 1 strong recommendation for use, 11 conditional recommendation for use, 2 abstentions due to insufficient expertise).
-
7)Recommends further research into the efficacy, effectiveness, and safety of pirfenidone to treat patients with SSc-ILD (13 votes: 2 conditional recommendation against use, 11 abstentions due to insufficient evidence).
-
8)Recommends further research into the efficacy, effectiveness, and safety of pirfenidone plus mycophenolate to treat patients with SSc-ILD (13 votes: 1 conditional recommendation against use, 12 abstentions due to insufficient evidence).
The above recommendations were not assessed as a stepwise algorithm. Clinicians are encouraged to use these recommendations in conjunction with shared decision-making with patients, incorporating side effects and personal values and preferences before administration.
Although the committee sought to differentiate SSc-ILD by initial diagnosis, stable SSc-ILD, and progressive SSc-ILD for each research question, disease status could not be extracted from any of the studies included in the systematic reviews. In addition, high-quality data were not available to assess the treatment effect for mycophenolate and cyclophosphamide by drug formulation or route of administration, respectively. Finally, the questions addressed by this guideline did not focus on comparing one therapeutic option against others. Therefore, the committee felt that a prescriptive decision tree would not be appropriate for this patient population with the evidence that was available.
While an algorithm for different options among the several treatment regimens available is what clinicians are looking for in a guideline, such an approach is ideal only if the evidence permits us to provide this approach. Because of the lack of data to support a preferential and/or stepwise approach to treatment interventions for SSc-ILD, the committee provided the scientific information as it unfolded in the careful review of the evidence to date to inform the treatment recommendations. The committee refrained from providing an algorithmic approach to treatment of SSc-ILD that would have been based solely on a consensus of opinions of the committee and not evidence based.
In addition to the limitations in the evidence, the committee also acknowledges the highly variable adverse event profiles, making it less than ideal to provide a hierarchical recommendation for these medications. Instead, the committee evaluated the medications independently, as some patients may prefer one over another based on their adverse effect profiles, pill burden, routes of administration, and/or costs. The committee believes the current approach in fact empowers the clinician to craft an algorithm tailored to the clinician’s opinion in partnership with the well-informed patient.
This guideline aims to serve as a starting point to highlight gaps in evidence to encourage future research into topics and comparisons that can then provide more prescriptive guidance. Further research is therefore needed to determine treatment efficacy, safety, and impact on patient QoL by disease status to determine if there exists a differential treatment effect going from initial diagnosis of SSc-ILD to development of progressive SSc-ILD. Furthermore, additional studies looking at combination therapy and the sequence of initiating each therapy would be of clinical benefit.
Conclusions
The recommendations in this document provide an evidence-based clinical practice guideline for the treatment of patients with SSc-ILD (Figure 1) and are intended to serve as the basis for informed and shared decision making by clinicians and patients. For treatment of patients with SSc-ILD, the committee: 1) recommends the use of mycophenolate; 2) recommends further research into the safety and efficacy of (a) pirfenidone and (b) the combination of pirfenidone plus mycophenolate; and 3) suggests the use of (a) cyclophosphamide, (b) rituximab, (c) tocilizumab, (d) nintedanib, and (e) the combination of nintedanib plus mycophenolate. Future research should investigate the efficacy and safety of these therapies on SSc-ILD subgroups by disease status (initial diagnosis, stable SSc-ILD, and progressive SSc-ILD) and in various combinations. This guideline was reviewed by the ATS Quality Improvement and Implementation Committee. None of the recommendations in this guideline are considered appropriate targets for a performance measure.
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Clinical Problems.
Members of the subcommittee are as follows:
Ganesh Raghu, M.D. (Chair)1,2,3*
Marya Ghazipura, Ph.D., M.S., M.Phil. (Co-Chair)4,5‡
Sydney B. Montesi, M.D. (Co-Chair)6*
Richard M. Silver, M.D. (Co-Chair)7§
Tanzib Hossain, M.D., M.S.8‡
Madalina Macrea, M.D., Ph.D.9,10‡
Derrick Herman, M.D.11‡
Hayley Barnes, M.B.B.S., Ph.D.12,13,14‡
Ayodeji Adegunsoye, M.D., M.S.15*
Arata Azuma, M.D., Ph.D.16,17*
Dee Burlile, M.Ed.18ǁ
Lorinda Chung, M.D.19§
Gregory C. Gardner, M.D.20§
Kristin B. Highland, M.D.21*§
Marie Hudson, M.D., M.P.H.22,23§
Robert J. Kaner, M.D.24*
Karen A. Kemper, M.S.P.H., Ph.D.25ǁ
Shanda L. Knight, M.L.S.26¶
Martin Kolb, M.D., Ph.D.27*
Mary Beth Scholand, M.D.28*
Virginia Steen, M.D.29§
Carey C. Thomson, M.D., M.P.H.30*
Elizabeth R. Volkmann, M.D., M.S.31§
Fredrick M. Wigley, M.D.32§
1Center for Interstitial Lung Diseases, Division of Pulmonary, Critical Care, and Sleep Medicine, 2Department of Medicine, and 3Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington; 4ZS Associates, Global Health Economics and Outcomes Research, New York, New York; 5Divisions of Epidemiology and Biostatistics, Department of Population Health, New York University Langone Health, New York, New York; 6Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Boston, Massachusetts; 7Department of Medicine, Division of Rheumatology and Immunology, Medical University of South Carolina, Charleston, South Carolina; 8Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Grossman School of Medicine, New York University Langone Health, New York, New York; 9Division of Pulmonary and Sleep Medicine, Salem Veterans Affairs Medical Center, Salem, Virginia; 10Department of Medicine, University of Virginia, Charlottesville, Virginia; 11Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Internal Medicine, The Ohio State Wexner Medical Center, Columbus, Ohio; 12Central Clinical School and 13Centre for Occupational and Environmental Health, Monash University, Melbourne, Australia; 14Department of Respiratory Medicine, Alfred Hospital, Melbourne, Australia; 15Section of Pulmonary and Critical Care, The University of Chicago School of Medicine, Chicago, Illinois; 16Mihara General Hospital, Pulmonary Medicine and Clinical Research Center, Saitama; 17Nippon Medical School, Tokyo, Japan; 18National Advocacy Committee Northwest Chapter Chair, Idaho; 19Division of Immunology and Rheumatology, Stanford University School of Medicine, Palo Alto, California; 20Division of Rheumatology, University of Washington Medical Center, Seattle, Washington; 21Respiratory Institute, Cleveland Clinic, Cleveland, Ohio; 22Division of Rheumatology, Department of Medicine, Jewish General Hospital and 23McGill University, Montreal, Quebec, Canada; 24Weill Cornell Medical Center, New York, New York; 25Department of Public Health Sciences, College of Behavioral, Social, and Health Sciences, Clemson University, Clemson, South Carolina; 26Strauss Health Sciences Library, University of Colorado Anschutz Medical Campus, Aurora, Colorado; 27Division of Respirology, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 28University of Utah, Salt Lake City, Utah; 29Georgetown University School of Medicine, Washington, D.C.; 30Department of Medicine, Mount Auburn Hospital/Beth Israel Lahey Health, Harvard Medical School, Cambridge, Massachusetts; 31Division of Rheumatology, Department of Medicine, University of California at Los Angeles, David Geffen School of Medicine, Los Angeles, California; 32Johns Hopkins University School of Medicine, Baltimore, Maryland
*Pulmonologist
‡Methodologist
§Rheumatologist
ǁPatient representative
¶Medical information scientist
Footnotes
This Official Clinical Practice Guideline of the American Thoracic Society was Approved May 2023
Supported by the American Thoracic Society.
An editorial, a clinical practice guideline summary for clinicians, and systematic reviews (https://www.atsjournals.org/doi/abs/10.1513/AnnalsATS.202301-053OC, https://www.atsjournals.org/doi/abs/10.1513/AnnalsATS.202301-054OC, https://www.atsjournals.org/doi/abs/10.1513/AnnalsATS.202301-055OC, https://www.atsjournals.org/doi/abs/10.1513/AnnalsATS.202301-056OC, https://www.atsjournals.org/doi/abs/10.1513/AnnalsATS.202301-081OC) related to this document will be published in upcoming issues of the Annals of the American Thoracic Society.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures: G.R. served on advisory board for Pulmonary Fibrosis Foundation; served as consultant for Boehringer Ingelheim, Bellerophan, Blade Therapeutics, Bristol Myers Squibb, Electra Therapeutics, Fibrogen, Nitto, Roche-Genentech, United Therapeutics, Veracyte, Zambon; served on data safety and monitoring board for Avalyn; served on steering committee for Bellerophan, Bristol Myers Squibb, Gilead, Fibrogen, Nitto, Novartis, Veracyte; received research support from Biogen, Boehringer Ingelheim, Fibrogen, NIH/NHLBI, Novartis; received royalties from UpToDate. S.B.M. served on advisory committee for APIE Therapeutics and Pliant; served as consultant for DevPro, Gilead, Pieris, Roche; served in leadership role for Massachusetts Pulmonary Society; received research support from Boehringer Ingelheim, Merck, National Scleroderma Foundation, NIH/NHLBI, Pliant, Three Lakes Foundation, United Therapeutics; received royalties from UpToDate; served as speaker for Cowen; received travel support from DevPro. R.M.S. served on advisory committee for Actelion/Jansen and Boehringer Ingelheim; served as speaker for Boehringer Ingelheim; received research support from Boehringer Ingelheim, Genentech, Horizon, Kadmon, Prometheus. H.B. received travel support from Boehringer Ingelheim, Janssen, United Therapeutics. A.Adegunsoye served as consultant for Boehringer Ingelheim, Genentech, Inogen; served as speaker for Boehringer Ingelheim; received research support from NIH/NHLBI and Pulmonary Fibrosis Foundation. A.Azuma served as consultant for Boehringer Ingelheim, Nippon Boehringer Ingelheim, Kyorin, Toray; served on data safety and monitoring board for Nippon Boehringer Ingelheim; served as speaker for Nippon Boehringer Ingelheim; received research support from Boehringer Ingelheim and Taiho. L.C. served as consultant for Eicos, Genentech, IgM, Jasper, Kyverna, Lilly, Mitsubishi Tanabe; received research support from Acceleron, Boehringer Ingelheim, Genentech, Horizon, Kadmon, Mitsubishi Tanabe, Prometheus. G.C.G. served in leadership role for American College of Rheumatology; provided expert panel testimony on SLE case for local attorney; served as speaker for American College of Physicians. K.B.H. served as consultant for Boehringer Ingelheim and Genentech; served as speaker for Actelion, Acceleron, Bayer Healthcare, United Therapeutics; served as speaker and received travel support from Boehringer Ingelheim; received research support from Acceleron, Actelion, Boehringer Ingelheim, E.I. Lilly, Gossamer Bio, NIH, Reata, United Therapeutics, Vela Bio. M.H. served on advisory committee for Alexion, Boehringer Ingelheim, Mallinckrodt; received honoraria from Boehringer Ingelheim; received research support from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Octapharma. R.J.K. served on advisory committee for AstraZeneca, Boehringer Ingelheim, Galapagos, Genentech, United Therapeutics; served as consultant for Boehringer Ingelheim, Galapagos, UpToDate; served on data safety and monitoring board for Pliant and PureTech; has financial stake in AirCycleSystems and Doximity; served in leadership role for Pulmonary Wellness Foundation and Stony World Foundation; served as medical writer for AstraZeneca, Boehringer Ingelheim, Galapagos, Genentech; served as speaker for Boehringer Ingelheim, France Foundation, Genentech, Vindico; received research support from Bellerophon, Boehringer Ingelheim, Chiesi, CSL Behring, Genentech, NIH, Respivant, Toray, US Department of Defense; received royalties from UpToDate; received travel support from Boehringer Ingelheim and United Therapeutics. M.K. served on advisory committee for Algernon; served as consultant for Abbvie, Algernon, AstraZeneca, Bellerophon, Boehringer Ingelheim, Cipla, CSL Behring, Horizon, LabCorp, Roche, Structure Therapeutics, United Therapeutics; served on data safety and monitoring board for LabCorp and United Therapeutics; provided expert testimony for Roche; served in leadership role for ERJ; served as speaker for Boehringer Ingelheim, Novartis, Roche; received research support from Boehringer Ingelheim, Structure Therapeutics, United Therapeutics. M.B.S. served on advisory committee for Bristol Myers Squibb, Genentech, Polarean, Tvardi, United Therapeutics, Veracyte; served as consultant for Boehringer Ingelheim, Bristol Myers Squibb, United Therapeutics, Veracyte; served as speaker for Boehringer Ingelheim, Genentech, United Therapeutics, Veracyte; received research and travel support from United Therapeutics. V.S. served on advisory board, as consultant, speaker, and received research support from Boehringer Ingelheim; served on advisory board and as consultant for CSL Behring and Eicos; served as speaker for Johnson & Johnson; received research support from Corbus, Eicos, Genentech, Kadman. C.C.T. served on advisory board and as consultant for Median Technology; served as chair for National Lung Cancer Roundtable Early Detection Task Group; served as editor for Lung Cancer Screening for Primary Care – Springer; received royalties from UpToDate; received research support from American Cancer Society and NIH. E.R.V. served as consultant for Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Roche; served as speaker for Boehringer Ingelheim; received research support from Boehringer Ingelheim, Forbius, Horizon, Prometheus. D.B. served on advisory committee and as chair for National Scleroderma Foundation; served as president for Scleroderma Outreach Northwest. K.A.K. served in leadership role for National Scleroderma Foundation; served as speaker for Boehringer Ingelheim. S.L.K. served as consultant for Guideline panel. M.G. employee of ZS Associates. T.H., M.M., D.H., F.M.W. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Preliminary criteria for the classification of systemic sclerosis (scleroderma): subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum . 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Scleroderma Lung Study Research Group Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med . 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 5. Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Sclerodema Lung Study II Investigators Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med . 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann-Vold AM, Allanore Y, Alves M, Brunborg C, Airó P, Ananieva LP, et al. EUSTAR collaborators Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis . 2021;80:219–227. doi: 10.1136/annrheumdis-2020-217455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis . 2010;69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 8. Roofeh D, Lescoat A, Khanna D. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol . 2021;33:240–248. doi: 10.1097/BOR.0000000000000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanna D, Lescoat A, Roofeh D, Bernstein EJ, Kazerooni EA, Roth MD, et al. Systemic sclerosis-associated interstitial lung disease: how to incorporate two Food and Drug Administration-approved therapies in clinical practice. Arthritis Rheumatol . 2022;74:13–27. doi: 10.1002/art.41933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahaghi FF, Hsu VM, Kaner RJ, Mayes MD, Rosas IO, Saggar R, et al. Expert consensus on the management of systemic sclerosis-associated interstitial lung disease. Respir Res . 2023;24:6. doi: 10.1186/s12931-022-02292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol . 2022;117:27–56. doi: 10.14309/ajg.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J . 2023;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 13. Trang G, Steele R, Baron M, Hudson M. Corticosteroids and the risk of scleroderma renal crisis: a systematic review. Rheumatol Int . 2012;32:645–653. doi: 10.1007/s00296-010-1697-6. [DOI] [PubMed] [Google Scholar]
- 14. Steen VD, Medsger TA., Jr Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum . 1998;41:1613–1619. doi: 10.1002/1529-0131(199809)41:9<1613::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. Clinical practice guidelines we can trust. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 16. Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. ATS Documents Development and Implementation Committee An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med . 2006;174:605–614. doi: 10.1164/rccm.200602-197ST. [DOI] [PubMed] [Google Scholar]
- 17.Barnes H, Ghazipura M, Herman D, Macrea M, Knight SL, Silver RM, et al. Cyclophosphamide in patients with systemic sclerosis-associated interstitial lung disease: a systematic review and meta-analysis. [DOI] [PubMed]
- 18.Herman D, Ghazipura M, Barnes H, Macrea M, Knight SL, Silver RM, et al. Mycophenolate in patients with systemic sclerosis-associated interstitial lung disease: a systematic review and meta-analysis. 2023. [DOI] [PubMed]
- 19.Macrea M, Ghazipura M, Herman D, Barnes H, Knight SL, Silver RM, et al. Rituximab in patients with systemic sclerosis-associated interstitial lung disease: a systematic review and meta-analysis. [DOI] [PubMed]
- 20.Ghazipura M, Macrea M, Herman D, Barnes H, Knight SL, Silver RM, et al. Tocilizumab in patients with systemic sclerosis-associated interstitial lung disease: a systematic review and meta-analysis. [DOI] [PubMed]
- 21.Herman D, Ghazipura M, Barnes H, Macrea M, Knight SL, Silver RM, et al. Nintedanib therapy alone and combined with mycophenolate in patients with systemic sclerosis-associated interstitial lung disease: systematic reviews and meta-analysis. [DOI] [PubMed]
- 22. Kowal-Bielecka O, Kowal K, Rojewska J, Bodzenta-Lukaszyk A, Siergiejko Z, Sierakowska M, et al. Cyclophosphamide reduces neutrophilic alveolitis in patients with scleroderma lung disease: a retrospective analysis of serial bronchoalveolar lavage investigations. Ann Rheum Dis . 2005;64:1343–1346. doi: 10.1136/ard.2004.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs . 1991;42:781–795. doi: 10.2165/00003495-199142050-00005. [DOI] [PubMed] [Google Scholar]
- 24. Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum . 2006;54:3962–3970. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 25. Shenoy PD, Bavaliya M, Sashidharan S, Nalianda K, Sreenath S. Cyclophosphamide versus mycophenolate mofetil in scleroderma interstitial lung disease (SSc-ILD) as induction therapy: a single-centre, retrospective analysis. Arthritis Res Ther . 2016;18:123. doi: 10.1186/s13075-016-1015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panopoulos ST, Bournia VK, Trakada G, Giavri I, Kostopoulos C, Sfikakis PP. Mycophenolate versus cyclophosphamide for progressive interstitial lung disease associated with systemic sclerosis: a 2-year case control study. Lung . 2013;191:483–489. doi: 10.1007/s00408-013-9499-8. [DOI] [PubMed] [Google Scholar]
- 27. Furst DE, Tseng CH, Clements PJ, Strange C, Tashkin DP, Roth MD, et al. Scleroderma Lung Study Adverse events during the Scleroderma Lung Study. Am J Med . 2011;124:459–467. doi: 10.1016/j.amjmed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 28. Volkmann ER, Tashkin DP, Roth MD, Goldin J, Kim GHJ. Early radiographic progression of scleroderma: lung disease predicts long-term mortality. Chest . 2022;161:1310–1319. doi: 10.1016/j.chest.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Scleroderma Lung Study Research Group Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med . 2007;176:1026–1034. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology . 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 31. Goldin JG, Kim GHJ, Tseng CH, Volkmann E, Furst D, Clements P, et al. Longitudinal changes in quantitative interstitial lung disease on computed tomography after immunosuppression in the Scleroderma Lung Study II. Ann Am Thorac Soc . 2018;15:1286–1295. doi: 10.1513/AnnalsATS.201802-079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naidu GSRSNK, Sharma SK, Adarsh MB, Dhir V, Sinha A, Dhooria S, et al. Effect of mycophenolate mofetil (MMF) on systemic sclerosis-related interstitial lung disease with mildly impaired lung function: a double-blind, placebo-controlled, randomized trial. Rheumatol Int . 2020;40:207–216. doi: 10.1007/s00296-019-04481-8. [DOI] [PubMed] [Google Scholar]
- 33. Volkmann ER, Tashkin DP, LeClair H, Roth MD, Kim G, Goldin J, et al. Treatment with mycophenolate and cyclophosphamide leads to clinically meaningful improvements in patient-reported outcomes in scleroderma lung disease: results of Scleroderma Lung Study II. ACR Open Rheumatol . 2020;2:362–370. doi: 10.1002/acr2.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Volkmann ER, Tashkin DP, Li N, Roth MD, Khanna D, Hoffmann-Vold AM, et al. Mycophenolate mofetil versus placebo for systemic sclerosis-related interstitial lung disease: an analysis of Scleroderma Lung Studies I and II. Arthritis Rheumatol . 2017;69:1451–1460. doi: 10.1002/art.40114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol . 2013;66:726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 36. Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford) . 2018;57:2106–2113. doi: 10.1093/rheumatology/key213. [DOI] [PubMed] [Google Scholar]
- 37. Boonstra M, Meijs J, Dorjée AL, Marsan NA, Schouffoer A, Ninaber MK, et al. Rituximab in early systemic sclerosis. RMD Open . 2017;3:e000384. doi: 10.1136/rmdopen-2016-000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) . 2010;49:271–280. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ebata S, Yoshizaki A, Oba K, Kashiwabara K, Ueda K, Uemura Y, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol . 2021;3:E489–E497. doi: 10.1016/S2665-9913(21)00107-7. [DOI] [PubMed] [Google Scholar]
- 40. Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, et al. focuSSced investigators Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med . 2020;8:963–974. doi: 10.1016/S2213-2600(20)30318-0. [DOI] [PubMed] [Google Scholar]
- 41. Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet . 2016;387:2630–2640. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 42. Khanna D, Denton CP, Lin CJF, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate) Ann Rheum Dis . 2018;77:212–220. doi: 10.1136/annrheumdis-2017-211682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khanna D, Lin CJF, Furst DE, Wagner B, Zucchetto M, Raghu G, et al. Long-term safety and efficacy of tocilizumab in early systemic sclerosis-interstitial lung disease: open-label extension of a phase 3 randomized controlled trial. Am J Respir Crit Care Med . 2022;205:674–684. doi: 10.1164/rccm.202103-0714OC. [DOI] [PubMed] [Google Scholar]
- 44. Roofeh D, Lin CJF, Goldin J, Kim GH, Furst DE, Denton CP, et al. focuSSced Investigators Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol . 2021;73:1301–1310. doi: 10.1002/art.41668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med . 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 46.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. SENSCIS Trial Investigators Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 47. Maher TM, Mayes MD, Kreuter M, Volkmann ER, Aringer M, Castellvi I, et al. SENSCIS Trial Investigators Effect of nintedanib on lung function in patients with systemic sclerosis-associated interstitial lung disease: further analyses of a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol . 2021;73:671–676. doi: 10.1002/art.41576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wells AU, Flaherty KR, Brown KK, Inoue Y, Devaraj A, Richeldi L, et al. INBUILD trial investigators Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med . 2020;8:453–460. doi: 10.1016/S2213-2600(20)30036-9. [DOI] [PubMed] [Google Scholar]
- 49.Kafaja S, Clements PJ, Wilhalme H, Tseng CH, Furst DE, Kim GH, et al. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS-I and SLS-II) Am J Respir Crit Care Med. 2018;197:644–652. doi: 10.1164/rccm.201709-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Highland KB, Distler O, Kuwana M, Allanore Y, Assassi S, Azuma A, et al. SENSCIS trial investigators Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med . 2021;9:96–106. doi: 10.1016/S2213-2600(20)30330-1. [DOI] [PubMed] [Google Scholar]
- 51. Allanore Y, Vonk MC, Distler O, Azuma A, Mayes MD, Gahlemann M, et al. SENSCIS-ON trial investigators Continued treatment with nintedanib in patients with systemic sclerosis-associated interstitial lung disease: data from SENSCIS-ON. Ann Rheum Dis . 2022;81:1722–1729. doi: 10.1136/ard-2022-222564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acharya N, Sharma SK, Mishra D, Dhooria S, Dhir V, Jain S. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease-a randomised controlled trial. Rheumatol Int. 2020;40:703–710. doi: 10.1007/s00296-020-04565-w. [DOI] [PubMed] [Google Scholar]
- 53. Khanna D, Albera C, Fischer A, Khalidi N, Raghu G, Chung L, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol . 2016;43:1672–1679. doi: 10.3899/jrheum.151322. [DOI] [PubMed] [Google Scholar]
- 54. Khanna D, Spino C, Bernstein E, Goldin J, Tashkin D, Roth M. Combination therapy of mycophenolate mofetil and pirfenidone vs. mycophenolate alone: results from the Scleroderma Lung Study III. Arthritis Rheumatol . [Google Scholar]