Summary
PPFIA3 encodes the protein-tyrosine phosphatase, receptor-type, F-polypeptide-interacting-protein-alpha-3 (PPFIA3), which is a member of the LAR-protein-tyrosine phosphatase-interacting-protein (liprin) family involved in synapse formation and function, synaptic vesicle transport, and presynaptic active zone assembly. The protein structure and function are evolutionarily well conserved, but human diseases related to PPFIA3 dysfunction are not yet reported in OMIM. Here, we report 20 individuals with rare PPFIA3 variants (19 heterozygous and 1 compound heterozygous) presenting with developmental delay, intellectual disability, hypotonia, dysmorphisms, microcephaly or macrocephaly, autistic features, and epilepsy with reduced penetrance. Seventeen unique PPFIA3 variants were detected in 18 families. To determine the pathogenicity of PPFIA3 variants in vivo, we generated transgenic fruit flies producing either human wild-type (WT) PPFIA3 or five missense variants using GAL4-UAS targeted gene expression systems. In the fly overexpression assays, we found that the PPFIA3 variants in the region encoding the N-terminal coiled-coil domain exhibited stronger phenotypes compared to those affecting the C-terminal region. In the loss-of-function fly assay, we show that the homozygous loss of fly Liprin-α leads to embryonic lethality. This lethality is partially rescued by the expression of human PPFIA3 WT, suggesting human PPFIA3 function is partially conserved in the fly. However, two of the tested variants failed to rescue the lethality at the larval stage and one variant failed to rescue lethality at the adult stage. Altogether, the human and fruit fly data reveal that the rare PPFIA3 variants are dominant-negative loss-of-function alleles that perturb multiple developmental processes and synapse formation.
Keywords: neurodevelopmental disorder, synaptic protein, active zone protein, Mendelian phenotypes, fruit flies
PPFIA3 is a scaffolding protein that mediates synaptic transmission. This study identified 20 individuals with PPFIA3 variants associated with developmental delay, intellectual disability, hypotonia, dysmorphisms, micro/macrocephaly, autistic features, and epilepsy. Functional analysis shows that PPFIA3 variants cause a syndromic neurodevelopmental disorder through a potential loss-of-function mechanism.
Introduction
Synapses are highly specialized communication junctions between neurons and their target cells where neurotransmitter release occurs in an intricately coordinated manner. In the presynaptic neuron, a key site for neurotransmitter release is the active zone, which is composed of a complex protein matrix.1,2,3,4 RIM, ELKS, Munc13, RIM-BP, Piccolo/Bassoon, and Liprin-α are the six major protein families comprising the active zone.5 These active zone proteins, along with other cytoskeletal proteins, Ca2+ channels, and soluble N-ethylmaleimide-sensitive fusion attachment protein receptors (SNAREs), form a tightly orchestrated unit to mediate synaptic vesicle docking, priming, fusion, and neurotransmitter release.5 Prior studies revealed that disruption of synapse structure or function leading to variable defects in neurotransmitter release contributes to neurodevelopmental and neuropsychiatric disorders including epilepsy, intellectual disability (ID), autism spectrum disorder (ASD), schizophrenia, and bipolar disorder.6,7,8,9,10
The network of multidomain proteins comprising the active zone falls into different categories such as cytoskeletal and scaffolding proteins, adhesion molecules, calcium channels, and synaptic vesicle release machinery. Liprins are scaffolding proteins found in the presynaptic active zone that are also known as protein tyrosine phosphatase receptor type F polypeptide (PTPRF)-interacting protein α (PPFIA) or β (PPFIB). Liprin family members interact with the adhesion molecule leukocyte antigen receptor-protein tyrosine phosphatases (LAR-PTPs) and are subdivided into liprin-α and liprin-β proteins.11,12 In conjunction with LAR-PTPs, liprins play a key role in the active zone organization and structure. Structural studies show that liprins, including PPFIA3, are comprised of an N-terminal coiled-coil domain and C-terminal sterile-α-motif (SAM) domain.11,12 The N-terminal coiled-coil domain mediates homodimerization and heterodimerization with other liprin-α members and interactions with other active zone proteins such as RIM and ELKS.13,14,15,16 The SAM domains are known for mediating protein-protein interactions and binding with RNA.17 The liprin-α SAM domain interacts with the LAR intracellular domain.18 Apart from these functional domains, liprins also contain intrinsically disordered regions that lack an ordered three-dimensional structure but were previously shown to be important for protein’s function.19,20 Additionally, liprins interact with kinesin motor proteins21,22,23 and are involved in the hedgehog signaling-dependent trafficking of Kif7 and Gli to the cilia in the context of embryonic development and cortical microtubule organization.21,22
Vertebrates have four Ppfia (1–4) proteins and two Ppfib (1–2) proteins that are encoded by Ppfia1–4 or Ppfib1–2, respectively.12 Expression studies in mice show that all four mouse Ppfia1–4 orthologs are expressed in the brain with differences in distribution and expression levels.24 Ppfia1 is found in the brain, lung, heart, liver, muscle, spleen, and testes.12,25,26 In the brain, Ppfia1 is predominantly localized to the cerebellum and olfactory bulb.24,25,26 In contrast, Ppfia2, Ppfia3, and Ppfia4 are predominantly found in the brain,12,25,26 including structures such as the olfactory bulb, striatum, cortex, hippocampus, thalamus, midbrain, cerebellum, and brainstem.24,25,26 A subcellular localization study showed that Ppfia2 and Ppfia3 are located in both the pre-synaptic and post-synaptic compartments.26 However, only Ppfia3 specifically colocalizes in the presynaptic compartment and mediates protein-protein interactions with the active zone proteins Bassoon, RIM, Munc-13, RIM-BP, and ELKS in hippocampal neurons.27 In humans, transcriptomic studies revealed that PPFIA3 (MIM: 603144) is similarly expressed in different brain regions like the neocortex, striatum, hippocampus, amygdala, mediodorsal nucleus of the thalamus, and cerebellar cortex from the early embryonic stage to late adulthood.28
Ppfia proteins are well conserved in both vertebrates and invertebrates. In C. elegans, the sole Ppfia ortholog, syd-2, plays a key role in presynaptic active zone organization.29,30 Studies showed that syd-2 recruits synaptic components to presynaptic sites and contributes to the formation of neuromuscular junctions (NMJs), along with active zone assembly and stabilization.30,31 Mutant syd-2 worms show presynaptic active zone defects due to disruption of syd-2 oligomerization.13,30,31 A similar role was found for the fruit fly ortholog Liprin-α where it is required for synapse formation, synaptic vesicular transport, active zone assembly, and axonal target selection from the retina to the medulla in the central nervous system.32,33,34 Consistent with the invertebrate models, synaptic ultrastructure and electrophysiological studies in Ppfia3 knockout mice found impaired presynaptic active zone assembly, synaptic vesicle docking, tethering, and exocytosis.27 Altogether, these studies reveal that Ppfia family members are integral scaffolding proteins for the assembly of intricate protein complexes involved in synapse formation, synaptic transmission, and protein trafficking.
Here, we report a cohort of 20 individuals from 18 families with rare variants in PPFIA3 associated with developmental delay (DD), ID, dysmorphisms, microcephaly, macrocephaly, hypotonia, ASD or autistic features, abnormal electroencephalogram (EEG), and epilepsy. The phenotypic consequences of rare variants in PPFIA3 have not been previously reported in Online Mendelian Inheritance in Man (OMIM).35 In a statistical model of de novo variants for ASDs/IDs, PPFIA3 was identified as one of ∼1,000 genes significantly lacking functional variation in non-ASD/ID individuals but enriched with de novo variants in individuals with ASD/ID.36 Furthermore, Genome Aggregation Database (gnomAD) version 2.1.1 analysis showed that PPFIA3 has a high probability of loss-of-function (LOF) intolerance (LOEUF = 0.12, pLI = 1.0), as 64.1 LOF variants were expected given the gene size and guanine-cytosine (GC) content, but only three LOF variants were observed.37 PPFIA3 is also a highly constrained gene with a missense Z score of 5.49, suggesting intolerance to missense variation, as 727.5 missense variants were expected but only 311 were observed.37 Together, these findings support that rare PPFIA3 variants may cause a neurodevelopmental phenotype.
The pathogenicity of the five PPFIA3 missense variants were tested using overexpression fly assays, revealing that the PPFIA3 variants are associated with behavioral, developmental, and NMJ defects. LOF assays with fly Liprin-α show that the human PPFIA3 wild type (WT) partially rescued the Liprin-α LOF embryonic lethality whereas three of the five tested variants exhibited impaired rescue of the LOF phenotype. Altogether, we show that rare PPFIA3 variants are deleterious to protein function with in vivo fruit fly assays and lead to a syndromic neurodevelopmental disorder characterized by DD, ID, hypotonia, ASD or autistic features, dysmorphisms, microcephaly or macrocephaly, abnormal EEG, and epilepsy in humans.
Material and methods
Study approval for identification of study participants and clinical phenotyping
Clinical data were acquired after written informed consent was obtained in accordance with the ethical standards of the participating institutional review boards (IRBs) on human research at each respective institution. GeneMatcher was used to form an international collaboration, allowing for comparison of individuals and their variants.38,39,40 Collection and analysis of the de-identified clinical cohort was approved by Baylor College of Medicine’s IRB. PPFIA3 heterozygous variants were identified by exome sequencing (ES) through each individual’s respective institution. DNA was extracted from peripheral blood mononuclear cells, buccal sample, or fetal skin for ES. ES or Sanger sequencing of the parental samples were performed when feasible to confirm de novo or inherited segregation. Paternity was confirmed by the inheritance of rare single-nucleotide polymorphisms from the parents. Sample swap was excluded. Participant identities are not known to anyone outside of the research group. Clinical phenotypes are ascertained by expert review of medical records and the most recent clinical assessment per each individual.
Molecular modeling
Molecular visualization of the PPFIA3 structure was completed with PyMol (the PyMOL Molecular Graphics System, v.2.5.2 Schrödinger, LLC). The crystal structure of PPFIA3 (GenBank: NP_003651.1, Uniprot ID: O75145) was used to build the PPFIA3 structure model in PyMol. Affected residues were altered to the corresponding human variants, and the mutation effects were modeled alongside the native protein. The changes in the PPFIA3 structure were assessed by displaying local polar contacts and residue interactions before and after mutagenesis.
Drosophila melanogaster stocks and maintenance
All the fruit fly stocks used in this study were reared in standard cornmeal- and molasses-based fly food at room temperature (RT, 20°C–21°C) unless otherwise noted. The fruit fly stocks used in the study were either obtained from Bloomington Drosophila Stock Center (BDSC) or generated at the Jan and Dan Duncan Neurological Research Institute. We generated transgenic fly alleles as previously described41 by utilizing the pUASg-HA-attB vector42 to express the human PPFIA3 WT and variant cDNAs with a region encoding a C-terminal hemagglutinin (HA) tag under the control of upstream activating sequence (UAS) elements by Gateway LR Cloning (LR Clonase II, Thermo Fisher Scientific, Cat #11791020). To generate the PPFIA3 variants, we utilized the human full-length cDNA of PPFIA3 (GenBank: NM_003660.4). PPFIA3 c.115C>T (p.Arg39Cys), PPFIA3 c.943G>T (p.Ala315Ser), PPFIA3 c.1243C>T (p.Arg415Trp), PPFIA3 c.1638G>T (p.Trp546Cys), and PPFIA3 c.2350C>T (p.Arg784Trp) were generated by Q5 site-directed mutagenesis (New England Biolabs, Cat #M0491S) in the pDONR221 Gateway compatible donor vector. The constructs were confirmed by Sanger sequencing. Primer sequences for the site-directed mutagenesis and Sanger sequencing are listed in Table S1. Human PPFIA3 WT and variant cDNAs were inserted into the chromosome-3 VK33 (PBac{y[+]-attP}VK00033) docking site by φC31-mediated recombination for fruit fly transgenesis.42 Transgenic UAS fly alleles generated in this study include UAS-PPFIA3-WT-HA, UAS-PPFIA3-p.Arg39Cys-HA, UAS-PPFIA3-p.Ala315Ser-HA, UAS-PPFIA3-p.Arg415Trp-HA, UAS-PPFIA3-p.Trp546Cys-HA, and UAS-PPFIA3-p.Arg784Trp-HA. Fly alleles from the stock centers include Liprin-αF3ex15/In(2LR)Gla (BDSC#8563), w[1118]; Df(2L)Exel7027/CyO (BDSC#7801), y[1] w[118]; PBac{y[+]-aatP-3B}-VK00033 (BDSC#9750), and elav-GAL4/CyO (BDSC#8765). UAS-empty-VK33, Actin-GAL4, and da-GAL4 lines were obtained from Dr. Hugo J. Bellen.
Larval brain and NMJ immunostaining and confocal microscopy
Fruit fly larval brains or whole-body wall muscles including the central nervous system were dissected from wandering third-instar larvae reared at 25°C in ice-cold 1X-PBS and fixed in 4% paraformaldehyde for 20 min at RT. The tissues were washed four times in Tri-PBS (1X-PBS +0.2% Triton X-100) with 1% bovine serum albumin (BSA) for 15 min each followed by incubation in blocking solution (Tri-PBS with 0.1% BSA and 8% normal donkey serum) for 30 min. Primary antibodies, rat anti-HA (1:50, clone 3F10, Millipore Sigma, Cat#11867423001), mouse anti-elav (1:100, Developmental Studies Hybridoma Bank, Cat#9F8A9), mouse anti-Bruchpilot (Brp) (1:50, Developmental Studies Hybridoma Bank, Cat#nc82), and goat anti-horseradish peroxidase (HRP) (1:1000, Jackson ImmunoResearch, Cat#123-005-021) were diluted in blocking solution, added to the tissues, and incubated overnight at 4°C. The tissues were rinsed three to four times in Tri-PBS with 1% BSA for 15 min each followed by incubation in blocking solution for 30 min at RT. The secondary antibodies, donkey anti-rat IgG antibody (Cy3) (1:300, Jackson ImmunoResearch, Cat#712-165-153), Alexa Fluor 488 Affinipure donkey anti-goat IgG (H + L) (1:300, Jackson ImmunoResearch, Cat#705-545-147), and Alexa Fluor 488 Affinipure donkey anti-mouse IgG (H + L) (1:300, Jackson ImmunoResearch, Cat#715-545-151) were diluted in blocking solution and added to the tissues for a 90-min incubation at RT on a rocker. For NMJ staining, phalloidin (Phalloidin-iFluor 405 Reagent, Abcam, Cat#ab176752) was added along with the secondary antibodies to visualize the muscles. After removing the secondary antibody, tissues were washed three times in Tri-PBS with 1% BSA for 15 min each and then rinsed in 1X-PBS at RT. For larval brains, this was followed by incubation in 406-diamidino-2-phenylindole dihydrochloride (DAPI, 1 mg/mL, Cayman Chemical, Cat#14285) for 30 min at RT. After removing DAPI, a final wash was completed with 1X-PBS for 15 min at RT. The tissues were mounted in Prolong Glass anti-fade mountant (Thermo Scientific, Cat#36984). Images were acquired on a Leica Sp8 laser-scanning confocal microscope. The same settings for laser power and detector gain were used for all genotypes. Third-instar larval brain images were acquired as a z stack with a z step of 1 μm and line average of four at 400 Hz with a 20× objective at 1024 × 1024 pixel resolution. NMJ images were acquired with a 40× objective. Maximum intensity projections were created from the z stack in ImageJ. All images were processed and assembled using ImageJ and Adobe Illustrator.
Western blotting
Adult fly heads were homogenized using cell lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.25% SDS, 0.25% sodium deoxycholate, 1 mM EDTA, and 1X liquid protease inhibitor [Gen DEPOT Cat#P3100-001]). The homogenized fly heads in cell lysis buffer were centrifuged at 13,000 revolutions per minute (rpm) for 10 min at 4°C. The supernatant was collected and mixed with Laemmli buffer containing β-mercaptoethanol and heated at 95°C for 10 min. The samples were loaded in 4%–20% gradient polyacrylamide gels (Bio-Rad MiniPROTEAN TGXTM Cat#4561086) followed by a transfer onto polyvinylidene difluoride membrane (Bio-Rad TransBlot Turbo mini-size LF PVDF membrane). The membrane was blocked using skim milk and treated overnight with the primary antibody (rat anti-HA 1:2000, clone 3F10, Millipore Sigma, Cat#11867423001). Anti-actin hFAB rhodamine antibody (Bio-Rad Cat#12004163, 1:5000) and goat anti-rat IgG polyclonal antibody (IRDye 800CW) (LI-COR Biosciences, Cat#926–32219) were used as the secondary antibodies. Images were acquired on the BioRad Chemidoc Imaging System (Cat#17001401). Quantification was done using ImageJ in which background-subtracted band intensity is acquired for both the actin and HA bands. The average intensity value is normalized and analyzed in GraphPad Prism8. Crosses for the western blot analysis were set up and maintained at 25°C.
Third-instar larval NMJ quantifications
The total number of boutons from abdominal segment 3 (A3) muscle 6/7 were counted semi-manually using Imaris. The spot function was used with point style sphere and radius scale 1.0 to count the number of boutons. The NMJ length was quantified using the HRP staining and measured in ImageJ.
Fruit fly behavioral assays
For the climbing assay, 5-day-old flies of both sexes were anesthetized with CO2 48 h prior to being tested, and two to three flies were housed in food-containing vials at 25°C. At the time of assay, these flies were transferred without anesthesia to a clear graduated cylinder with a 15-cm mark. The flies were tapped three times to the bottom of the cylinder to examine the climbing ability. The cutoff time to reach the 15-cm mark was 30 s. A total of 55–75 flies of both sexes were tested for each genotype. Crosses for the climbing assay were set up at 25°C and the assay was performed at 20°C–21°C. The climbing assay for 15-day-old flies was done with the flies anesthetized with CO2 24 h prior to being tested and kept at RT until the behavioral test was done. The cutoff time for the 15-day-old flies to reach the 20-cm mark was 40 s. A total of 40–55 flies of both sexes were tested for each genotype for the 15-day-old flies.
For the bang-sensitivity assay, 5-day-old flies of both sexes were anesthetized with CO2 48 h prior to being tested, and two to three flies were housed in food-containing vials at 25°C. At the time of assay, these flies were transferred without anesthesia to an empty food vial and vortexed for 10 s. Flies were observed for time to recover from the vortexing. The cutoff time to recover was 30 s. Recovery was defined as being upright and mobile. Flies were considered bang sensitive if they remained upside down, immobile, or showed rhythmic involuntary movements suggestive of electrophysiological abnormalities in the nervous system. A total of 55–75 flies of both sexes were tested for each genotype. Crosses for the bang-sensitivity assay were set up at 25°C, and the assay was performed at 20°C–21°C. The bang-sensitivity assay for 15-day-old flies was done with the flies anesthetized with CO2 24 h prior to being tested and kept at RT until the behavioral test was done. The vortexing time for the 15-day-old flies was 15 s. A total of 40–55 flies of both sexes were tested for each genotype for the 15-day-old flies.
Liprin-α LOF lethality rescue with human PPFIA3 WT and variants
Df(Liprin-α)/CyO act-GFP; UAS-cDNA/TM6B flies were crossed with Liprin-α F3ex15/CyO act-GFP; da-GAL4/TM6B flies. The UAS-cDNA lines used were UAS-empty, UAS-PPFIA3 WT, UAS-PPFIA3 p.Arg39Cys, UAS-PPFIA3 p.Arg415Trp, UAS-PPFIA3 p.Trp546Cys, and UAS-PPFIA3 p.Arg784Trp. Rescue larvae with the genotype Df(Liprin-α)/Liprin-α F3ex15; UAS-cDNA/da-GAL4 were selected (GFP negative and Tubby [Tb] negative) and kept in a new vial to assess the development. The experiment was done in three biological replicates. The crosses were set and maintained at 20°C as the higher temperatures (25°C and above) were embryonic lethal with all cDNAs.
Pupal lethality and eclosion defect assessment
Actin-GAL4/Cyo,Tb females were crossed with the homozygous UAS-PPFIA3 WT and UAS-PPFIA3 p.Arg39Cys, p.Ala315Ser, p.Arg415Trp, p.Trp546Cys, and p.Arg784Trp males at 25°C. The overexpression progenies were identified based on the absence of the markers, CyO (visible in adults) and Tb (visible in larvae, pupae, and adults). Sample sizes are shown in Table S2.
Adult fruit fly leg mounting
Adult flies were fixed overnight in ethanol at RT, and the legs were dissected and mounted using CMCP-10 Macroinvertebrate High Viscosity Mountant (D/S259) (Electron Microscopy Sciences, Cat#18004-02). Leg images were taken using the Leica MZ16 stereomicroscope. Images were processed and assembled using Adobe Photoshop CS5.1 and Adobe Illustrator. Crosses were set up at 25°C. Sample sizes are shown in Table S2.
Genomic DNA isolation and qPCR
Genomic DNA was extracted by homogenizing four whole flies in 50 mM sodium hydroxide and heating the samples at 95°C for 30 min followed by the addition of 1 M Tris-HCl (pH 7.5) to stop the lysis. Equal amounts (50 ng) of DNA for each genotype were used for amplification. qPCR was performed with the BioRad SsoAdvanced Universal SYBR-Green Supermix (Cat#1725274) and the BioRad CFX96 Touch Real-Time PCR Detection System (Cat#1845096). The relative change in gene expression was determined by the Livak method, and fold changes were calculated using the 2−ΔΔCT formula. The experiment was repeated in three independent biological replicates. HA and PPFIA3 band intensity were quantified by normalizing to the band intensity of the endogenous reference rps17 and plotted as fold change relative to the control. Flies were maintained at 20°C–21°C. Primer sequences are listed in Table S1.
Statistics
Data was collected and analyzed blinded to genotypes. Statistical analysis between the control and experimental groups was conducted with one-way ANOVA and Tukey’s post-hoc analysis in GraphPad Prism 8. Statistical summary is in Table S3.
Results
Identification of rare PPFIA3 variants in individuals with neurodevelopmental phenotypes
An international collaboration through the Undiagnosed Diseases Network (UDN)43 and GeneMatcher38,39,40 led to the identification of 20 individuals from 18 families with neurodevelopmental phenotypes and 17 rare missense, frameshift deletion, exonic deletion, or consensus splice site variants in PPFIA3 (Table 1; Figures 1A and 1B, see supplemental information). The cases were ascertained in individuals with phenotypes including DD, ID, ASD or autistic features, epilepsy, abnormal EEG, hypotonia, dysmorphisms, microcephaly, and macrocephaly. Heterozygous PPFIA3 variants were identified in nineteen individuals (1–19). One individual (20) was found to harbor compound heterozygous variants with concordant phenotypes. The variants from all affected individuals were identified through ES or Sanger sequencing.
Table 1.
Genetic and neurologic findings in individuals with rare PPFIA3 variants and neurodevelopmental disorders
| Individual | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Family | F1 | F2 | F3 | F4 | F5 | F5 | F6 | F7 | F8 | F9 |
| cDNA (GenBank: NM_003660.4) | c.115C>T | c.115C>T | c.118G>A | c.239A>C | c.240+1G>A | c.240+1G>A | c.943G>T | c.1243C>T | c.1243C>T | c.1285C>T |
| Protein (GenBank: NP_003651.1) | p.Arg39Cys | p.Arg39Cys | p.Glu40Lys | p.Gln80Pro | N/A | N/A | p.Ala315Ser | p.Arg415Trp | p.Arg415Trp | p.Arg429Trp |
| Human reference genome | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) |
| Variant inheritance | de novo | de novo | not from mother; father unavailable for testing | unknown | inherited from affected mother (individual 6) | unknown | de novo | de novo | unknown | de novo |
| gnomAD (v.2.1.1) | not present | not present | not present | not present | not present | not present | not present | not present | not present | not present |
| Mosaicism | no | no | no | no | no | no | no | no | no | yes |
| Sex | male | male | female | female | female | female | female | female | female | female |
| Age at most recent assessment | 16 years | 13 years | 22 years | 1 year and 10 months | 5 years | 35 years | neonatal | 8 years | 10 years and 9 months | N/A |
| Racial and ethnic categories (NIH) | White | family declined to answer | White | White | White | White | Latino | mixed European and Asian | Asian | White |
| Status | alive | alive | alive | alive | alive | alive | deceased | alive | alive | elective pregnancy termination |
| Abnormal EEG | yes | yes | yes | N/A | N/A | N/A | N/A | yes | yes | N/A |
| Epilepsy | yes | yes | yes | no | no | no | N/A | yes | N/A | N/A |
| Autism or autistic features | no | no | no | suspected | no | N/A | N/A | autistic features | autistic features | N/A |
| Dysmorphisms | yes | yes | yes | yes | yes | yes | yes | yes | yes | N/A |
| Individual | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| Family | F10 | F11 | F12 | F13 | F13 | F14 | F15 | F16 | F17 | F18 |
| cDNA (GenBank: NM_003660.4) | c.1492C>T | c.1638G>T | c.2350C>T | c.2609T>A | c.2609T>A | c.2706dup | c.2717C>T | c.3307del | deletion exons 22–30 | c.[2377C>A]; [c.2276A>G] |
| Protein (GenBank: NP_003651.1) | p.Arg498Trp | p.Trp546Cys | p.Arg784Trp | p.Ile870Asn | p.Ile870Asn | p.Ser903Leu fs∗86 | p.Ser906Leu | p.Glu1103Asnfs∗8 | N/A | p.[Pro793Thr]; p.[Lys759Arg] |
| Human reference genome | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh38 (hg38) | GRCh37 (hg19) |
| Variant inheritance | de novo | de novo | de novo | de novo (monozygotic twin of individual 15) | de novo monozygotic twin of individual 14) | unknown | de novo | unknown | inherited, affected mother | inherited from unaffected mother and father |
| gnomAD (v.2.1.1) | not present | not present | frequency of 3.19 × 10−5 (1/31,386) | not present | not present | not present | not present | not present | N/A | p.Pro793Thr frequency of 7.61 × 10−4 (215/282,366); p.Lys759Arg frequency of 4.77 × 10−5 (13/282,880) |
| Mosaicism | no | no | no | no | no | N/A | no | no | N/A | none |
| Sex | male | male | female | female | female | male | female | female | male | male |
| Age at most recent assessment | 6 years and 11 months | 11 years | 16 years | 5 years | 5 years | 23 years | 13 years and 11 months | 9 years and 9 months | 7 years and 8 months | 9 years |
| Racial and ethnic categories (NIH) | Asian | White | White | White | White | White | White | White | White | White |
| Status | alive | alive | alive | alive | alive | alive | alive | alive | alive | alive |
| Abnormal EEG | N/A | no | yes | N/A | N/A | N/A | yes | yes | no | yes |
| Epilepsy | no | no | yes | no | no | N/A | no | no | no | yes |
| Autism or autistic features | yes, autistic features improved over last few years | yes | no | yes | no | autistic features | yes | no | N/A | autistic features |
| Dysmorphisms | Yes | no | no | no | no | N/A | yes | yes | yes | yes |
Abbreviations: electroencephalogram (EEG), no information available (N/A).
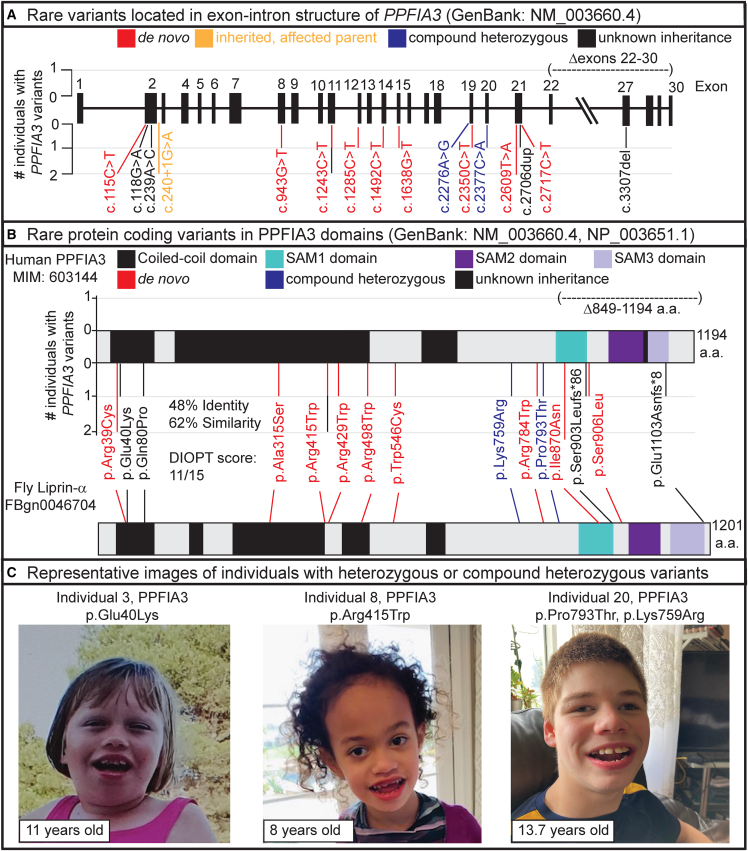
Figure 1.
Variant location and images of individuals with PPFIA3 variants
(A) Location of PPFIA3 variants in the genomic locus corresponding to the exon-intron structure. Number of individuals with the rare PPFIA3 variant shown in the y axis.
(B) Location of PPFIA3 variants in the corresponding protein domains. Number of individuals with the variant shown in the y axis. The fruit fly ortholog Liprin-α shows 48% identity and 62% similarity with the human PPFIA3. Sterile alpha motif, SAM.
(C) Images of individuals with heterozygous or compound heterozygous PPFIA3 variants. The three individuals shown have dysmorphic features such as wide mouth, widely spaced teeth, prominent forehead, and hypotonic facies.
Eleven of the individuals harbored de novo (1–2, 7–8, 10–15, and 17) missense variants. The de novo p.Arg429Trp variant was seen in individual 10 in a mosaic state (present in 26% of the ES reads, suggesting heterozygosity in ∼52% of cells) from DNA analysis of fetal skin. Individuals 14 and 15 are monozygotic twins from family 13, and one individual (5) inherited a consensus splice variant from a similarly affected parent (6) (both from family 5). Individual 19 inherited a deletion of exons 22–30 from an affected parent, individual 20 inherited compound heterozygous variants from unaffected parents, and the inheritance pattern for six affected individuals is unknown (3, 4, 6, 9, 16, and 18) (Table 1, see supplemental information). The individuals with unknown inheritance pattern have either a missense variant (3, 4, and 9), a consensus splice variant (6), or a frameshift deletion (16 and 18).
To determine the potential pathogenicity of the PPFIA3 variants, we examined the combined annotation-depletion (CADD) score where scores above 20 are considered to be deleterious.44 The CADD scores for the PPFIA3 variants ranged from 22.6 to 54, suggesting they are potentially deleterious (Table 2). Fourteen out of 17 variants were absent from the gnomAD (v.2.1.1).37 The p.Arg784Trp variant had a frequency of 3.19 × 10−5 (1/31,386) in gnomAD v.2.1.1 and was identified as a de novo finding in individual 13 with mild ID and Landau-Kleffner epilepsy syndrome. The p.Pro793Thr variant had a frequency of 7.61 × 10−4 (215/282,366) in gnomAD v.2.1.1 and was identified as a maternally inherited variant in trans with a paternally inherited p.Lys759Arg variant in individual 20 with DD, ID, hypotonia, epilepsy, microcephaly, and autistic features. The p.Lys759Arg variant has a frequency of 4.77 × 10−5 (13/282,880) in gnomAD v.2.1.1. Differences in variant frequencies were documented in gnomAD v.4.0.0, encompassing variants reported in this study that were either submitted to ClinVar or identified from the UK Biobank (Tables S4 and S5).
Table 2.
In silico predictions for PPFIA3 variants
| Individuals | 1 and 2 | 3 | 4 | 5 and 6 | 7 | 8 and 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|
| Human reference genome | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) |
| PPFIA3 variant cDNA (GenBank: NM_003660.4) | c.115C>T | c.118G>A | c.239A>C | c.240+1G>A | c.943G>T | c.1243C>T | c.1285C>T | c.1492C>T |
| PPFIA3 variant protein (GenBank: NP_003651.1) | p.Arg39Cys | p.Glu40Lys | p.Gln80Pro | N/A | p.Ala315Ser | p.Arg415Trp | p.Arg429Trp | p.Arg498Trp |
| REVEL (v.1) | 0.316 | 0.271 | 0.338 | N/A | 0.218 | 0.21 | 0.295 | 0.273 |
| CADD (v.1.6) | 31 | 30 | 26.8 | 34 | 24 | 33 | 29.2 | 25.9 |
| GERP | 4.19 | 4.19 | 4.34 | 4.34 | 4.29 | 2.12 | 2.95 | 1.41 |
| M-CAP (v.1.4) | possibly pathogenic | possibly pathogenic | possibly pathogenic | N/A | possibly pathogenic | likely benign | possibly pathogenic | possibly pathogenic |
| PolyPhen2 HumDiv | probably damaging | probably damaging | benign | N/A | benign | probably damaging | probably damaging | probably damaging |
| PolyPhen2 HumVar | probably damaging | probably damaging | benign | N/A | benign | probably damaging | probably damaging | probably damaging |
| Phylop Vertebrate | 0.945 | 9.447 | 9.02 | N/A | 9.4308 | 0.0498 | 2.25 | 2.58 |
| SIFT | damaging | damaging | damaging | N/A | damaging | damaging | damaging | damaging |
| Individuals | 12 | 13 | 14 and 15 | 16 | 17 | 18 | 19 | 20 | 20 |
|---|---|---|---|---|---|---|---|---|---|
| Human reference genome | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh37 (hg19) | GRCh38 (hg38) | GRCh37 (hg19) | GRCh37 (hg19) |
| PPFIA3 variant cDNA (GenBank: NM_003660.4) | c.1638G>T | c.2350C>T | c.2609T>A | c.2706dup | c.2717C>T | c.3307del | deletion exons 22–30a | c.2377C>A | c.2276A>G |
| PPFIA3 variant protein (GenBank: NP_003651.1) | p.Trp546Cys | p.Arg784Trp | p.Ile870Asn | p.Ser903Leu fs∗86 | p.Ser906Leu | p.Glu1103Asnfs∗8 | N/A | p.Pro793Thr | p.Lys759Arg |
| REVEL (v.1) | 0.186 | 0.158 | 0.41 | N/A | 0.207 | N/A | N/A | 0.092 | 0.183 |
| CADD (v.1.6) | 25.6 | 26.5 | 31 | N/A | 29.3 | 54 | 49 | 22.6 | 26.8 |
| GERP | 3.87 | 3.63 | 4.45 | 4.45 | 3.31 | 4.29 | N/A | 4.91 | 4.17 |
| M-CAP (v.1.4) | possibly pathogenic | possibly pathogenic | possibly pathogenic | N/A | likely benign | N/A | N/A | possibly pathogenic | possibly pathogenic |
| PolyPhen2 HumDiv | probably damaging | probably damaging | probably damaging | N/A | tolerated | N/A | N/A | probably damaging | probably damaging |
| PolyPhen2 HumVar | probably damaging | benign | probably damaging | N/A | probably damaging | N/A | N/A | probably damaging | probably damaging |
| Phylop Vertebrate | 4 | 1.609 | 7.855 | N/A | 5.756 | N/A | N/A | 3.7 | 9.17 |
| SIFT | tolerated | damaging | damaging | N/A | damaging | N/A | N/A | damaging | damaging |
Abbreviations: combined annotation-dependent depletion (CADD); genomic evolutionary rate profiling (GERP); Mendelian clinically applicable pathogenicity (M-CAP); polymorphism phenotyping v2 (PolyPhen2); sorting intolerant from tolerant (SIFT); not applicable (N/A)ano further information is available for the breakpoints for deletion in Individual 19.
Eighteen individuals in the cohort had DD and ID (1–6, 8–9, 11–20) (Tables S4 and S5, see supplemental information), while two individuals (7 and 10) could not be assessed for this feature due to premature mortality. Individual 7 had renal failure, severe anorectal malformation with complete anal atresia, absent bladder, dysmorphisms, and passed away at 5 months of age. Individual 10 had a prenatal diagnosis of abnormal gyration and ventriculomegaly, which led to elective pregnancy termination. Abnormal EEG (seen in 9/20; 1–3, 8, 9, 13, 17, 18, 20) and epilepsy (seen in 6/20; 1–3, 8, 13, 20) were found in a total of nine individuals (Table 1, see supplemental information). The affected individuals had multiple seizure semiologies including focal clonic seizures, atonic seizures, absence seizures, and focal tonic-clonic seizures with secondary generalization (Tables S4 and S5, see supplemental information). Six individuals had neuroanatomical changes detected by magnetic resonance imaging (MRI) (2, 3, 8, 10, 15, 20), which included flattening of the posterior globes at the level of optic nerve insertion, abnormal gyration with ventriculomegaly, and mild periventricular leukomalacia with mild white matter volume loss (Tables S4 and S5). Delayed speech development was present in 16 individuals (1–5, 8, 9, 11, 13–20) with absent speech in two individuals (8 and 20) (Table S4. General information and clinical findings in individuals 1–10 with PPFIA3 variants, Table S5. General information and clinical findings in individuals 11–20 with PPFIA3 variants, Table S6. Motor, language, and social milestones in individuals 1–20, see supplemental information). Hypotonia was present in eight individuals (1, 4, 8, 15, 17–20) (Tables S4 and S5, see supplemental information). Co-morbid ASD diagnosis was reported in four individuals (11, 12, 14, 17) (Table 1, see supplemental information). Five of the individuals had autistic features, but no formal diagnosis of autism was made (4, 8, 9, 16, 20) (Table 1, see supplemental information). Gastrointestinal dysmotility characterized by constipation, difficulty feeding, and dysphagia was present in ten individuals (1, 3, 4, 7–9, 13–15, 20) (Tables S4 and S5, see supplemental information). Dysmorphic facial features were described in 13 individuals, which included prominent forehead, plagiocephaly, triangular face, clinodactyly, strabismus, wide mouth, widely spaced teeth, and bilateral epicanthal folds (1–5, 7–9, 11, 17–20) (Tables 1, S4 and S5; Figure 1C, see supplemental information). Macrocephaly or microcephaly were present in nine individuals (5–8, 14, 15, 17, 19, 20) (Tables S4 and S5, see supplemental information).
Conservation analysis and molecular modeling of PPFIA3 variants
The predicted pathogenicity of PPFIA3 variants was validated in vivo using D. melanogaster (fruit fly). The fly ortholog of PPFIA3 is Liprin-α, and the fly protein shows an overall 48% identity and 62% similarity with the human protein (Figure 1B). Like the human PPFIA3, the fruit fly Liprin-α contains N-terminal coiled-coil domains and three C-terminal SAM domains (Figure 1B). Seven of the variants, p.Arg39Cys, p.Glu40Lys, p.Gln80Pro, p.Ala315Ser, p.Arg415Trp, p.Arg429Trp, and p.Arg498Trp, are located in the N-terminal coiled-coil domain (Figure 1B). The variants p.Ile870Asn and p.Ser903Leufs∗86 are located in the SAM1 domain (Figure 1B). The variants p.Trp546Cys, p.Lys759Arg, p.Arg784Trp, p.Pro793Thr, and p.Ser906Leu are in the intrinsically disordered region of the protein; however, p.Ser906Leu is located near the SAM1 domain (Figure 1B). Conservation analysis of p.Arg39, p.Gln80, p.Ala315, p.Arg415, and p.Arg429 reveals these coiled-coil domain residues are well conserved in invertebrates and vertebrates. However, p.Glu40 is only conserved in mice and p.Arg498 is only conserved in mice and worms (Figure S1). The affected residues in the SAM1 domain, p.Ile870 and p.Ser903, and the residue near the SAM1 domain, p.Ser906, are also conserved across species. In the intrinsically disordered region, p.Trp546 is conserved in mice but not in fruit flies and worms; p.Lys759 is conserved in mice and fruit flies; p.Arg784 is conserved only in mice; and p.Pro793 is conserved only in mice (Figure S1). The affected variant in the SAM3 domain, p.Glu1103, is conserved in mice and fruit flies but not in worms (Figure S1).
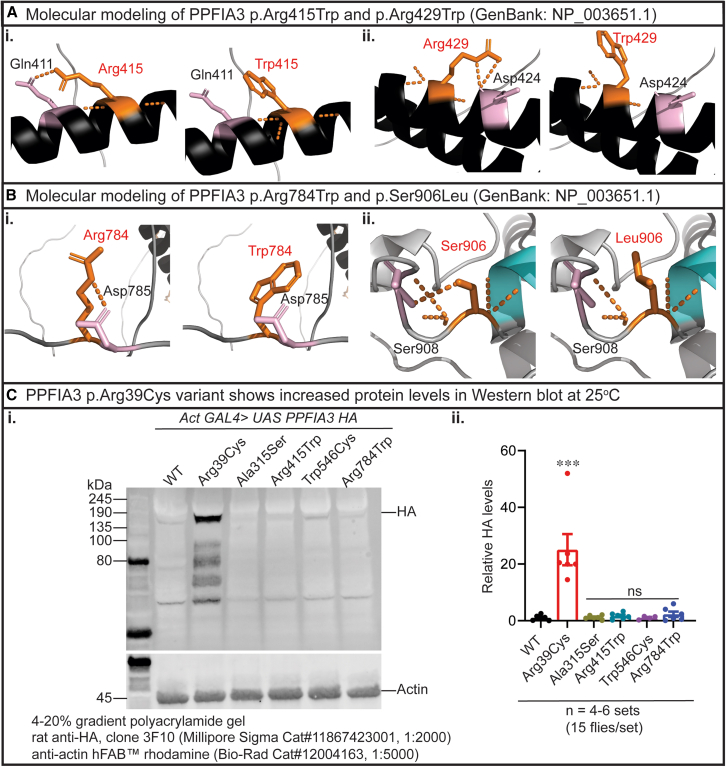
Molecular modeling was completed for the missense variants using PyMol to determine whether the amino acid changes affect protein function in silico (Figures 2A and 2B). Regarding the coiled-coil variants, the variant p.Arg415Trp introduces a bulky side chain predicted to disrupt the interaction with p.Gln411 (Figure 2Ai). Similarly, the variant p.Arg429Trp introduces a bulky side chain that may disrupt the interaction with p.Asp424 (Figure 2Aii). The variant p.Arg784Trp introduces a bulky side chain predicted to disrupt the polar interaction with p.Asp785 (Figure 2Bi). The variant p.Ser906Leu is near the SAM1 domain and disrupts the interaction with the neighboring residue p.Ser908 (Figure 2Bii). Together, the molecular modeling suggests that these rare variants may hinder PPFIA3 function by disrupting the polar interactions with neighboring residues.
Figure 2.
Molecular modeling of PPFIA3 missense variants and protein levels associated with PPFIA3 variants
(A and B) PPFIA3 missense variants are modeled in PyMol (version 2.5.2) with GenBank: NP_003651.1. Human PPFIA3 WT residues are modeled in gray with coiled coils displayed in black and affected residues highlighted in orange. Local polar contacts (orange dashed lines) and residue interactions (highlighted in pink) are displayed before and after mutagenesis for (Bi) p.Arg415Trp, (Bii) p.Arg429Trp, (Ci) p.Arg784Trp, and (Cii) p.Ser906Leu.
(C) (i) Western blot from PPFIA3 WT and variants show higher levels of HA in PPFIA3 p.Arg39Cys compared to WT.
(ii) Quantification of relative HA in 4–6 sets of biological replicates show higher level of HA in PPFIA3 p.Arg39Cys flies. Statistical analysis conducted with one-way ANOVA and Tukey’s post-hoc analysis. Data shown as mean ± SEM. Significance shown as ∗∗∗p < 0.001. Non-significance shown as ns.
In vivo functional analysis of PPFIA3 missense variants in fruit flies
To study the functional consequences of PPFIA3 variants in vivo, we selected five of the missense variants to generate transgenic fruit flies using human cDNAs. We generated UAS-PPFIA3-WT-HA, UAS-PPFIA3-p.Arg39Cys-HA, UAS-PPFIA3-p.Ala315Ser-HA, UAS-PPFIA3-p.Arg415Trp-HA, UAS-PPFIA3-p.Trp546Cys-HA, and UAS-PPFIA3-p.Arg784Trp-HA fly alleles with C-terminal HA epitope tags (Table S7). The GAL4-UAS expression system was used to express PPFIA3 WT and variant cDNAs under the spatiotemporal regulation of the transactivator protein GAL4 (Figure S2A). A pan-neuronal driver on the second chromosome, elav-GAL4, was used to express PPFIA3 cDNAs in neurons, and a ubiquitous driver on the second chromosome, Actin-GAL4, was used to express PPFIA3 cDNAs in the whole fly (Figure S2A). We found that elav-GAL4 and Actin-GAL4 produced the HA-tagged PPFIA3 WT and variants in third-instar larval brains (Figure S2B) and adult fly heads (Figure 2C). Interestingly, we observed elevated protein levels for PPFIA3 p.Arg39Cys compared to PPFIA3 WT and the other missense variants (Figures 2Ci, 2Cii, and S3). To confirm the cDNA copy-number insertions are consistent between the PPFIA3 WT and variant fly lines, genomic DNA qPCR using SYBR Green was performed in UAS-PPFIA3-WT-HA, UAS-PPFIA3-p.Arg39Cys-HA, UAS-PPFIA3-p.Ala315Ser-HA, UAS-PPFIA3-p.Arg415Trp-HA, UAS-PPFIA3-p.Trp546Cys-HA, and UAS-PPFIA3-p.Arg784Trp-HA. Genomic DNA regions for HA epitope tag, PPFIA3, and rps17 were amplified, and the expression levels of either HA or PPFIA3 were quantified using rps17 as the internal control (Figures S2Ci–S2Cii). We also performed semi-quantitative genomic DNA PCR using Taq polymerase, and the PCR product band intensity was quantified using rps17 as the internal control (Figures S4 and S5). No significant difference in the relative expression of HA and PPFIA3 was observed by both genomic DNA qPCR and PCR analyses, indicating the cDNA copy number is similar across all the UAS-PPFIA3 WT and variant fly lines. Hence, the higher protein levels observed for p.Arg39Cys may be due to increased protein stability from the missense variant.
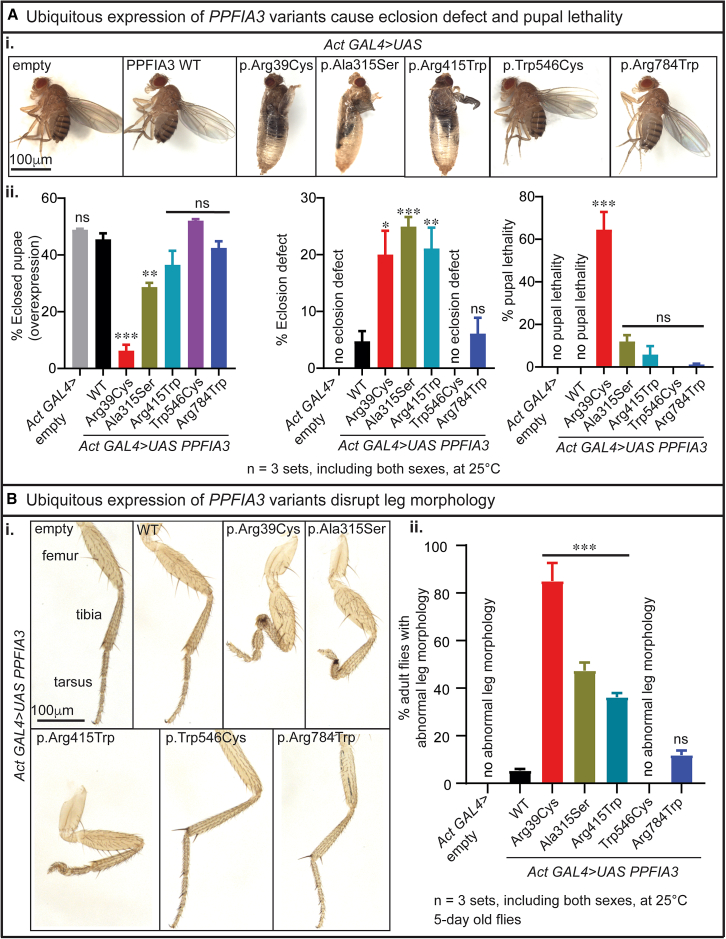
To determine whether expression of PPFIA3 WT and missense variants are deleterious to developmental processes, we ubiquitously expressed PPFIA3 cDNAs using Actin-GAL4 at 25°C and analyzed the fly development from pupal stage. PPFIA3 cDNAs were expressed in the presence of endogenous fly Liprin-α. We found that PPFIA3 p.Arg39Cys causes pupal lethality and eclosion defects, whereas p.Ala315Ser and p.Arg415Trp cause only eclosion defect (Figures 3Ai and 3Aii). However, these phenotypes were not observed for the PPFIA3 p.Trp546Cys and p.Arg784Trp variants that are located in the intrinsically disordered region (Figures 3Ai–3Aii). In the eclosed adult flies, we observed a reduced penetrance of leg dysmorphology. The typical WT morphology is comprised of three pairs of legs with each leg containing three segments: femur, tibia, and tarsus (Figure 3Bi). We found morphological defects in these segments in either the first, second, third, or all leg pairs with expression of the PPFIA3 missense variants (Figure 3Bi). Leg dysmorphology was observed in 80% of PPFIA3 p.Arg39Cys flies, 50% of PPFIA3 p.Ala315Ser flies, and 40% of PPFIA3 p.Arg415Trp flies (Figures 3Bi–3Bii). In PPFIA3 p.Arg784Trp flies, 10% had leg defects but the phenotype was not significant compared to that of PPFIA3 WT flies (Figures 3Bi–3Bii). In contrast, the leg dysmorphology phenotype was absent in the PPFIA3 p.Trp546Cys flies (Figures 3Bi–3Bii).
Figure 3.
Actin-GAL4-mediated ubiquitous expression of PPFIA3 variants cause developmental and anatomical defects in fruit flies
(A) Pupal lethality and eclosion defect associated with Actin-GAL4-mediated overexpression of PPFIA3 variants.
(i) Images showing overexpression of PPFIA3 p.Arg39Cys cause pupal lethality and eclosion defect, and p.Ala315Ser and p.Arg415Trp cause eclosion defect compared to the PPFIA3 WT and UAS-empty control. Uneclosed flies from p.Arg39Cys, p.Ala315Ser, and p.Arg415Trp remain in the pupal case. PPFIA3 p.Trp546Cys and p.Arg784Trp overexpression does not cause a difference in pupal lethality and eclosion defect compared to PPFIA3 WT and UAS-empty control flies. Scale bar = 100 μm.
(ii) Bar graphs showing the percentage of eclosed pupae (overexpression), eclosion defect, and pupal lethal. Statistical analysis conducted with one-way ANOVA and Tukey’s post-hoc analysis. Data shown as mean ± SEM with the sample size of total number of pupae in three sets. Significance shown as ∗∗p < 0.01 and ∗∗∗p < 0.001. Non-significance shown as ns.
(B) Images of leg morphology associated with Actin-GAL4-mediated overexpression of PPFIA3 variants.
(i) Empty control and PPFIA3 WT flies have typical legs with three segments. PPFIA3 p.Arg39Cys, p.Ala315Ser, and p.Arg415Trp result in pronounced leg segment developmental defects compared to PPFIA3 WT. Mild leg segmental developmental defects found with PPFIA3 p.Arg784Trp but not significant compared to PPFIA3 WT. No leg defects were found in PPFIA3 p.Trp546Cys flies. Scale bar = 100 μm.
(ii) Bar graph showing the percentage of flies with abnormal leg morphology. Statistical analysis conducted with one-way ANOVA and Tukey’s post-hoc analysis. Data shown as mean ± SEM with the sample size of total number of adult flies in three sets. Significance shown as ∗∗∗p < 0.001. Non-significance shown as ns.
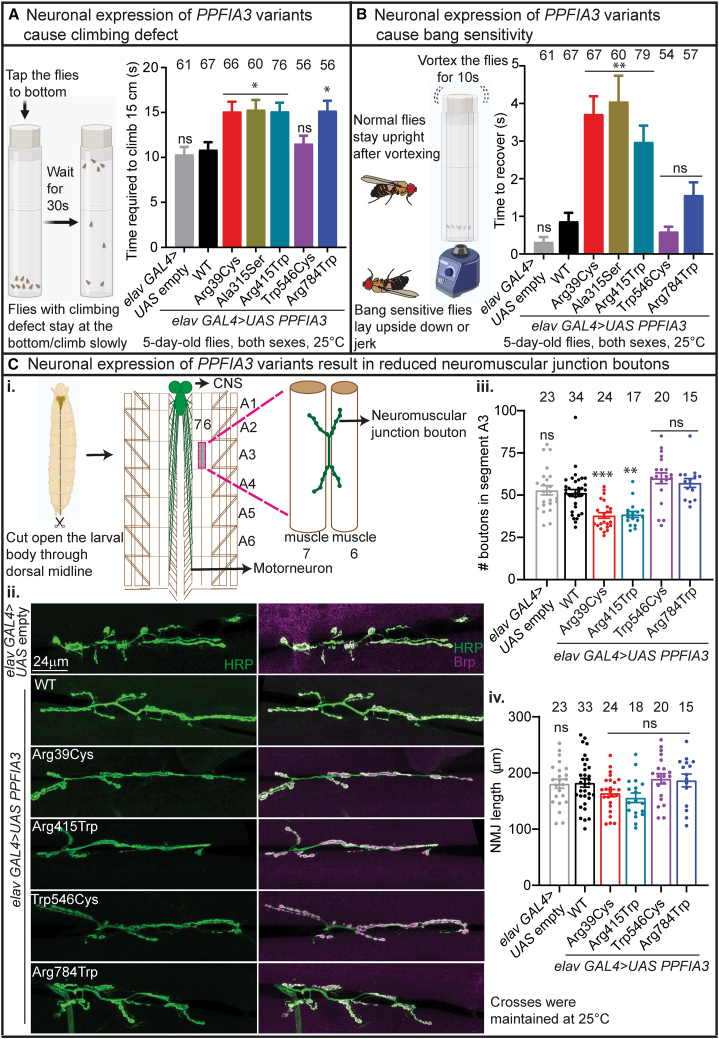
Next, to determine whether the neuronal expression of PPFIA3 variants by elav-GAL4 at 25°C impaired nervous system development and function, we conducted climbing behavior, bang-sensitivity behavior, and NMJ-morphology assays. First, we performed a climbing assay in 5-day-old flies to assess for motor defects. The standard behavior of the flies is to climb upward, and any increase in time to climb represents a potential defect in either motor coordination or negative geotaxis. Therefore, we used the climbing assay primarily as a screening tool to assess motor function. We found that elav-GAL4>UAS-PPFIA3 WT flies had motor function like control flies that do not produce human PPFIA3 (elav-GAL4>UAS-empty) (Figure 4A). However, climbing behavior was impaired in PPFIA3 p.Arg39Cys-, p.Ala315Ser-, p.Arg415Trp-, and p.Arg784Trp-expressing 5-day-old flies (Figure 4A). Second, to determine whether the PPFIA3 variants have electrophysiological abnormalities in the nervous system, we used bang sensitivity as a screening tool in the 5-day-old flies with 10-s vortexing. We found that elav-GAL4>UAS-PPFIA3 WT flies were not bang sensitive and recovered similarly to the elav-GAL4>UAS-empty control. However, p.Arg39Cys, p.Ala315Ser, and p.Arg415Trp flies exhibited bang sensitivity with an increased recovery time (Figure 4B). When we examined the recovery time by sex, we found that p.Arg39Cys shows increased recovery time in males (Figure S6A), p.Ala315Ser shows increased recovery time both in males and females (Figure S6A), and p.Arg415Trp shows increased recovery time in females (Figure S6A). To determine whether there is an age-dependent effect, the climbing and bang assays were also performed in 15-day-old flies. We found that both p.Arg39Cys and p.Arg415Trp flies had climbing defects (Figure S6B). The bang-sensitivity assay for 15-day-old flies with 15-s vortexing showed recovery times similar to the bang-sensitivity assay in the 5-day-old flies (Figure S6B). Third, to explore the consequence of PPFIA3 variants at the synapse, we examined the fruit fly third-instar larval NMJ morphology in muscle 6/7 of A3 (Figures 4Ci–4Cii). The fly NMJ is a glutamatergic synapse and a well-established model for excitatory glutamatergic synapse development and function.45,46 We found a reduced number of boutons (presynaptic contacts) with elav-GAL4-mediated production of the PPFIA3 p.Arg39Cys and p.Arg415Trp variants (Figure 4Ciii), indicating that these variants perturb synapse formation. Total NMJ length associated with the PPFIA3 variants were like the PPFIA3 WT and UAS-empty controls (Figure 4Civ).
Figure 4.
elav-GAL4-mediated neuronal overexpression of PPFIA3 variants result in climbing defect, bang sensitivity, and neuromuscular junction (NMJ) bouton loss
(A) elav-GAL4 mediated neuronal expression of PPFIA3 p.Arg39Cys, PPFIA3 p.Ala315Ser, PPFIA3 p.Arg415Trp, and PPFIA3 p.Arg784Trp result in impaired motor coordination on the climbing assay compared to PPFIA3 WT and empty control flies. Crosses were set and maintained at 25°C. Behavioral testing was conducted at 20°C–21°C with both sexes.
(B) elav-GAL4-mediated neuronal expression of PPFIA3 p.Arg39Cys, PPFIA3 p.Ala315Ser, and PPFIA3 p.Arg415Trp have bang sensitivity with delayed recovery from vortexing compared to PPFIA3 WT and UAS-empty control flies. Crosses were set and maintained at 25°C. Behavioral test was conducted at 20°C–21°C with both sexes.
(C) elav-GAL4-mediated neuronal overexpression of PPFIA3 variants result in NMJ bouton loss without a significant change in NMJ length.
(i) Model depicting the method for visualizing the NMJ in fruit fly third-instar larva.
(ii) Representative images of third-instar larval NMJs of each genotype including elav GAL4>UAS empty, elav GAL4>PPFIA3 WT, p.Arg39Cys, p.Arg415Trp, p.Trp546Cys, and p.Arg784Trp are shown. Horseradish peroxidase (HRP) is a pan-neuronal marker (green) and Brp (Bruchpilot) is an active zone marker (magenta). Scale bar is 24 μm.
(iii) Quantification of total number of boutons in the muscle 6/7 (abdominal segment 3) NMJ show that PPFIA3 p.Arg39Cys and p.Arg415Trp result in bouton loss compared to PPFIA3 WT and empty control. In contrast, PPFIA3 p.Trp546Cys and p.Arg784Trp show no alteration in bouton numbers.
(iv) Quantification of total NMJ length in each genotype is shown, and there is no significant difference between PPFIA3 WT, variants, and UAS-empty control. Crosses were set and maintained at 25°C. Statistical analysis conducted with one-way ANOVA and Tukey’s post-hoc analysis. Data shown as mean ± SEM with the sample size of total number of quantified NMJs shown above the bars. Significance shown as ∗∗p < 0.01, ∗∗∗p < 0.001. Non-significance shown as ns.
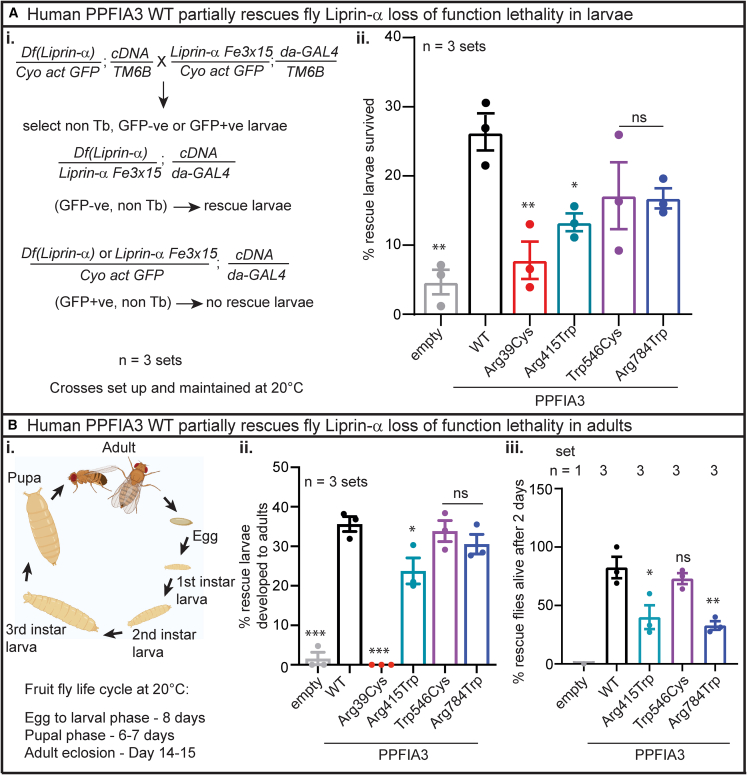
To determine the functional nature of the human PPFIA3 variants in the absence of WT fly Liprin-α, we performed in vivo rescue experiments at 20°C–21°C with a previously established Liprin-α LOF allele,32 Liprin-αF3ex15, and a Liprin-α deficiency allele, Df(2L)Exel7027/CyO (Figure 5Ai). To express PPFIA3 cDNAs in the background of Liprin-α LOF, we used the ubiquitously expressing daughterless-GAL4 (da-GAL4). First, we observed that complete loss of Liprin-α function (Liprin-αF3ex15/Df(2L)Exel7027) is embryonic lethal in control da-GAL4>UAS-empty flies, with a few escapers reaching larval stage (Figure 5Aii). We expressed the human PPFIA3 WT or variant cDNAs in the background of Liprin-α LOF using a ubiquitously expressed da-GAL4 at 20°C and assessed whether human PPFIA3 WT or variants rescued the embryonic lethality. We found a ∼25% larval rescue of embryonic lethality with PPFIA3 WT, indicating functional conservation in fruit flies (Figure 5Aii). PPFIA3 p.Arg39Cys and p.Arg415Trp resulted in significantly reduced larval rescue compared to WT (8% and 13%, respectively) (Figure 5Aii). However, the PPFIA3 p.Trp546Cys and p.Arg784Trp variants resulted in ∼17% rescue efficiency of the embryonic lethality, which was similar to the PPFIA3 WT rescue efficiency. Second, we assessed the survival of the rescued larvae to the adult stage (Figure 5Bi). We found that ∼35% of PPFIA3 WT larvae reached the adult stage; however, none of the PPFIA3 p.Arg39Cys larvae reached the adult stage (Figure 5Bii). In contrast, we found that 23% of PPFIA3 p.Arg415Trp larvae reached the adult stage, which is significantly reduced compared to PPFIA3 WT (Figure 5Bii). However, the frequency of PPFIA3 p.Trp546Cys and PPFIA3 p.Arg784Trp larvae reaching the adult stage was similar to PPFIA3 WT (33% and 30%, respectively) (Figure 5Bii). Third, we assessed the survival of these rescue adult flies in the 48-h post-eclosion. We found that 75% of PPFIA3 WT rescue flies were alive 48 h post-eclosion, indicating PPFIA3 WT in the Liprin-α LOF background is capable of restoring viability and survival. In contrast, only 35% of the PPFIA3 p.Arg415Trp and 30% of the PPFIA3 p.Arg784Trp rescue flies were alive 48 h post-eclosion (Figure 5Biii). However, PPFIA3 p.Trp546Cys rescue flies had a survival rate similar to PPFIA3 WT rescue flies (Figure 5Biii).
Figure 5.
PPFIA3 WT partially rescues the fly Liprin-α LOF lethality
(A) Human PPFIA3 WT in the background of fly Liprin-α LOF results in a partial rescue of embryonic lethality.
(i) Crossing scheme to delete fly Liprin-α and express human PPFIA3 WT and variants. The scheme describes the rescue larvae selection strategy. Crosses were set and maintained at 20°C.
(ii) Quantification of n = 3 sets per genotype showing % GFP-negative larvae (rescue larvae) that survive to the larval stage. PPFIA3 WT expression can partially rescue larval viability compared to empty control. PPFIA3 p.Arg39Cys and p.Arg415Trp show impaired ability to rescue larval viability.
(B) Human PPFIA3 WT expression partially rescues the lethality in adult stage.
(i) Representative illustration of the different stages of fruit fly development.
(ii) Quantification of 3 sets of rescued larvae per genotype that survive to the adult stage. PPFIA3 WT expression can partially rescue adult viability compared to empty control. PPFIA3 p.Arg39Cys and p.Arg415Trp show impaired ability to rescue adult viability.
(iii) Quantification of 1–3 sets of rescued larvae per genotype that survived after 48 h post-eclosion. For the empty control larvae, only one escaper rescue larvae survived to adult stage but died within 2 days post-eclosion. None of the PPFIA3 p.Arg39Cys rescue larvae survived to adult stage. Due to the lack of any PPFIA3 p.Arg39Cys rescue larvae surviving to the adult stage, this variant was not quantifiable for the adult survival phenotype. PPFIA3 p.Arg415Trp and PPFIA3 p.Arg784Trp show impaired ability to rescue adult viability compared to the PPFIA3 WT. Sample size is shown in Table S2. Statistical analysis with one-way ANOVA and Tukey’s post-hoc analysis. Data shown as mean ± SEM with the sample size of flies scored shown in (Table S2). Significance shown as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Non-significance shown as ns.
Finally, we analyzed the number of NMJ boutons and NMJ length with da-GAL4-mediated production of PPFIA3 WT and variants in the Liprin-α LOF background at 20°C (Figure S7A). Although the total number of boutons is significantly reduced compared to da-GAL4>UAS empty controls, there is no significant difference in the number of boutons between PPFIA3 WT and variants (Figure S7Bi). We quantified the total length of the NMJ and found no significant difference between genotypes (Figure S7Bii). Interestingly, we observed a significantly reduced ratio of bouton numbers per muscle 6/7 NMJ (segment A3) length in both PPFIA3 WT and variants compared to the da-GAL4>UAS empty control (Figure S7Bii). However, the bouton-to-NMJ-length ratio remained unchanged between PPFIA3 WT and variants (Figure S7Bii). This indicates that there is a significant loss of bouton density in the background of complete Liprin-α LOF compared to the da-GAL4>UAS empty control. However, neither PPFIA3 WT nor variants were able to rescue the loss of NMJ boutons in the Liprin-α LOF background (Figure S7Bi, iii). It is possible that due to the severity of the complete Liprin-α LOF, the PPFIA3 WT or variants expressing larvae examined for NMJ morphology represent a healthier subset of larvae capable of developing to the third-instar stage. Therefore, we may not be capturing PPFIA3 WT or variants expressing larvae with more severe NMJ phenotypes. This would limit our ability to identify a morphological difference between PPFIA3 WT and variants in the background of complete Liprin-α LOF. Together, the in vivo fly functional experiments demonstrate that rare PPFIA3 variants p.Arg39Cys, p.Arg415Trp, and p.Arg784Trp result in loss of PPFIA3 function and are deleterious to multiple developmental processes. The clinical findings and fruit fly functional assays show that the PPFIA3 variants have a variable spectrum of severity. The variants in the coiled-coil domains are associated with multiple neurodevelopment phenotypes in the affected individuals, and these variants cause severe phenotypes in the fruit flies as well (Table 3). These findings show that rare autosomal-dominant or autosomal-recessive PPFIA3 variants in key functional domains may lead to a syndromic neurodevelopmental disorder.
Table 3.
Comparison of clinical phenotypes and findings in fruit flies expressing PPFIA3 missense variants
|
PPFIA3 variants (GRCh37, hg19) |
c.115C>T (p.Arg39Cys) |
c.943G>T (p.Ala315Ser) |
c.1243C>T (p.Arg415Trp) |
c.1638G>T (p.Trp546Cys) |
c.2350C>T (p.Arg784Trp) |
|||
|---|---|---|---|---|---|---|---|---|
| Individual | 1 | 2 | 7 | 8 | 9 | 12 | 13 | |
| Key clinical phenotypes in individuals with PPFIA3 variants | location | coiled-coil | coiled-coil | coiled-coil | disordered region | disordered region | ||
| abnormal EEG | + | + | N/A | + | + | − | + | |
| epilepsy | + | − | N/A | + | − | − | + | |
| autism/autistic features | − | − | N/A | + | + | + | − | |
| DD/ID | + | + | N/A | + | + | + | + | |
| hypotonia | + | N/A | N/A | + | − | − | − | |
| dysmorphisms | + | + | + | + | N/A | − | − | |
| micro- or macrocephaly | − | − | + | + | N/A | − | − | |
| clinical features (# present/total reported) | 5/7 | 3/6 | 2/2 | 7/7 | 3/5 | 2/7 | 3/7 | |
| Findings in fruit flies expressing PPFIA3 variants | eclosion defect | + | + | + | − | − | − | + |
| abnormal leg morphology | + | + | + | − | − | − | + | |
| climbing defect | + | + | + | − | + | − | + | |
| bang sensitivity | + | + | + | − | − | − | + | |
| NMJ defect | + | N/A | + | − | − | − | + | |
| liprin-α LOF rescue defect | + | N/A | + | − | + | − | + | |
| fly phenotypes (# present/total assays) | 6/6 | 4/4 | 6/6 | 0/6 | 2/6 | 0/6 | 6/6 | |
| variant severity according to the number of phenotypes in fly assays (0: no effect in fly assays; 1–2: mild; 3–4: moderate; 5–6: severe) | severe | at least moderate | severe | no effect | mild | no effect | severe | |
Abbreviations: electroencephalogram (EEG), delayed development (DD), intellectual disability (ID), no information available (n/a), loss of function (LOF), neuromuscular junction (NMJ).
Discussion
We describe 20 individuals from 18 families with 17 rare variants in PPFIA3 who have neurodevelopmental phenotypes including DD, ID, hypotonia, ASD or autistic features, dysmorphisms, microcephaly or macrocephaly, abnormal EEG, and epilepsy (see supplemental information). The results of our clinical analysis, in silico molecular modeling, and in vivo functional studies in fruit flies show that rare PPFIA3 variants lead to a syndromic neurodevelopmental disorder. PPFIA3 domain analysis and molecular modeling revealed that seven of the PPFIA3 missense variants, p.Arg39Cys, p.Glu40Lys, p.Gln80Pro, p.Ala315Ser, p.Arg415Trp, p.Arg429Trp, and p.Arg498Trp, are located in the N-terminal coiled-coil domain. The coiled-coil domain is critical for PPFIA3’s homodimerization and interaction with active zone proteins, such as RIM and ELKS, to regulate active zone organization and synaptic vesicle release.13,14,15,16 Five PPFIA3 missense variants, p.Trp546Cys, p.Lys759Arg, p.Arg784Trp, p.Pro793Thr, and p.Ser906Leu, are located in the intrinsically disordered region of the protein. The p.Pro793Thr and p.Lys759Arg variants were inherited in trans from unaffected parents. One PPFIA3 missense variant, p.Ile870Asn, is located in the SAM1 domain. The SAM domains are known to bind to RNA, lipid membranes, and the adhesion molecule LAR-RPTP.18,47,48 Finally, the PPFIA3 frameshift deletion variants (p.Ser903Leufs∗86 and p.Glu1103Asnfs∗8) and the exonic deletion variant (Δexons 22–30) may result in nonsense-mediated decay followed by reduced protein levels.
Phenotypic assessment of available clinical information revealed seven commonly reported neurodevelopmental features in the 20 individuals. These seven features include DD, ID, hypotonia, dysmorphisms, microcephaly or macrocephaly, ASD or autistic features, abnormal EEG, and epilepsy (Tables 1, S4, and S5, see supplemental information). We found that out of the nine individuals with missense variants in the coiled-coil domain (1 and 2, p.Arg39Cys; 3, p.Glu40Lys; 4, p.Gln80Pro; 7, p.Ala315Ser; 8 and 9, p.Arg415Trp; 10, p.Arg429Trp; and 11, p.Arg498Trp), two individuals had premature mortality (7 and 11). Individuals 1 and 2 have the same variant (p.Arg39Cys) and share similar clinical features such as abnormal EEG, epilepsy, DD, and ID. Individual 3 had DD, severe ID, hypertonia, dysmorphisms, and epilepsy. Individual 4 had autistic features, DD, ID, hypotonia, and dysmorphisms. Individuals 8 and 9 have the same variant (p.Arg415Trp), in which individual 8 had abnormal EEG, epilepsy, autistic features, DD, ID, hypotonia, dysmorphisms, and macrocephaly. Individual 9 had bilateral epileptiform discharges on EEG, autistic features, DD, and ID. Individual 11 had autism, DD, and ID. Our amino acid conservation analysis showed that affected residues in the PPFIA3 coiled-coil domain are highly conserved, except for the residues p.Glu40 and p.Arg498, across mice, fruit flies, and C. elegans (Figures S1A and S1B). These findings suggest the affected residues are critical for the protein’s function across different species. Furthermore, molecular modeling of p.Arg415Trp and p.Arg429Trp variants suggested that the missense variants would hinder PPFIA3 function.
Three individuals have de novo PPFIA3 missense variants (12, p.Trp546Cys; 13, p.Arg784Trp; and 17, p.Ser906Leu) in the intrinsically disordered region of the protein. The amino acid conservation analysis suggests that the affected residues are not well conserved across species except for p.Ser906 that is near the SAM1 domain. Individual 12 had autism, DD, and ID, whereas individual 13 had epilepsy, DD, and ID. Individual 17 (p.Ser906Leu) had abnormal EEG, autism, DD, ID, hypotonia, dysmorphisms, and micro/macrocephaly. Together, these findings suggest that variants in the intrinsically disordered regions are associated with variable disease severity, which may be due to the lack of association in this region with functional domains for PPFIA3. Two monozygotic twins had PPFIA3 missense variants in the SAM1 domain (14 and 15, p.Ile870Asn). Individual 14 had autism, DD, ID, and microcephaly, and individual 15 had DD, ID, hypotonia, and microcephaly. Individual 18 has a PPFIA3 frameshift variant in the SAM3 domain, p.Glu1103Asnfs∗8, with abnormal EEG, DD, ID, hypotonia, and dysmorphisms. There were two related individuals (5 and 6) with a PPFIA3 intronic splice variant (c.240+1G>A) and DD, ID, dysmorphisms, and microcephaly. Individual 5 inherited the PPFIA3 variant from the affected parent, individual 6. The inheritance of the PPFIA3 variant in individual 6 is unknown. One individual (20) was identified with compound heterozygous variants, p.Pro793Thr and p.Lys759Arg, which were inherited from asymptomatic parents. This individual has DD, ID, epilepsy, autistic features, hypotonia, dysmorphism, and microcephaly. As this is the only individual with biallelic variants in PPFIA3 described to date in GeneMatcher,38,39,40 the significance of these variants is uncertain until additional individuals with similar features and biallelic PPFIA3 variants are identified. However, there are striking similarities in facial features and clinical phenotypes between individual 20 and other individuals in the cohort. Finally, we also identified a rare missense PPFIA3 p.Arg559Trp (c.1675C>T [GenBank: NM_003660.4]) variant of unknown inheritance in an individual with a discordant severe neurodegeneration phenotype and family history of consanguinity (data not shown). This variant is not present in gnomAD v.2.1.1 and has a CADD (v.1.6) score of 24.6, suggesting this variant could be potentially damaging. However, the severe neurodegeneration phenotype was not observed in the 20 individuals in our cohort. This suggests that PPFIA3 p.Arg559Trp is either a more severe deleterious variant or there are other genetic alterations, including alterations that may be related to the family history of consanguinity, contributing to this individual’s clinical findings.
Our conservation analysis revealed that the PPFIA3 domains are well conserved in the fruit fly ortholog Liprin-α, which is primarily found in the fruit fly embryonic and larval nervous system. We utilized the evolutionary conservation to assess the impact of PPFIA3 variants on development and behavior in the fruit fly. We found that ubiquitous expression of PPFIA3 missense variants in the coiled-coil domain (p.Arg39Cys, p.Ala315Ser, and p.Arg415Trp) resulted in pupal lethality and/or eclosion defects. Interestingly, p.Arg39Cys caused severe pupal lethality, whereas p.Ala315Ser and p.Arg415Trp had milder effects on pupal lethality. All three tested variants in the coiled-coil domain showed abnormal leg morphology. Neuron-specific expression of PPFIA3 p.Arg39Cys and p.Arg415Trp variants showed bang sensitivity and climbing defects. Furthermore, as Liprin-α is known to regulate NMJ development in fruit flies, we examined the effect of human PPFIA3 WT and variant cDNA expression in the larval NMJ. We found that PPFIA3 pArg39Cys and p.Arg415Trp caused a reduction in bouton number compared to PPFIA3 WT, which would potentially impair neurotransmission. Our fly overexpression findings reveal the PPFIA3 missense variants in the coiled-coil domain cause lethality, suggesting these variants are dominant-negative alleles. Interestingly, ubiquitous or neuronal overexpression of the PPFIA3 variants in the intrinsically disordered region (p.Arg784Trp and p.Trp546Cys) had either mild or no phenotypes in the fruit flies, which may be due to the variants not being conserved in the flies, causing mild protein dysfunction that is tolerated in the fly model, or these variants are benign and the phenotypes are due to other genetic etiologies. However, knowledge regarding the presence of Liprin-α outside the nervous system during Drosophila postembryonic development is limited.32 Therefore, the genetic mechanism underlying the PPFIA3 variant-associated phenotypes in the fruit flies may be more complex.
To further determine whether the variants are LOF or gain of function (GOF) in nature and the functional conservation between human PPFIA3 and fly Liprin-α, we performed an LOF lethality rescue assay using Liprin-α mutants and PPFIA3 WT and variants. Our LOF rescue assay showed that human PPFIA3 WT partially rescued the Liprin-α LOF embryonic lethality and development to the adult stage, suggesting that human PPFIA3 WT function is partially conserved in fruit flies. Interestingly, the coiled-coil variants, PPFIA3 p.Arg39Cys and p.Arg415Trp, showed reduced rescue efficiency of the Liprin-α LOF embryonic lethality, indicating these variants are strong LOF variants. In contrast, the intrinsically disordered region variants, PPFIA3 p.Trp546Cys and p.Arg784Trp, showed a similar rescue efficiency of the embryonic lethality as compared to PPFIA3 WT. However, we found in the adult stage that PPFIA3 p.Arg784Trp significantly reduced the lifespan of rescue flies compared to PPFIA3 WT, suggesting that p.Arg784Trp is a hypomorphic LOF variant. These findings are consistent with the clinical findings in individuals 11 (p.Trp546Cys) and 12 (p.Arg784Trp). Both individuals had fewer clinical features reported. In contrast, individuals 1 and 2 (p.Arg39Cys) and individuals 8 and 9 (p.Arg415Trp) had more clinical features reported. Together, our overexpression and LOF rescue assays in fruit flies reveal that rare PPFIA3 variants cause a neurodevelopmental disorder through an LOF mechanism and suggest that disease severity correlates with the degree of LOF.
Together, the clinical phenotypes and functional assays in fruit flies point toward a possible domain-specific disease-severity mechanism where the variants in the coiled-coil domains might lead to relatively more severe phenotypes in both affected individuals and fruit flies. Notably, ten individuals in the cohort have a family history of neurologic findings, which include ID, autism, NDD, dyslexia, muscular dystrophy, and psychiatric illnesses (Tables S4 and S5, see supplemental information). These familial findings raise the possibility that genetic background effects may also contribute to the severity and penetrance of PPFIA3-related phenotypes. However, the current number of participants and tested variants limits the prediction of genotype-phenotype correlations. Further studies in a larger sample size of affected individuals will be required to elucidate potential genotype-phenotype correlations.
Finally, to determine whether PPFIA3 copy-number variants (CNVs) may potentially be contributory to neurodevelopmental phenotypes, we examined CNVs involving the PPFIA3 locus in DECIPHER v.11.21, gnomAD structural variants (SVs) v.2.1, and ClinVar.37,49,50 Across all three databases, seven individuals were reported with PPFIA3 CNV deletions, but phenotypic information is limited to one individual reported with autistic behavior and mild microcephaly and one individual reported with a progressive familial heart block type IB (Figure S8A; Table S8). There are 82 individuals reported with chromosomal duplications in these databases (Figure S8A; Table S8). However, in gnomAD SV v.2.1, there were three individuals found to be homozygous and 38 individuals found to be heterozygous for a 42.9-kb duplication involving both the PPFIA3 and TRPM4 loci (Table S8), suggesting that PPFIA3 duplication may be tolerated. Altogether, the phenotypes associated with the reported CNV deletions and duplications involving the PPFIA3 locus include DD, ID, seizures, dysmorphisms, autistic behavior, microcephaly, and behavioral abnormalities (Figure S8; Table S8). Together, these findings suggest that PPFIA3 deletions may contribute to the pathogenesis of neurodevelopmental disorders, but further studies will be needed to determine the significance of PPFIA3 haploinsufficiency in human disease.
In summary, our study provides clinical and functional evidence that rare PPFIA3 variants cause a syndromic neurodevelopmental disorder characterized by DD, ID, hypotonia, ASD or autistic features, dysmorphisms, and epilepsy. The reduced penetrance of features in affected family members suggests a complex relationship between PPFIA3 germline variants and developmental features. Together, our in vivo functional modeling in fruit flies reveals that PPFIA3 variants may contribute to disease pathogenesis through LOF mechanisms related to the location of the affected residues in the PPFIA3 functional domains. These findings and our clinical characterizations show that rare PPFIA3 variants lead to a syndromic neurodevelopmental disorder. Future approaches to further elucidate the mechanistic underpinnings of PPFIA3 variants in disease pathogenesis may include synaptic ultrastructural analysis with transmission electron microscopy, electrophysiology to analyze the synaptic recycling system, and genotype-to-phenotype correlations with fruit fly functional assays for the 17 identified PPFIA3 variants. Longitudinal assessments in a larger sample size of affected individuals and mechanistic studies in model organisms will advance our understanding of disease pathogenesis, improve prognostication based on variant type and location, and identify potential therapeutic avenues.
Consortia
Members of the Undiagnosed Diseases Network (UDN) include Maria T. Acosta, Margaret Adam, David R. Adams, Raquel L. Alvarez, Justin Alvey, Laura Amendola, Ashley Andrews, Euan A. Ashley, Carlos A. Bacino, Guney Bademci, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Michael Bamshad, Deborah Barbouth, Pinar Bayrak-Toydemir, Anita Beck, Alan H. Beggs, Edward Behrens, Gill Bejerano, Hugo J. Bellen, Jimmy Bennett, Beverly Berg-Rood, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, Stephanie Bivona, Elizabeth Blue, John Bohnsack, Devon Bonner, Lorenzo Botto, Brenna Boyd, Lauren C. Briere, Gabrielle Brown, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, Peter Byers, William E. Byrd, John Carey, Olveen Carrasquillo, Thomas Cassini, Ta Chen Peter Chang, Sirisak Chanprasert, Hsiao-Tuan Chao, Ivan Chinn, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, Matthew Coggins, F. Sessions Cole, Heather A. Colley, Heidi Cope, Rosario Corona, William J. Craigen, Andrew B. Crouse, Michael Cunningham, Precilla D’Souza, Hongzheng Dai, Surendra Dasari, Joie Davis, Jyoti G. Dayal, Esteban C. Dell'Angelica, Katrina Dipple, Daniel Doherty, Naghmeh Dorrani, Argenia L. Doss, Emilie D. Douine, Dawn Earl, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Marni Falk, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, William A. Gahl, Ian Glass, Bernadette Gochuico, Page C. Goddard, Rena A. Godfrey, Katie Golden-Grant, Alana Grajewski, Don Hadley, Sihoun Hahn, Meghan C. Halley, Rizwan Hamid, Kelly Hassey, Nichole Hayes, Frances High, Anne Hing, Fuki M. Hisama, Ingrid A. Holm, Jason Hom, Martha Horike-Pyne, Alden Huang, Sarah Hutchison, Wendy Introne, Rosario Isasi, Kosuke Izumi, Fariha Jamal, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Orpa Jean-Marie, Vaidehi Jobanputra, Lefkothea Karaviti, Shamika Ketkar, Dana Kiley, Gonench Kilich, Shilpa N. Kobren, Isaac S. Kohane, Jennefer N. Kohler, Susan Korrick, Mary Kozuira, Deborah Krakow, Donna M. Krasnewich, Elijah Kravets, Seema R. Lalani, Byron Lam, Christina Lam, Brendan C. Lanpher, Ian R. Lanza, Kimberly LeBlanc, Brendan H. Lee, Roy Levitt, Richard A. Lewis, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Audrey Stephannie Maghiro, Rachel Mahoney, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Kenneth Maravilla, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Jacob McCauley, Allyn McConkie-Rosell, Alexa T. McCray, Elisabeth McGee, Heather Mefford, J. Lawrence Merritt, Matthew Might, Ghayda Mirzaa, Eva Morava, Paolo Moretti, John Mulvihill, Mariko Nakano-Okuno, Stanley F. Nelson, John H. Newman, Sarah K. Nicholas, Deborah Nickerson, Shirley Nieves-Rodriguez, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina G.S. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, Lorraine Potocki, Barbara N. Pusey Swerdzewski, Aaron Quinlan, Deepak A. Rao, Anna Raper, Wendy Raskind, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Natalie Rosenwasser, Francis Rossignol, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Mario Saporta, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, C. Ron Scott, Elaine Seto, Vandana Shashi, Jimann Shin, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Edward C. Smith, Kevin S. Smith, Lilianna Solnica-Krezel, Ben Solomon, Rebecca C. Spillmann, Joan M. Stoler, Kathleen Sullivan, Jennifer A. Sullivan, Angela Sun, Shirley Sutton, David A. Sweetser, Virginia Sybert, Holly K. Tabor, Queenie K.-G. Tan, Amelia L. M. Tan, Arjun Tarakad, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Rachel A. Ungar, Tiina K. Urv, Adeline Vanderver, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Melissa Walker, Stephanie Wallace, Nicole M. Walley, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Daniel Wegner, Monika Weisz Hubshman, Mark Wener, Tara Wenger, Monte Westerfield, Matthew T. Wheeler, Jordan Whitlock, Lynne A. Wolfe, Kim Worley, Changrui Xiao, Shinya Yamamoto, John Yang, Zhe Zhang, and Stephan Zuchner.
Data and code availability
The de-identified data supporting the current study are available from the corresponding author on request. The submission and accession numbers for the variants reported to ClinVar are (1) individuals 1 and 2, ClinVar: SCV003804191.1; GenBank: NM_003660.4 (PPFIA3); c.115C>T (p.Arg39Cys); (2) individual 4, ClinVar: SCV003804194.1; c.239A>C (p.Gln80Pro); (3) individuals 8 and 9, ClinVar: SCV003804192.1; c.1243C>T (p.Arg415Trp); (4) individual 10, ClinVar: SCV003801340; c.1285C>T (p.Arg429Trp); (5) individual 11, ClinVar: SCV003840201; c.1492C>T (p.Arg498Trp); (6) individual 12, ClinVar: SCV003804193.1; c.1638G>T (p.Trp546Cys); (7) individual 13, ClinVar: SCV003801341; c.2350C>T (p.Arg784Trp); (8) individuals 14 and 15, ClinVar: SCV004042691.1; c.2609T>A (p.Ile870Asn); (9) individual 17, ClinVar: SCV003035511; c.2717C>T (p.Ser906Leu); and (10) individual 18, ClinVar: SCV003035512; c.3307del (p.Glu1103Asnfs∗8).
The following variants have been submitted to the ClinVar and the data are scheduled to be released publicly by April 29, 2024: (1) individual 3, ClinVar: SCV004041597; c.118G>A (p.Glu40Lys); (2) individuals 5 and 6, ClinVar: SCV004041598; c.240+1G>A; (3) individual 7, ClinVar: SCV004041599; c.943G>T; (p.Ala315Ser); (4) individual 16, ClinVar: SCV004171207; c.2706dup (p.Ser903Leufs∗86); (5) individual 19, ClinVar: SCV004041600; deletion exons 22–30; (6) individual 20, ClinVar: SCV004041615; c.2377C>A; (p.Lys759Arg); and (7) individual 20, ClinVar: SCV004041614; c.2276A>G; (p.Pro793Thr).
Acknowledgments
We thank the families and clinical staff at each location for participation in this study. In addition, we thank Mingshan Xue, Dongwon Lee, Kailin Mao, Wu (Charles) Chen, Brooke Horist, and Cole Deisseroth for critical feedback on the manuscript. H.-T.C.’s research work is supported by the McNair Medical Institute at The Robert and Janice McNair Foundation, the Burroughs Wellcome Fund, Child Neurology Foundation and Society, the Gordan and Mary Cain Foundation, Annie and Bob Graham, The Elkins Foundation, and the Mark A. Wallace Endowment Award. M.S.P.’s research effort is supported in part by the National Ataxia Foundation and the Burroughs Wellcome Fund. J.M.P.’s research effort was supported in part by the Burroughs Wellcome Fund. V.C.L. and S.L.M. were supported in part by The Gordan and Mary Cain Foundation and Annie and Bob Graham. S.L.M. was also supported in part by the Mark A. Wallace Endowment Award. This work was also supported by Texas Children’s Hospital, the Jan and Dan Duncan Neurological Research Institute, and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number P50HD103555 for use of the Clinical Translational Core and Microscopy Core facilities. Research reported in this manuscript was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under award number U01HG007709. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
M.S.P. and H.-T.C. contributed to the conception and design of the study, the acquisition and analysis of data, drafting the text, and preparing figures and tables. S.L.M., H.P., V.C.L., A.T., M.A.L., M.W.-H., R.A.L., M.R.B., N.B., L.M., S.P., J.T., J.L.M., S.R.C., L.L., T.P., D.Z., L.F., S.M., G.V., S.R.-H., G.L.C., C.A.B., B.H.L., H.Y.K., A.P., J.B., C.P., D.S.J.S., A.M., M.B.T., E.G.K., R.M., G.B.S., V.R., P.V., C.P., and M.O. contributed to the acquisition and analysis of data. J.M.P. and H.C. contributed to analysis of data and preparing figures. J.A.R., M.Z., M.W., H.E., K.C., E.M., M.J.G.S., R.P., P.P.M., A.S.A.C., J.-B.L.P., K.E., C.R., A-S.D.-P., S.S., I.E.S., and H.M. contributed to the acquisition and analysis of data, drafting the text, and preparing tables.
Declaration of interests
The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the clinical exome sequencing services offered at Baylor Genetics. J.L.M., M.J.G.S., and R.P. are employees of GeneDx, LLC.
Published: January 4, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2023.12.004.
Contributor Information
Hsiao-Tuan Chao, Email: chao-lab@bcm.edu.
Undiagnosed Diseases Network:
Maria T. Acosta, Margaret Adam, David R. Adams, Raquel L. Alvarez, Justin Alvey, Laura Amendola, Ashley Andrews, Euan A. Ashley, Carlos A. Bacino, Guney Bademci, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Michael Bamshad, Deborah Barbouth, Pinar Bayrak-Toydemir, Anita Beck, Alan H. Beggs, Edward Behrens, Gill Bejerano, Hugo J. Bellen, Jimmy Bennett, Beverly Berg-Rood, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, Stephanie Bivona, Elizabeth Blue, John Bohnsack, Devon Bonner, Lorenzo Botto, Brenna Boyd, Lauren C. Briere, Gabrielle Brown, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, Peter Byers, William E. Byrd, John Carey, Olveen Carrasquillo, Thomas Cassini, Ta Chen Peter Chang, Sirisak Chanprasert, Hsiao-Tuan Chao, Ivan Chinn, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, Matthew Coggins, F. Sessions Cole, Heather A. Colley, Heidi Cope, Rosario Corona, William J. Craigen, Andrew B. Crouse, Michael Cunningham, Precilla D’Souza, Hongzheng Dai, Surendra Dasari, Joie Davis, Jyoti G. Dayal, Esteban C. Dell'Angelica, Katrina Dipple, Daniel Doherty, Naghmeh Dorrani, Argenia L. Doss, Emilie D. Douine, Dawn Earl, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Marni Falk, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, William A. Gahl, Ian Glass, Bernadette Gochuico, Page C. Goddard, Rena A. Godfrey, Katie Golden-Grant, Alana Grajewski, Don Hadley, Sihoun Hahn, Meghan C. Halley, Rizwan Hamid, Kelly Hassey, Nichole Hayes, Frances High, Anne Hing, Fuki M. Hisama, Ingrid A. Holm, Jason Hom, Martha Horike-Pyne, Alden Huang, Sarah Hutchison, Wendy Introne, Rosario Isasi, Kosuke Izumi, Fariha Jamal, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Orpa Jean-Marie, Vaidehi Jobanputra, Lefkothea Karaviti, Shamika Ketkar, Dana Kiley, Gonench Kilich, Shilpa N. Kobren, Isaac S. Kohane, Jennefer N. Kohler, Susan Korrick, Mary Kozuira, Deborah Krakow, Donna M. Krasnewich, Elijah Kravets, Seema R. Lalani, Byron Lam, Christina Lam, Brendan C. Lanpher, Ian R. Lanza, Kimberly LeBlanc, Brendan H. Lee, Roy Levitt, Richard A. Lewis, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Audrey Stephannie Maghiro, Rachel Mahoney, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Kenneth Maravilla, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Jacob McCauley, Allyn McConkie-Rosell, Alexa T. McCray, Elisabeth McGee, Heather Mefford, J. Lawrence Merritt, Matthew Might, Ghayda Mirzaa, Eva Morava, Paolo Moretti, John Mulvihill, Mariko Nakano-Okuno, Stanley F. Nelson, John H. Newman, Sarah K. Nicholas, Deborah Nickerson, Shirley Nieves-Rodriguez, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina G.S. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, Lorraine Potocki, Barbara N. Pusey Swerdzewski, Aaron Quinlan, Deepak A. Rao, Anna Raper, Wendy Raskind, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Natalie Rosenwasser, Francis Rossignol, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Mario Saporta, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, C. Ron Scott, Elaine Seto, Vandana Shashi, Jimann Shin, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Edward C. Smith, Kevin S. Smith, Lilianna Solnica-Krezel, Ben Solomon, Rebecca C. Spillmann, Joan M. Stoler, Kathleen Sullivan, Jennifer A. Sullivan, Angela Sun, Shirley Sutton, David A. Sweetser, Virginia Sybert, Holly K. Tabor, Queenie K.-G. Tan, Amelia L.M. Tan, Arjun Tarakad, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Rachel A. Ungar, Tiina K. Urv, Adeline Vanderver, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Melissa Walker, Stephanie Wallace, Nicole M. Walley, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Daniel Wegner, Monika Weisz Hubshman, Mark Wener, Tara Wenger, Monte Westerfield, Matthew T. Wheeler, Jordan Whitlock, Lynne A. Wolfe, Kim Worley, Changrui Xiao, Shinya Yamamoto, John Yang, Zhe Zhang, and Stephan Zuchner
Web resources
CLUSTAL Omega, https://www.ebi.ac.uk/Tools/msa/clustalo/
Decipher, https://www.deciphergenomics.org/
GeneMatcher, https://genematcher.org/
ImageJ, https://github.com/imagej/ImageJ
Imaris, https://imaris.oxinst.com/
OMIM, https://www.omim.org/
PyMOL, https://pymol.org/2/
SIFT, https://sift.bii.a-star.edu.sg/www/Extended_SIFT_chr_coords_submit.html
UCSC genome browser, https://genome.ucsc.edu/
Supplemental information
References
- 1.Südhof T.C. Neurotransmitter Release: The Last Millisecond in the Life of a Synaptic Vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Südhof T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 3.Zhai R.G., Bellen H.J. The Architecture of the Active Zone in the Presynaptic Nerve Terminal. Physiology. 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- 4.Xue M., Giagtzoglou N., Bellen H.J. Dueling Ca2+ Sensors in Neurotransmitter Release. Cell. 2011;147:491–493. doi: 10.1016/j.cell.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Südhof T.C. The Presynaptic Active Zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoischen A., Krumm N., Eichler E.E. Prioritization of neurodevelopmental disease genes by discovery of new mutations. Nat. Neurosci. 2014;17:764–772. doi: 10.1038/nn.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mencacci N.E., Brockmann M.M., Dai J., Pajusalu S., Atasu B., Campos J., Pino G., Gonzalez-Latapi P., Patzke C., Schwake M., et al. Biallelic variants in TSPOAP1, encoding the active-zone protein RIMBP1, cause autosomal recessive dystonia. J. Clin. Invest. 2021;131 doi: 10.1172/JCI140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepeta K., Lourenco M.V., Schweitzer B.C., Martino Adami P.V., Banerjee P., Catuara-Solarz S., de La Fuente Revenga M., Guillem A.M., Haidar M., Ijomone O.M., et al. Synaptopathies: synaptic dysfunction in neurological disorders - A review from students to students. J. Neurochem. 2016;138:785–805. doi: 10.1111/jnc.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taoufik E., Kouroupi G., Zygogianni O., Matsas R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: an overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018;8 doi: 10.1098/rsob.180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W., Cai Z.-L., Chao E.S., Chen H., Longley C.M., Hao S., Chao H.-T., Kim J.H., Messier J.E., Zoghbi H.Y., et al. Stxbp1/Munc18-1 haploinsufficiency impairs inhibition and mediates key neurological features of STXBP1 encephalopathy. Elife. 2020;9 doi: 10.7554/eLife.48705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serra-Pagès C., Kedersha N.L., Fazikas L., Medley Q., Debant A., Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serra-Pagès C., Medley Q.G., Tang M., Hart A., Streuli M. Liprins, a Family of LAR Transmembrane Protein-tyrosine Phosphatase-interacting Proteins. J. Biol. Chem. 1998;273:15611–15620. doi: 10.1074/jbc.273.25.15611. [DOI] [PubMed] [Google Scholar]
- 13.Taru H., Jin Y. The Liprin Homology Domain Is Essential for the Homomeric Interaction of SYD-2/Liprin-α Protein in Presynaptic Assembly. J. Neurosci. 2011;31:16261–16268. doi: 10.1523/JNEUROSCI.0002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoch S., Castillo P.E., Jo T., Mukherjee K., Geppert M., Wang Y., Schmitz F., Malenka R.C., Südhof T.C. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 15.Ko J., Na M., Kim S., Lee J.-R., Kim E. Interaction of the ERC Family of RIM-binding Proteins with the Liprin-α Family of Multidomain Proteins. J. Biol. Chem. 2003;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y., Taru H., Deken S.L., Grill B., Ackley B., Nonet M.L., Jin Y. SYD-2 Liprin-α organizes presynaptic active zone formation through ELKS. Nat. Neurosci. 2006;9:1479–1487. doi: 10.1038/nn1808. [DOI] [PubMed] [Google Scholar]
- 17.Kim C.A., Bowie J.U. SAM domains: uniform structure, diversity of function. Trends Biochem. Sci. 2003;28:625–628. doi: 10.1016/j.tibs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Pulido R., Serra-Pagès C., Tang M., Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc. Natl. Acad. Sci. USA. 1995;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Der Lee R., Buljan M., Lang B., Weatheritt R.J., Daughdrill G.W., Dunker A.K., Fuxreiter M., Gough J., Gsponer J., Jones D.T., et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014;114:6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babu M.M. The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem. Soc. Trans. 2016;44:1185–1200. doi: 10.1042/BST20160172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y.C., Couzens A.L., Deshwar A.R., B McBroom-Cerajewski L.D., Zhang X., Puviindran V., Scott I.C., Gingras A.C., Hui C.C., Angers S. The PPFIA1-PP2A protein complex promotes trafficking of Kif7 to the ciliary tip and Hedgehog signaling. Sci. Signal. 2014;7 doi: 10.1126/scisignal.2005608. ra117-13. [DOI] [PubMed] [Google Scholar]
- 22.van der Vaart B., van Riel W.E., Doodhi H., Kevenaar J.T., Katrukha E.A., Gumy L., Bouchet B.P., Grigoriev I., Spangler S.A., Yu K.L., et al. CFEOM1-Associated Kinesin KIF21A Is a Cortical Microtubule Growth Inhibitor. Dev. Cell. 2013;27:145–160. doi: 10.1016/j.devcel.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Shin H., Wyszynski M., Huh K.-H., Valtschanoff J.G., Lee J.-R., Ko J., Streuli M., Weinberg R.J., Sheng M., Kim E. Association of the Kinesin Motor KIF1A with the Multimodular Protein Liprin-α. J. Biol. Chem. 2003;278:11393–11401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- 24.Spangler S.A., Jaarsma D., De Graaff E., Wulf P.S., Akhmanova A., Hoogenraad C.C. Differential expression of liprin-α family proteins in the brain suggests functional diversification. J. Comp. Neurol. 2011;519:3040–3060. doi: 10.1002/cne.22665. [DOI] [PubMed] [Google Scholar]
- 25.Zürner M., Schoch S. The mouse and human Liprin-α family of scaffolding proteins: Genomic organization, expression profiling and regulation by alternative splicing. Genomics. 2009;93:243–253. doi: 10.1016/j.ygeno.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Zürner M., Mittelstaedt T., tom Dieck S., Becker A., Schoch S. Analyses of the spatiotemporal expression and subcellular localization of liprin-α proteins. J. Comp. Neurol. 2011;519:3019–3039. doi: 10.1002/cne.22664. [DOI] [PubMed] [Google Scholar]
- 27.Wong M.Y., Liu C., Wang S.S.H., Roquas A.C.F., Fowler S.C., Kaeser P.S. Liprin-α3 controls vesicle docking and exocytosis at the active zone of hippocampal synapses. Proc. Natl. Acad. Sci. USA. 2018;115:2234–2239. doi: 10.1073/pnas.1719012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang H.J., Kawasawa Y.I., Cheng F., Zhu Y., Xu X., Li M., Sousa A.M.M., Pletikos M., Meyer K.A., Sedmak G., et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhen M., Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]
- 30.Kittelmann M., Hegermann J., Goncharov A., Taru H., Ellisman M.H., Richmond J.E., Jin Y., Eimer S. Liprin-α/SYD-2 determines the size of dense projections in presynaptic active zones in C. elegans. J. Cell Biol. 2013;203:849–863. doi: 10.1083/jcb.201302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel M.R., Shen K. RSY-1 Is a Local Inhibitor of Presynaptic Assembly in C. elegans. Science. 2009;323:1500–1503. doi: 10.1126/science.1169025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann N., DeProto J., Ranjan R., Wan H., Van Vactor D. Drosophila Liprin-α and the Receptor Phosphatase Dlar Control Synapse Morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- 33.Astigarraga S., Hofmeyer K., Farajian R., Treisman J.E. Three Drosophila Liprins Interact to Control Synapse Formation. J. Neurosci. 2010;30:15358–15368. doi: 10.1523/JNEUROSCI.1862-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmeyer K., Maurel-Zaffran C., Sink H., Treisman J.E. Liprin-α has LAR-independent functions in R7 photoreceptor axon targeting. Proc. Natl. Acad. Sci. USA. 2006;103:11595–11600. doi: 10.1073/pnas.0604766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amberger J., Bocchini C.A., Scott A.F., Hamosh A. McKusick’s Online Mendelian Inheritance in Man (OMIM) Nucleic Acids Res. 2009;37:D793–D796. doi: 10.1093/nar/gkn665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnström K., Mallick S., Kirby A., et al. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arachchi H., Wojcik M.H., Weisburd B., Jacobsen J.O.B., Valkanas E., Baxter S., Byrne A.B., O’Donnell-Luria A.H., Haendel M., Smedley D., et al. matchbox : An open-source tool for patient matching via the Matchmaker Exchange. Hum. Mutat. 2018;39:1827–1834. doi: 10.1002/humu.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: A Matching Tool for Connecting Investigators with an Interest in the Same Gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philippakis A.A., Azzariti D.R., Beltran S., Brookes A.J., Brownstein C.A., Brudno M., Brunner H.G., Buske O.J., Carey K., Doll C., et al. The Matchmaker Exchange: A Platform for Rare Disease Gene Discovery. Hum. Mutat. 2015;36:915–921. doi: 10.1002/humu.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao H.-T., Davids M., Burke E., Pappas J.G., Rosenfeld J.A., McCarty A.J., Davis T., Wolfe L., Toro C., Tifft C., et al. A Syndromic Neurodevelopmental Disorder Caused by De Novo Variants in EBF3. Am. J. Hum. Genet. 2017;100:128–137. doi: 10.1016/j.ajhg.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bischof J., Sheils E.M., Björklund M., Basler K. Generation of a transgenic ORFeome library in Drosophila. Nat. Protoc. 2014;9:1607–1620. doi: 10.1038/nprot.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gahl W.A., Mulvihill J.J., Toro C., Markello T.C., Wise A.L., Ramoni R.B., Adams D.R., Tifft C.J., UDN The NIH Undiagnosed Diseases Program and Network: Applications to modern medicine. Mol. Genet. Metabol. 2016;117:393–400. doi: 10.1016/j.ymgme.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon K.P., Carrillo R.A., Zinn K. Development and plasticity of the Drosophila larval neuromuscular junction: Development and plasticity of the neuromuscular junction. WIREs Dev Biol. 2013;2:647–670. doi: 10.1002/wdev.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins C.A., DiAntonio A. Synaptic development: insights from Drosophila. Curr. Opin. Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Green J.B., Gardner C.D., Wharton R.P., Aggarwal A.K. RNA Recognition via the SAM Domain of Smaug. Mol. Cell. 2003;11:1537–1548. doi: 10.1016/s1097-2765(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 48.Barrera F.N., Poveda J.A., González-Ros J.M., Neira J.L. Binding of the C-terminal Sterile α Motif (SAM) Domain of Human p73 to Lipid Membranes. J. Biol. Chem. 2003;278:46878–46885. doi: 10.1074/jbc.M307846200. [DOI] [PubMed] [Google Scholar]
- 49.MacDonald J.R., Ziman R., Yuen R.K.C., Feuk L., Scherer S.W. The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42:D986–D992. doi: 10.1093/nar/gkt958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landrum M.J., Lee J.M., Benson M., Brown G.R., Chao C., Chitipiralla S., Gu B., Hart J., Hoffman D., Jang W., et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de-identified data supporting the current study are available from the corresponding author on request. The submission and accession numbers for the variants reported to ClinVar are (1) individuals 1 and 2, ClinVar: SCV003804191.1; GenBank: NM_003660.4 (PPFIA3); c.115C>T (p.Arg39Cys); (2) individual 4, ClinVar: SCV003804194.1; c.239A>C (p.Gln80Pro); (3) individuals 8 and 9, ClinVar: SCV003804192.1; c.1243C>T (p.Arg415Trp); (4) individual 10, ClinVar: SCV003801340; c.1285C>T (p.Arg429Trp); (5) individual 11, ClinVar: SCV003840201; c.1492C>T (p.Arg498Trp); (6) individual 12, ClinVar: SCV003804193.1; c.1638G>T (p.Trp546Cys); (7) individual 13, ClinVar: SCV003801341; c.2350C>T (p.Arg784Trp); (8) individuals 14 and 15, ClinVar: SCV004042691.1; c.2609T>A (p.Ile870Asn); (9) individual 17, ClinVar: SCV003035511; c.2717C>T (p.Ser906Leu); and (10) individual 18, ClinVar: SCV003035512; c.3307del (p.Glu1103Asnfs∗8).
The following variants have been submitted to the ClinVar and the data are scheduled to be released publicly by April 29, 2024: (1) individual 3, ClinVar: SCV004041597; c.118G>A (p.Glu40Lys); (2) individuals 5 and 6, ClinVar: SCV004041598; c.240+1G>A; (3) individual 7, ClinVar: SCV004041599; c.943G>T; (p.Ala315Ser); (4) individual 16, ClinVar: SCV004171207; c.2706dup (p.Ser903Leufs∗86); (5) individual 19, ClinVar: SCV004041600; deletion exons 22–30; (6) individual 20, ClinVar: SCV004041615; c.2377C>A; (p.Lys759Arg); and (7) individual 20, ClinVar: SCV004041614; c.2276A>G; (p.Pro793Thr).