Abstract
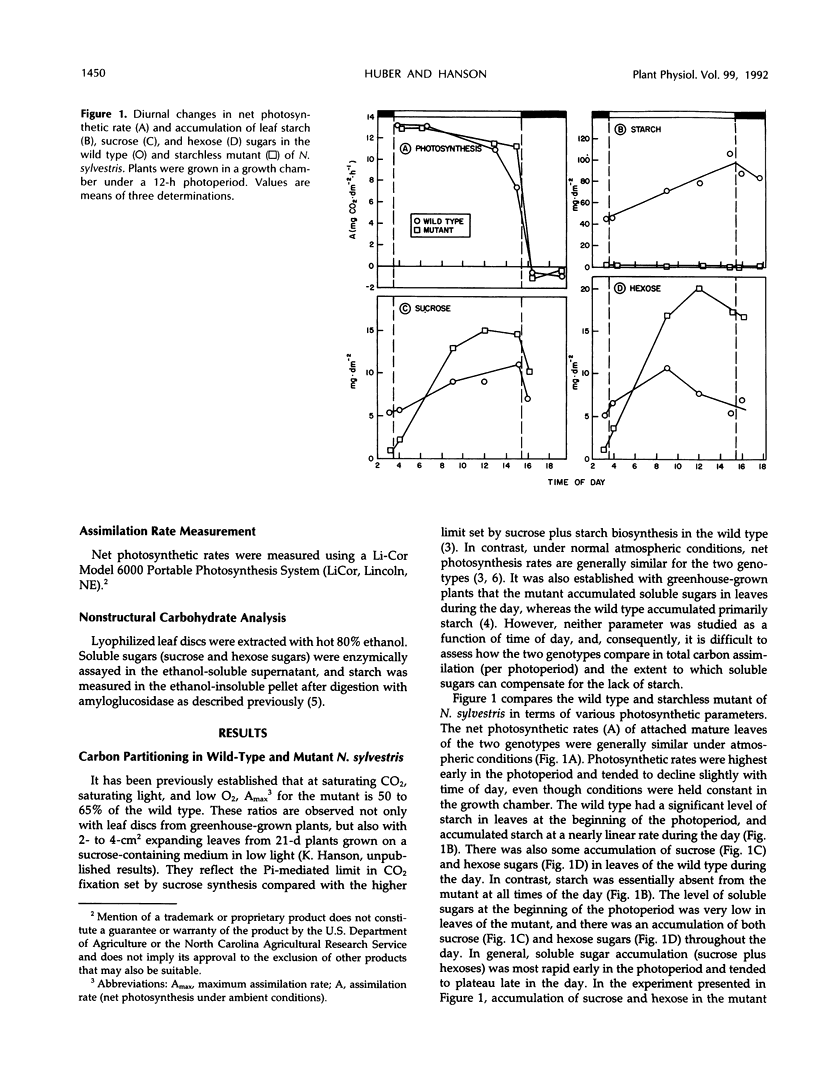
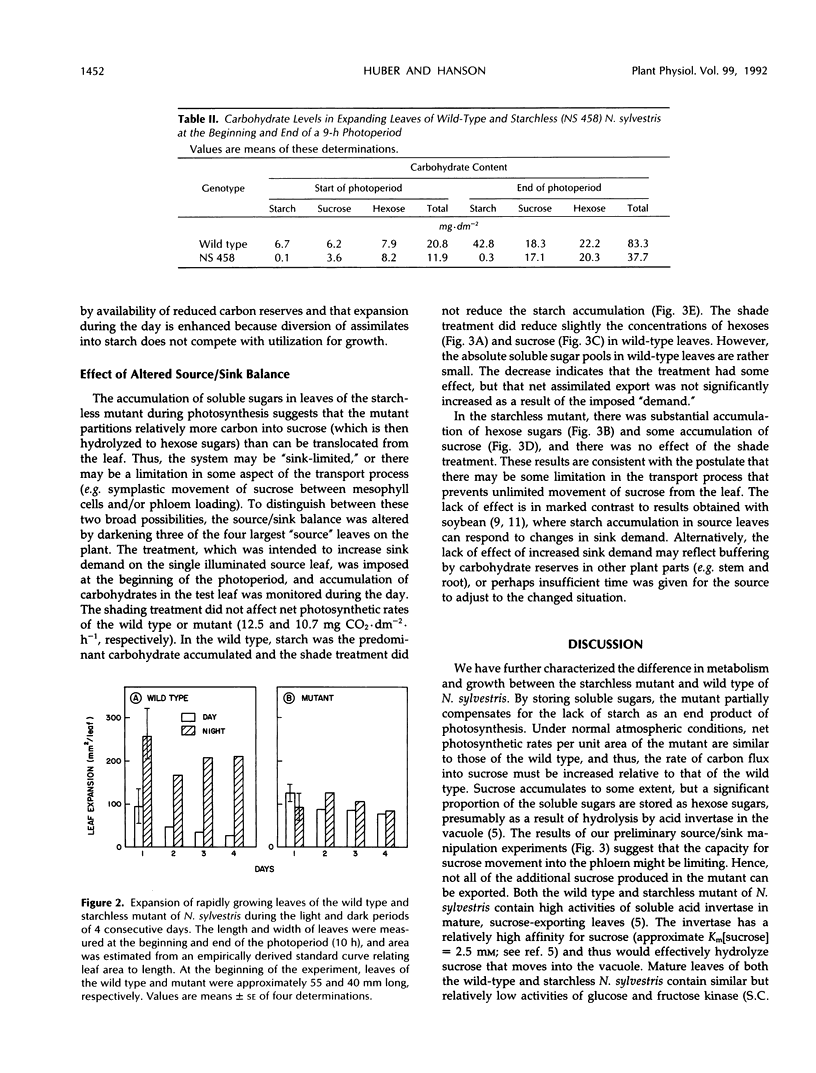
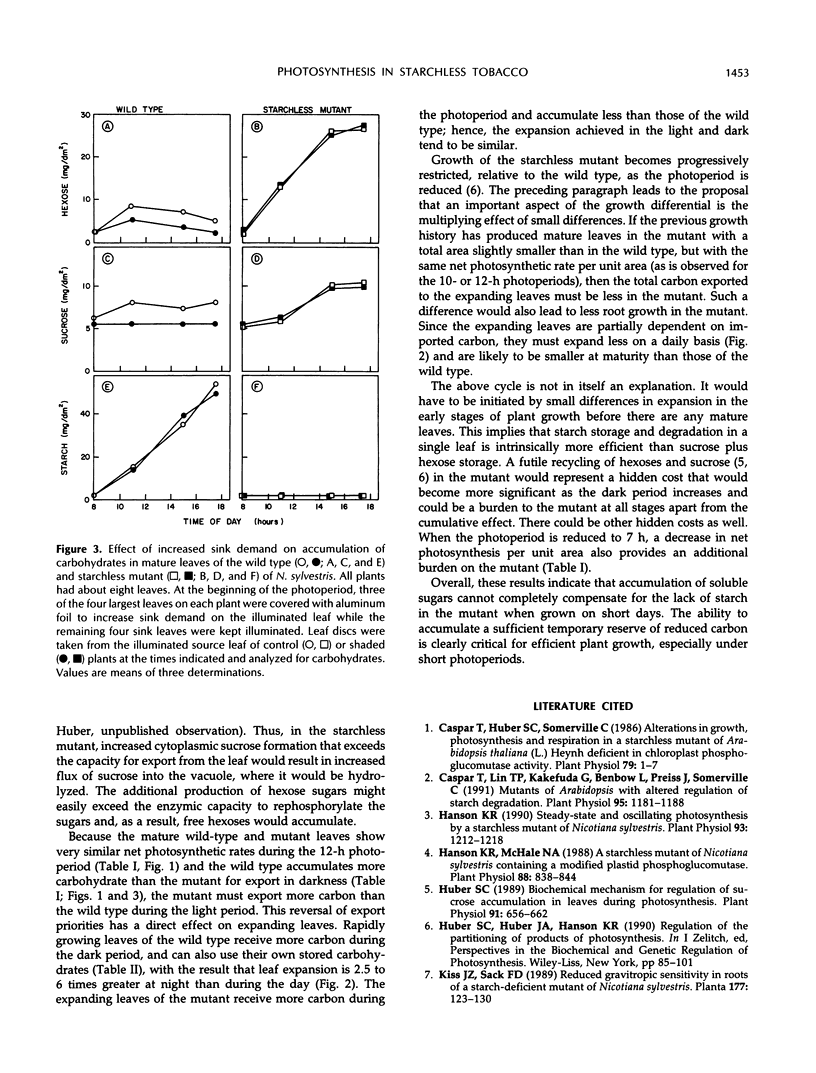
We have further characterized the photosynthetic carbohydrate metabolism and growth of a starchless mutant (NS 458) of Nicotiana sylvestris that is deficient in plastid phosphoglucomutase (Hanson KR, McHale NA [1988] Plant Physiol 88: 838-844). In general, the mutant had only slightly lower rates of photosynthesis under ambient conditions than the wild type. However, accumulation of soluble sugars (primarily hexose sugars) in source leaves of the mutant compensated for only about half of the carbon stored as starch in the wild type. Therefore, the export rate was slightly higher in the mutant relative to the wild type. Starch in the wild type and soluble sugars in the mutant were used to support plant growth at night. Growth of the mutant was progressively restricted, relative to wild type, when plants were grown under shortened photoperiods. When grown under short days, leaf expansion of the mutant was greater during the day, but was restricted at night relative to wild-type leaves, which expanded primarily at night. We postulate that restricted growth of the mutant on short days is the result of several factors, including slightly lower net photosynthesis and inability to synthesize starch in both source and sink tissues for use at night. In short-term experiments, increased “sink demand” on a source leaf (by shading all other source leaves) had no immediate effect on starch accumulation during the photoperiod in the wild type or on soluble sugar accumulation in the mutant. These results would be consistent with a transport limitation in N. sylvestris such that not all of the additional carbon flux into sucrose in the mutant can be exported from the leaf. Consequently, the mutant accumulates hexose sugars during the photoperiod, apparently as the result of sucrose hydrolysis within the vacuole by acid invertase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspar T., Lin T. P., Kakefuda G., Benbow L., Preiss J., Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol. 1991 Apr;95(4):1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R., McHale N. A. A Starchless Mutant of Nicotiana sylvestris Containing a Modified Plastid Phosphoglucomutase. Plant Physiol. 1988 Nov;88(3):838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R. Steady-State and Oscillating Photosynthesis by a Starchless Mutant of Nicotiana sylvestris. Plant Physiol. 1990 Jul;93(3):1212–1218. doi: 10.1104/pp.93.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Biochemical Mechanism for Regulation of Sucrose Accumulation in Leaves during Photosynthesis. Plant Physiol. 1989 Oct;91(2):656–662. doi: 10.1104/pp.91.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J. Z., Sack F. D. Reduced gravitropic sensitivity in roots of a starch-deficient mutant of Nicotiana sylvestris. Planta. 1989;180:123–130. [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Huber S. C. Changes in Starch Formation and Activities of Sucrose Phosphate Synthase and Cytoplasmic Fructose-1,6-bisphosphatase in Response to Source-Sink Alterations. Plant Physiol. 1983 Jun;72(2):474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze W., Stitt M., Schulze E. D., Neuhaus H. E., Fichtner K. A Quantification of the Significance of Assimilatory Starch for Growth of Arabidopsis thaliana L. Heynh. Plant Physiol. 1991 Mar;95(3):890–895. doi: 10.1104/pp.95.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


