Abstract
Objectives
To evaluate the effectiveness and safety of upadacitinib in treatment-refractory inflammatory myositis.
Methods
Patients with refractory inflammatory myositis treated with upadacitinib from a single urban centre in Vancouver, British Columbia, Canada, were included from September 2020 to June 2023. The medical records of these patients were retrospectively reviewed.
Results
10 total patients were identified for review, including 5 classic dermatomyositis (DM), 3 amyopathic DM (ADM) and 2 antisynthetase syndrome. The patients failed an average of four immunosuppressants before initiation of upadacitinib. Three had prior Janus kinase inhibitor therapy with tofacitinib. In the classic DM and ADM aggregate group, upadacitinib offered clinically and statistically significant cutaneous improvement. Lack of active muscle disease at baseline precluded analysis of the effect of upadacitinib on muscle weakness. Nine patients remained on upadacitinib at the end of the study period. One patient discontinued upadacitinib due to severe facial acne.
Conclusion
Upadacitinib appears to be effective in targeting cutaneous manifestations of refractory inflammatory DM. Further research is still needed to validate its efficacy on a broader population scale.
Keywords: Dermatomyositis, Autoimmune Diseases, Polymyositis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Janus kinase (JAK) inhibitors have shown benefit in the treatment of refractory dermatomyositis (DM) cutaneous disease, with tofacitinib (JAK1/2/3) most commonly used in clinical setting. Upadacitinib with high JAK1 selectivity has not been studied for myositis.
WHAT THIS STUDY ADDS
In this case series, upadacitinib improves cutaneous disease in patients with refractory DM despite failing average 3–4 immunosuppressants. In addition, upadacitinib may further improve cutaneous disease activity following incomplete response to tofacitinib.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This represents the first report of selective JAK1 inhibitor in DM and antisynthetase syndrome. Phase 2 and 3 studies to evaluate effectiveness and safety of JAK1 and/or TYK2 inhibitors for DM are currently underway.
Introduction
Idiopathic inflammatory myositis (IIM) is a heterogeneous family of systemic autoimmune diseases that includes dermatomyositis (DM), immune-mediated necrotising myopathy, antisynthetase syndrome (ASSD) and sporadic inclusion body myositis.1 Tofacitinib, an oral Janus kinase (JAK) inhibitor (JAK1/JAK2/JAK3), has shown benefit in the treatment of refractory DM cutaneous disease,2–6 calcinosis7 8 and rapid progressive (RP) interstitial lung disease (ILD) in antimelanoma differentiation-associated gene 5 antibody (anti-MDA5)-positive DM.8–11 Other JAK inhibitors including baricitinib (JAK1/JAK2) and ruxolitinib (JAK1/JAK2) have also been used for treating refractory DM.12 The approval of upadacitinib with high JAK1 selectivity introduces a second generation of JAK inhibitors with promise for minimisation of JAK2-related and JAK3-related side effects.13 Upadacitinib has not previously been used in IIM. In this study, we reported a case series of 10 patients with IIM who were treated with upadacitinib. To our knowledge, this represents the first report on the use of upadacitinib in myositis.
Methods
Study population
Patients were identified through the tertiary myositis rheumatology and dermatology clinics at Vancouver General Hospital, British Columbia, Canada, from September 2020 to January 2023. To be included, patients had to be over 18 years old, with a diagnosis of inflammatory myositis based on expert opinion of either a rheumatologist or dermatologist and treated with upadacitinib for any duration of time. The clinic records of the myositis patients were retrospectively reviewed.
Data collection
All available clinical, laboratory, treatment and outcome data were systematically collected through retrospective chart review. Autoantibodies were detected by line immunoassay (Euroimmun GmbH, Luebeck, Germany) and results reported for myositis specific antibodies (Anti-Jo‐1, Mi2‐α, Mi2-β, MDA5, NXP2, TIF1γ, PL7, PL12, SRP, EJ, OJ, HMGCR, NT5C1 A/Mup44) or myositis associated antibodies (anti- PM/Scl75, PM/Scl100, Ku and Ro52), to further characterise participants.
Prior trials of therapy were summarised in aggregate form, including conventional disease-modifying antirheumatic drugs (cDMARDs) (ie, methotrexate, azathioprine, mycophenolate mofetil, cyclosporin A and cyclophosphamide), intravenous immunoglobulin (IG), rituximab and tofacitinib.
The assessment of the effectiveness of upadacitinib was reported via pretreatment and post-treatment Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI)10 and Manual Muscle Testing Subset of Eight Muscles (MMT8)11 scores using median and IQR and p values. A p value<0.05 was considered statistically significant, as calculated by the Wilcoxon Rank-Sum test. When missing data did not allow the type of response to be quantified, this response was considered not available in final quantitative analysis. Safety was assessed through reporting of all adverse events.
Results
In total, 10 patients treated with upadacitinib for myositis were identified for inclusion. Their baseline clinical and serological characteristics, treatments, as well as responses in CDASI and MMT-8 are detailed in table 1 and online supplemental table 1. Five patients were classified as having classic DM, three amyopathic DM (ADM) and two ASSD. The patients had failed an average of 3.9 (SD 1.91) immunosuppressants before initiation of upadacitinib. Three (30.0%) patients had undergone prior JAK inhibitor therapy with tofacitinib. Median months of upadacitinib therapy was 9.5 months, ranging from 2 to 33 months (online supplemental table 1).
Table 1.
Patient baseline characteristics, treatments and individual response to upadacitinib therapy
| Age (current), sex | Race | Disease Phenotype | Duration of disease, years | Myositis Ab identified | Prior DMARDs | Prednisone dose (daily), pre-UPA | Current DMARDs | Prednisone dose (daily), current | Time on UPA (months)† | CDASI, pre-UPA | CDASI, current | MMT8, pre-UPA | MMT8, current |
| 50, F | East Asian | CDM | 2 | Anti-MDA5, anti-Ro52 | HCQ*, intravenous IG*, MMF*, MTX, TAC* | 10 mg | CP, TAC | 2.5 mg | 8 | 12 | 4 | 126 | 150 |
| 39, F | East Asian | CDM | 7 | Negative | AZA, HCQ*, MMF, MTX*, QUI, TOF* |
0 | UPA, MTX | 0 | 13 | 7 | 1 | 150 | 150 |
| 36, M | South Asian | CDM | 4 | Anti-SAE1, anti-Ro52 | AZA, HCQ, intravenous IG*, MMF, RTX*, TAC, TOF | 15 mg | UPA, intravenous IG | 4 mg | 10 | 27 | 5 | 150 | 150 |
| 86, F | Southeast Asian | CDM | 3 | Anti-TIF-1 | AZA, MMF* | 50 mg | UPA | 5 mg | 4 | 50 | 31 | 127 | 131 |
| 49, F | Southeast Asian | CDM | 2 | Anti-MDA5, anti-Ro52 | CP, intravenous IG, MMF*, MTX, TAC* | 10 mg | UPA, MMF | 0 | 3 | 2 | 0 | 150 | 150 |
| 77, F | White | ADM | 1 | Anti-MDA5 | MMF*, MTX* | 0 | UPA | 0 | 11 | 8 | 1 | NA | NA |
| 31, F | East Asian | ADM | 1 | Anti-MDA5, anti-Ro52 | AZA*, MTX* | 5 mg | UPA, MTX | 0 | 6 | 9 | 0 | NA | NA |
| 57, F | East Asian | ADM | 11 | Not done | HCQ, intravenous IG, MMF*, MTX, TOF | 0 | UPA, MMF | 0 | 9 | 14 | 7 | NA | NA |
| 64, F | Southeast Asian | ASSD | 6 | Anti-PL12, anti- Ro52 |
AZA*, HCQ*, MTX* | 10 mg as needed | UPA | 0 | 33 | 0 | 0 | NA | NA |
| 76, M | East Asian | ASSD | 2 | Anti-Jo1, anti-Ro52 | LEF*, MTX* | 5 mg daily | UPA, MTX | 0 | 18 | 0 | 0 | NA | NA |
*Denotes active medication regimen at start of upadacitinib trial.
†At time of final follow-up or discontinuation of treatment.
ADM, amyopathic dermatomyositis; Anti-MDA5, antimelanoma differentiation-associated gene 5 antibody; ASSD, antisynthetase syndrome; AZA, azathioprine; CDASI, Cutaneous Dermatomyositis Disease Area and Severity Index; CDM, classic dermatomyositis; CP, cyclophosphamide; DMARDs, disease-modifying antirheumatic drugs; F, female; HCQ, hydroxychloroquine; IG, immunoglobulin; LEF, leflunomide; M, male; MMF, mycophenolate mofetil; MMT8, Manual Muscle Testing Subset of Eight Muscles; MTX, methotrexate; NA, not available; QUI, quinacrine; RTX, rituximab; TAC, tacrolimus; TOF, tofacitinib; UPA, upadacitinib.
rmdopen-2023-003837supp001.pdf (58.6KB, pdf)
At the end of the study, nine patients remained on upadacitinib with no reported side effects, including two as monotherapy and seven with concurrent cDMARDs. The disease profiles and response of individual patients is shown in table 1. One patient with anti-MDA5 classic DM discontinued treatment due to severe facial acneiform eruption shortly after starting upadacitinib, which only partially responded to topical benzoyl peroxide. Of the seven patients on prednisone at baseline, four were able to discontinue steroid therapy, while the other three tapered their dose of prednisone while on upadacitinib. Of the two patients reliant on intravenous IG therapy at baseline, one was able to discontinue intravenous IG after starting upadacitinib.
Table 2 summarised quantitative analysis of the overall response to upadacitinib in patients with cutaneous and muscular manifestations of disease. The two patients with ASSD who tested positive for anti-PL12 and anti-Jo1, respectively, shared manifestations of inflammatory arthritis and ILD with no clinical evidence of myositis or DM rash. As these two patients’ CDASI and MMT8 scores were normal at baseline, they were excluded from final quantitative analysis. Both achieved remission in inflammatory arthritis and improvement in ILD, defined by improvement in pulmonary function test (PFT) and CT of the chest.
Table 2.
Summary of response to upadacitinib
| Pre upadacitinib | Current | P value | |
| Classic DM subtype | |||
| CDASI, median (IQR) (n=5) | 12 (7–27) | 4 (1–5) | 0.0625 |
| MMT8, median (IQR) (n=5) | 150 (127–150) | 150 (150–150) | 0.371 |
| Amyopathic DM subtype | |||
| CDASI, mean (IQR) (n=3) | 9 (8.5–11.5) | 1 (0.5–4) | 0.174 |
| Aggregate classic DM and ADM | |||
| CDASI, median (IQR) (n=8) | 10.5 (7.8–12.2) | 2.5 (0.75–5.5) | 0.014 |
ADM, amyopathic dermatomyositis; CDASI, Cutaneous Dermatomyositis Disease Area and Severity Index; DM, dermatomyositis; MMT8, Manual Muscle Testing Subset of Eight Muscles.
Self-reported clinical improvement in rash and reduction in CDASI were noted in both subgroups of classic DM and ADM, but statistical significance was not achieved in individual subgroups. Aggregate analysis of the classic DM and ADM subgroups revealed statistically significant improvement in CDASI score from median of 10.5 (IQR 7.8–12.2) to 2.5 (IQR 0.75–5.5) (p value=0.014) (table 2).
Assessment of muscular response of upadacitinib was limited due to the near complete recovery of muscle strength at baseline prior to upadacitinib treatment.
Five total patients had ILD at the time of diagnosis including two ASSD, and three anti-MDA5 DM. Three patients had mild dyspnoea at baseline, and their respiratory status stabilised or improved on upadacitinib, measured by clinical status, PFT and CT of the chest. One anti-MDA5 patient had asymptomatic mild radiographic progression in ILD while on upadacitinib. Another anti-MDA5 patient presented with RP ILD requiring mechanical ventilation and extracorporeal membrane oxygenation. After completing cyclophosphamide and tacrolimus induction therapy, her ILD improved drastically. Following induction, she was first on mycophenolate and tacrolimus maintenance. Due to recurrent intense pruritus and inflammatory arthritis, her immunosuppressant was switched to upadacitinib and mycophenolate combination therapy which led to clinical improvement of cutaneous symptoms and arthritis. Her ILD remained in remission with stability in PFT and CT of the chest.
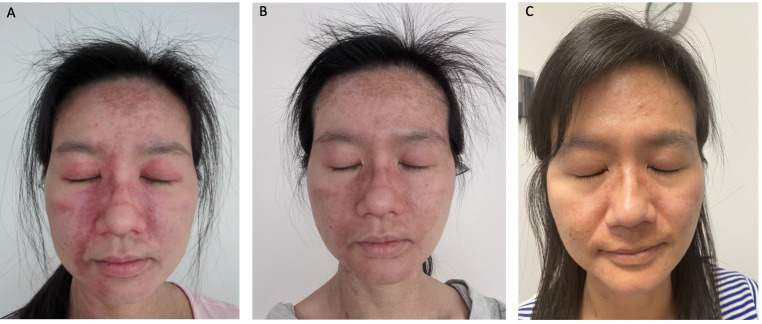
Three patients had previously undergone trials of tofacitinib at a dose of 5 mg two times per day. Two were classified as classic DM and one as ADM. They had either no or incomplete response to tofacitinib. After switching to upadacitinib, their individual CDASI scores improved from 7 to 1, 27 to 5 and 14 to 7, respectively. Figure 1 demonstrates the improvement in cutaneous disease activity in a DM patient who transitioned to upadacitinib after failing tofacitinib.
Figure 1.
The improvement in cutaneous disease activity in a dermatomyositis patient who transitioned to upadacitinib after failing tofacitinib, including photos at assessment pre tofacitinib (A), post tofacitinib (B) and post upadacitinib (C).
Discussion
The pathogenesis underlying the different IIM subtypes is not completely understood. It is well established that there is marked upregulation of interferon type 1 (IFN1)-induced genes and proteins in DM.14–17 IFN1 pathway signalling is mediated through JAK1 and TYK2 activation, and the interferon type II (IFN2) pathway through JAK1 and JAK2. These findings are consistent with a systemic review reporting on the effectiveness of JAK/STAT inhibitors in patients with DM.12 There is also evidence of modest activation of IFN1 signature and robust IFN2 signature in ASSD.18 However, selective agents are likely more effective than the pan-JAK inhibitors with have less effective INF1 blockade.19 Therefore, further research is also needed to assess whether there is clinically significant difference in response to JAK1 inhibitors (which block IFN1 and IFN2) and TYK2 inhibitors (which block primarily INF1) in the treatment of DM and ASSD. Indeed, a phase 3 study of brepocitinib (a dual JAK1 and TYK2 inhibitor) and a phase 2 study of GLPG3667 (a TYK2 inhibitor) for DM are currently underway.
We report the first retrospective case series of upadacitinib, a selective JAK1 inhibitor, in the treatment of patients with refractory DM and ASSD who had failed an average of four steroid-sparing agents. Despite the small sample size and refractory diseases, upadacitinib improved cutaneous disease activity, both clinically and statistically, in the aggregate analysis of patients with classic DM and ADM.
Unfortunately, impact on muscle strength could not be adequately assessed due to the lack of patients with clinically significant weakness at the start of upadacitinib therapy. The improvement shown in the single patient with weakness at the start of treatment may point towards the effectiveness of upadacitinib for treatment of muscle weakness when used early in the course of disease, but this will need to be validated through further research in a larger cohort with muscle weakness at baseline.
Our case series included three patients on upadacitinib who demonstrated significant improvement in cutaneous disease activity after failing tofacitinib, suggesting switching to upadacitinib may be worthwhile for those who have incomplete response to tofacitinib.
Upadacitinib may also have some benefit in myositis-associated ILD. In this study, two patients with ASSD and three with anti-MDA5 DM, had ILD at the time of diagnosis. Aside from one anti-MDA5 patient who had mild asymptomatic progression in ILD while on upadacitinib, the other four patients demonstrated improvement on PFTs and disease stability on CT. IL-4, IL-6, IL-8 and IL-10 are significantly higher in patients with myositis-associated ILD than those without.20 As signalling by these cytokines (except IL-8) are mediated by JAK1, JAK3 and TYK2, it is not surprising that JAK inhibitors may be effective in myositis-associated ILD.
Our study is limited by the small sample size, the retrospective case series study design, and inherent lack of a comparator group. Despite these limitations, this represents the first report of the effect of upadacitinib on DM and ASSD and provides a starting point for development of larger studies to evaluate efficacy and safety on a broader scale. Upadacitinib was well tolerated among study patients, bolstering its potential for future use in the most challenging cases of DM.
Acknowledgments
We thank Mr. Anthony Obrzut’s assistance in the statistical analysis.
Footnotes
Contributors: KH, MB and JD had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. KH designed the study. MB, JD and KH acquired, analysed and interpreted the data. MB and KH drafted the manuscript. All authors participated in the critical revision of the manuscript for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JD received speaker’s honoraria from AbbVie, Amgen, Bausch, Leo, Janssen, Novartis, Sanofi and Pfizer; he participated a Data Safety Monitoring Board for Bristol Myers Squib and is on the advisory board for Solius and Boehringer Ingelheim. He performs clinical research with Corbus, Eli Lilly and company. MB and KH have no conflict of interest to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by University of British Columbia (#H20-03777). Individual patient’s consent is not required by REB with the exception of the figure 1. Participant signed informed consent form for the publication of figure 1.
References
- 1.Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, et al. Classification and management of adult inflammatory myopathies. Lancet Neurol 2018;17:816–28. 10.1016/S1474-4422(18)30254-0 [DOI] [PubMed] [Google Scholar]
- 2.Paik JJ, Casciola-Rosen L, Shin JY, et al. Study of tofacitinib in refractory dermatomyositis: an open-label pilot study of ten patients. Arthritis Rheumatol 2021;73:858–65. 10.1002/art.41602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min MS, Alsarheed A, Kassamali B, et al. Tofacitinib as treatment for refractory dermatomyositis: a retrospective study from 2 academic medical centers. J Am Acad Dermatol 2022;86:423–5. 10.1016/j.jaad.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Kurtzman DJB, Wright NA, Lin J, et al. Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol 2016;152:944–5. 10.1001/jamadermatol.2016.0866 [DOI] [PubMed] [Google Scholar]
- 5.Paik JJ, Shneyderman M, Gutierrez-Alamillo L, et al. Long-term extension study of tofacitinib in refractory dermatomyositis. Arthritis Rheumatol 2022;74:371–2. 10.1002/art.41944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckett M, Tan J, Bonnardeaux E, et al. Tofacitinib therapy in refractory inflammatory myositis: a retrospective cohort study of 41 patients. Rheumatology (Oxford) 2023:kead404. 10.1093/rheumatology/kead404 [DOI] [PubMed] [Google Scholar]
- 7.Shneyderman M, Ahlawat S, Christopher-Stine L, et al. Calcinosis in refractory dermatomyositis improves with tofacitinib monotherapy: a case series. Rheumatology (Oxford) 2021;60:e387–8. 10.1093/rheumatology/keab421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendel S, Venhoff N, Frye BC, et al. Successful treatment of extensive calcifications and acute pulmonary involvement in dermatomyositis with the janus-kinase inhibitor tofacitinib - a report of two cases. J Autoimmun 2019;100:131–6. 10.1016/j.jaut.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 9.Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford) 2018;57:2114–9. 10.1093/rheumatology/key188 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med 2019;381:291–3. 10.1056/NEJMc1900045 [DOI] [PubMed] [Google Scholar]
- 11.Takanashi S, Kaneko Y, Takeuchi T. Tofacitinib in interstitial lung disease complicated with anti-Mda5 antibody-positive dermatomyositis: a literature review. Mod Rheumatol 2022;32:231–7. 10.1080/14397595.2021.1906505 [DOI] [PubMed] [Google Scholar]
- 12.Paik JJ, Lubin G, Gromatzky A, et al. Use of Janus kinase inhibitors in dermatomyositis: a systematic literature review. Clin Exp Rheumatol 2023;41:348–58. 10.55563/clinexprheumatol/hxin6o [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traves PG, Murray B, Campigotto F, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis 2021;80:865–75. 10.1136/annrheumdis-2020-219012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg SA, Pinkus JL, Pinkus GS, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol 2005;57:664–78. 10.1002/ana.20464 [DOI] [PubMed] [Google Scholar]
- 15.Walsh RJ, Kong SW, Yao Y, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum 2007;56:3784–92. 10.1002/art.22928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baechler EC, Bauer JW, Slattery CA, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 2007;13:59–68. 10.2119/2006-00085.Baechler [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong D, Kea B, Pesich R, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS ONE 2012;7:e29161. 10.1371/journal.pone.0029161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology 2019;93:e1193–204. 10.1212/WNL.0000000000008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther 2019;21. 10.1186/s13075-019-1964-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford) 2014;53:2196–203. 10.1093/rheumatology/keu258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003837supp001.pdf (58.6KB, pdf)