Abstract
Objective
Gastrointestinal (GI) involvements were scarcely reported in adult anti-nuclear matrix protein 2 (NXP2) dermatomyositis (NXP2+DM). In this study, we investigated the clinical, pathological and molecular features as well as treatment options of this rare yet life-threatening disease.
Methods
We retrospectively collected the data of the cohort of NXP2+ DM from 2012 to 2022 in our hospital. RNA sequencing was performed in intestinal samples of perforated patients compared with healthy controls data set.
Results
A total of 56 patients with adult NXP2+DM were collected including 10 cases with GI involvements. Abdominal pain and melena were the initial manifestations for GI involvements with a median 10-month time lag after the diagnosis of NXP2+DM when myositis largely subsided. Within weeks, GI perforation occurred in 8 of 10 patients, while five patients underwent eight surgical interventions subsequently. The short-term mortality was observed in four patients. NXP2+DM with GI involvements presented with more extramuscular systemic manifestations such as interstitial lung disease and subcutaneous calcinosis. The GI pathological features encompassed vasculitis/vasculopathy with high MxA expression, intestinal smooth muscle necrosis and serosal calcinosis. Gene expression profile validated the type-I interferon activation and revealed that epithelial mesenchymal transition and focal adhesion pathway may also contribute. Finally, vedolizumab, an anti-α4β7-integrin monoclonal antibody, exhibited promising therapeutic signals which should be further investigated.
Conclusions
GI involvement is a unique complication in patients with adult NXP2+DM. Timely recognition and targeted therapy may turn out to be lifesaving.
Keywords: dermatomyositis, autoimmunity, inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Severe gastrointestinal involvements are rare but fatal complication of dermatomyositis and is often overlooked in clinical practice.
WHAT THIS STUDY ADDS
The clinical, pathological and molecular features of gastrointestinal involvements in nuclear matrix protein 2+ dermatomyositis were summarised. Activation of type-I interferon in gastrointestinal smooth muscle may be critical for the pathophysiology.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Early diagnosis and correct recognition of gastrointestinal involvements would improve the adverse prognosis. Some novel targeted therapy would be promising but need further investigations by restricting clinical trials.
Introduction
Dermatomyositis (DM) is a group of heterogeneous autoimmune diseases affecting skin, muscles and multiple organs. Currently, five myositis-specific autoantibodies, namely anti-transcriptional intermediary factor1γ (TIF1γ), anti-melanoma differentiation-associated protein 5 (MDA5), anti-Mi2, anti-small ubiquitin-like modifier activating enzyme (SAE) and anti-nuclear matrix protein 2 (NXP2), have been well-recognised as serological benchmarks to differentiate different clinical phenotypes of DM.1 2
NXP2 is mainly involved in transcriptional regulation and RNA metabolism. Anti-NXP2 antibody was first described and commonly detected in juvenile DM, while also being present by 2–25% in adult DM.3 4 Severe muscle weakness, calcinosis cutis and peripheral oedema were the typical clinical manifestations across all ages. It has been reported that anti-NXP2 antibody is associated with malignancies in adult patients, while more gastrointestinal (GI) involvements were identified in juvenile NXP2+DM.5–7 Severe GI involvements such as perforation and bleeding can have devastating outcomes.8
A recent report from a large Chinese adult NXP2+DM cohort (n=70) further divided the disease into two clusters, which were younger adult patients (<40 years of age) with high incidence of subcutaneous calcinosis and older patients (40 years of age or older) with worse prognosis carrying higher frequency of interstitial lung disease (ILD).9 To our surprise, no GI involvement was documented. In our practice, GI involvement in adult NXP2+DM occurs with a low but significant frequency, and considering its grave implications it should not be overlooked. Herein, we present a case series of 10 adult NXP2+DM with severe GI involvement, throughout which the clinical, pathological and molecular signatures were explored, heralding unprecedently targeted treatments.
Methods
Patients
Abide by the 2017 EULAR/American College of Rheumatology Classification Criteria10 for idiopathic inflammatory myopathies and 2019 European Neuromuscular Centre (ENMC) criteria1 for DM, we retrospectively collected 10 patients with GI involvements out of 56 patients with adult NXP2+DM, during 2012–2022 at the Rheumatology Department of Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. A 16 myositis-specific antibodies line-blot panel (EUROIMMUN) were implemented with a validation ELISA for anti-NXP2 autoantibody. All patients were 18 years or older and malignancy diagnosed within 2 years of NXP2+DM was excluded. The definition of GI involvement was with abdominal pain and objective findings of intestinal oedema, perforation, GI bleeding, with or without surgical interventions. Clinical and laboratory data were summarised and analysed. The study followed local ethics committee regulations with informed consent obtained from all participants.
Pathological analysis
Histochemical staining of H&E was performed in intestinal sections of surgery samples. Immunohistochemical staining using anti-MxA (clone M143; Millipore), anti-integrin alpha4 beta7 (clone 111D9.03; Novus Biologicals) and anti-MAdCAM1 (clone ab307734; Abcam) were applied for further analysis.
RNA sequencing analysis
Total RNA was extracted from formalin fixation and paraffin embedding sections of surgical samples from patients with NXP2+DM with perforations. Complementary DNA library was generated using random hexamer-primed reverse transcription and PCR amplification as described.11 The RNA sequencing (RNA-seq) data used as healthy controls were collected from GSE189820.12 Raw FASTQ files of RNA-seq was aligned to human reference genome GRCh38 (release 40). DESeq2 was used to evaluate the gene expression level by normalising the length of genes.13 Functional enrichment analysis using Gene Set Enrichment Analysis (GSEA) and the gene sets were obtained from the Molecular Signatures Database (MSigDB, V.2022.1).14 All sequencing data included in this study are available at the National Omics Data Encyclopedia database (OEP004694).
Statistical analysis
Categorical variables were expressed as number or percentage and compared using Fisher’s exact test. Continuous variables were described as median and IQR and calculated using Mann-Whitney test. P values<0.05 were considered as statistical significance. Survival analysis was estimated using Kaplan-Meier method. All statistical calculations were performed in SPSS statistical software (IBM SPSS V.26) and GraphPad Prism V.9.0. RNA-seq data analyses were performed using R V.4.2.1.
Results
Clinical characteristics at the diagnosis of NXP2+DM between patients with and without GI involvements
A total of 56 patients were included in this adult NXP2+DM cohort, 10 of which (17.86%) developed severe GI involvements and 9 of 10 were women. The median age at NXP2+DM diagnosis was 45 years old (table 1). There is no significant difference in demographic features, symptoms such as DM rash (either Gottron’s sign or Gottron’s papules or Heliotrope rash), and musculoskeletal involvements between GI and non-GI groups. Of note, the GI group tended to have more systemic disease, for example, ILD (60.0% vs 23.9%, p=0.024) and subcutaneous calcinosis (60.0% vs 4.34%, p=0.000). Laboratory findings showed no significant differences in inflammatory markers and peak levels of creatine kinases between the two groups. However, the GI group suffered anaemia at diagnosis of NXP2+DM (90 vs 115 g/L, p=0.000), and lymphopenia (570 vs 870/µL, p=0.033) was also more prominent compared to the non-GI group. More importantly, GI involvements incurred an unfavourable prognosis in overall survival (deceased rate 40.0% vs 13%, p=0.044) (table 1). Cytomegalovirus that could induce GI diseases were screened by DNA copies in blood among all patients and results showed no significant difference between GI and non-GI group (1/10 vs 3/46, p=0.699).
Table 1.
Clinical characteristics at the diagnosis of NXP2+ DM between patients with and without gastrointestinal involvements
| Total (n=56) | GI involvement (n=10) | Non-GI involvement (n=46) | P value | |
| Sex (male) | 18 (32.1%) | 1 (10%) | 17 (36.4%) | 0.076 |
| Age, mean±SD | 45.1±19.4 | 45.1±19.6 | 45.1±19.6 | 0.995 |
| Clinical manifestations | ||||
| Rash | 48 | 9 (90.0%) | 39 (84.8%) | 0.669 |
| Myalgia | 35 | 8 (80.0%) | 27 (58.7) | 0.207 |
| Muscle weakness | 49 | 10 (100%) | 39 (84.8%) | 0.330 |
| Arthralgia | 11 | 1 (10.0%) | 10 (21.7%) | 0.397 |
| Dysphagia | 31 | 7 (70.0%) | 24 (52.2%) | 0.304 |
| Subcutaneous oedema | 24 | 6 (60.0%) | 18 (39.1%) | 0.277 |
| Interstitial lung disease | 17 | 6 (60.0%) | 11 (23.9%) | 0.024* |
| Calcinosis | 8 | 6 (60.0%) | 2 (4.34%) | 0.000** |
| Laboratory findings | ||||
| CRP, median (IQR) | 5.21 (2.7–17.46) | 5.21 (2.7–17.46) | 4.61 (1.1–9.74) | 0.533 |
| ESR, median (IQR) | 21 (11–40) | 32 (13–50) | 20 (9–39) | 0.305 |
| SF, median (IQR) | 515 (261–1295) | 646 (305–1356) | 479 (252–1277) | 0.476 |
| CKmax, median (IQR) | 3000 (751–7488) | 2558 (1322–4017) | 3602 (605–8607) | 0.600 |
| WBC, median (IQR) | 8.05 (6.07–11.75) | 8.14 (5.17–10.98) | 8.05 (6.27–11.83) | 0.803 |
| Neu, median (IQR) | 6.77 (4.49–9.54) | 7.10 (3.92–10.18) | 6.67 (4.48–9.18) | 0.973 |
| Lym, median (IQR) | 0.86 (0.57–1.26) | 0.57 (0.43–0.88) | 0.87 (0.68–1.35) | 0.033* |
| Hb, median (IQR) | 113 (103–132) | 90 (88–99) | 115 (108–135) | 0.000** |
| Plt, median (IQR) | 212 (144–249) | 152 (96–268) | 216 (167–248) | 0.203 |
| Treatment | ||||
| GCmax mg/d | 110.5±100 | 111.7±73.3 | 110.3±101.6 | 0.969 |
| Immunosuppressants | 1.6±1 | 1.5±0.9 | 2.1±2.0 | 0.175 |
| IVIg | 27 | 8 (80%) | 20 (45.4%) | 0.073 |
| The autoantibodies | 29 | 5 | 24 | 0.956 |
| Deceased | 10 | 4 (40.0%) | 6 (13.0%) | 0.044* |
Age was presented as mean and SD, while other variables as median and IQR.
*P<0.05. **p<0.01.
CKmax, creatine kinase maximum; CRP, C-reactive protein; DM, dermatomyositis; ESR, erythrocyte sedimentation rate; GCmax, glucocorticoids maximum (prednisolone equivalent); Hb, haemoglobin; IVIg, intravenous immunoglobulin; Lym, lymphocyte; Neu, neutrophil; NXP2, nuclear matrix protein 2; Plt, platelet; SF, serum ferritin; WBC, white blood cell.
Clinical features of GI involvements
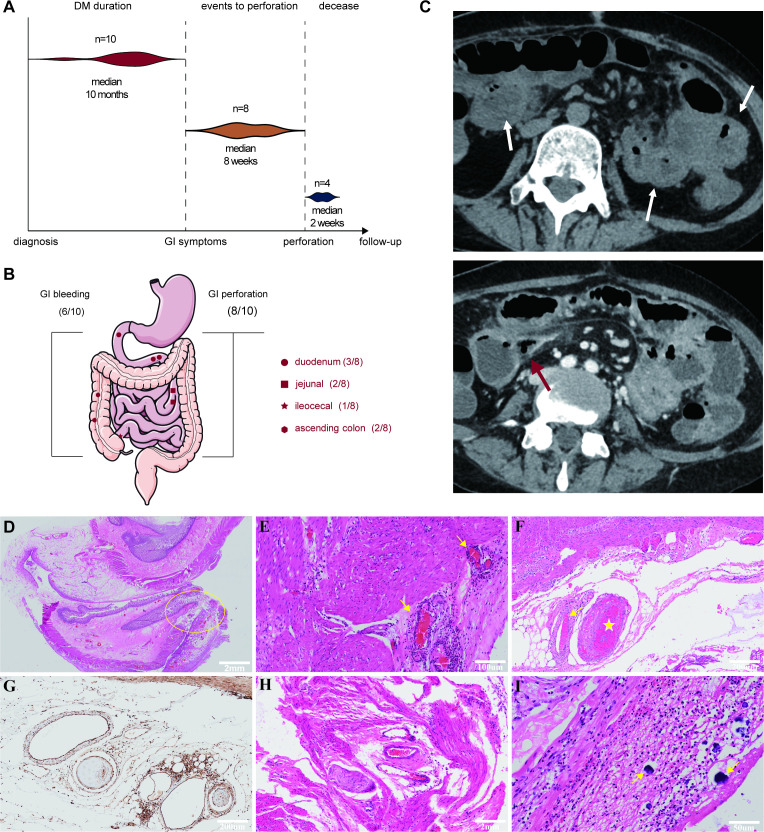
The median time frame between NXP2+ DM diagnosis and the onset of GI symptoms was 10 months (figure 1A), typically after muscle involvement started to subside and when creatine kinase was significantly reduced from the peak. The most common initial symptoms were abdominal pain and melena accompanied by upregulation of inflammatory markers such as C reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Figure 1.
(A) Timeline from NXP2+ DM diagnosis to GI events. (B) Bleeding and perforation sites of patients with NXP2+ DM in gastrointestinal tracts. (C) CT scan indicated diffuse oedema of intestinal wall (white arrow) and perforation (red arrow). (D) Histopathology of the perforation site showed all layers of ulceration (yellow cycle). (E) Vasculitis and perivasculitis were accompanied by intravascular thrombosis in intestinal arteries (yellow arrow) and (F) narrowing (yellow arrow) or occlusion (yellow star) of small and medium arteries. (G) MxA was positive in vascular smooth muscle. (H) Intestinal smooth muscle necrosis. (I) Calcinosis (yellow arrow) on the serosal surface. DM, dermatomyositis; GI, gastrointestinal; NXP2, nuclear matrix protein 2.
GI perforation occurred in 8 of 10 patients (Case1–5, 8–10) 2 weeks to 3 months after the onset of GI symptoms (figure 1A, online supplemental table S1). Most of them were receiving more than 1 mg/kg/day prednisolone equivalent at the time of perforation. Non-steroidal anti-inflammatory drugs were not used in these patients. The small intestine was the most frequent perforation site in our study; however, the stomach and ascending colon also exhibited signs of perforation (figure 1B). CT scan revealed diffuse oedema of intestinal wall with accumulation of fluid and blood in the intestinal lumen (figure 1C), which recapitulates the typical presentation of GI involvements. One case in the GI group did not progress to perforation, but underwent severe GI bleeding instead (Case6), while Case8 had both perforation and bleeding. Endoscopy identified gastroduodenum as the bleeding site for both cases. Case10 had neither perforation nor bleeding, but intractable abdominal pain. Capsule endoscopy and abdominal CT scan revealed multiple jejunal ulcer, extensive intestinal oedema and features of mesenteric panniculitis.
rmdopen-2023-003901supp001.pdf (1.6MB, pdf)
Histopathological features of GI tissues
Histochemical and immunohistochemical staining in intestinal surgical specimens were performed in four perforated patients. Histopathology of the resected intestine revealed that the perforation sites was accompanied by profound inflammation of all layers including mucosal ulceration, submucosal oedema and heavy lymphoid tissues infiltration in all patients (figure 1D). Three relatively specific features were captured: (1) Significant vasculitis, perivasculitis, along with intravascular thrombosis in intestinal arteries (figure 1E). Narrowing or occlusion of small and medium arteries without vessel wall immune cell infiltration could also be appreciated (figure 1F). In addition, MxA was universally positive in vascular smooth muscle (figure 1G), connotating that type I interferon (IFN) pathway may underlie the vasculopathy/vasculitis. (2) Intestinal smooth muscle necrosis was detected with inflammatory cells infiltration, which suggested a possible smooth muscle myositis (figure 1H). (3) Calcinosis similar to subcutaneous calcinosis in NXP2+DM was found on the serosal surface of intestine (figure 1I).
Gene expression profile of intestine in NXP2+ DM
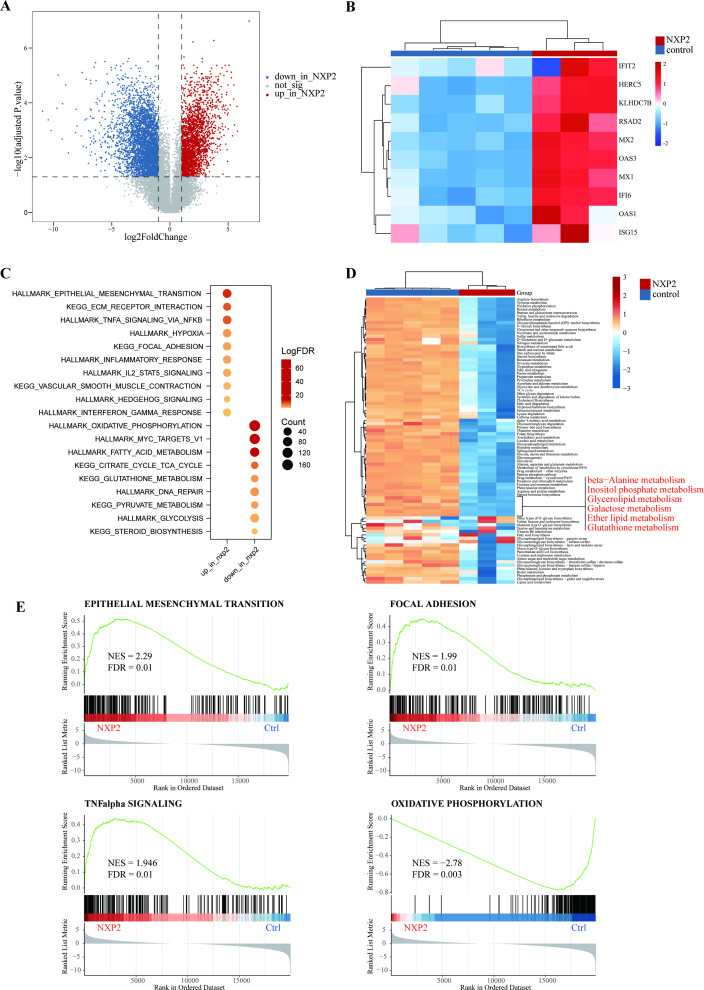
To characterise the underlying molecular changes in GI tracts, bulk RNA-seq was performed for samples from three patients with perforation in comparison to healthy control data set of intestinal samples. According to the principal component analysis (PCA) plot and heating maps, the transcriptomic profiles of patients with NXP2+DM were significantly altered (online supplemental figure S1A,B). A total of 5974 differentially expressed genes (DEGs) were identified including 2339 upregulated and 3634 downregulated genes (absolute logFC>1 and p<0.05) (figure 2A). Consistent with prior study in skeletal muscle biopsies,15 key players in the type I IFN pathway (eg, ISG15, MX1 and IFI6) were the most representative DEGs in the GI samples from patients with NXP2+DM (figure 2B), underpinning the role of type I IFN signalling in such severe GI involvements. The results echoed the findings in previous pathological studies. Functional enrichment analysis using DEGs uncovered other significantly upregulated gene pathways in patients with NXP2+DM, including epithelial mesenchymal transition, ECM receptor interaction and focal adhesion (figure 2C). The activation of focal adhesion involved both upstream ECM-receptor and cytokine–cytokine receptor interactions and therefore lead to cell adhesion, migration and proliferation (online supplemental figure S1C). Tumor necrosis factor (TNF)-α and interleukin (IL)-2-signal transducer and activator of transcription (STAT)5 signalling were also activated as well as hypoxia and inflammatory response. Upregulated vascular smooth muscle contraction pathway may be relevant to vasculopathy. On the other hand, significantly downregulated pathways were mainly oxidative phosphorylation and metabolism-relevant, as shown by enrichment scores of metabolic genes. The most representative of the inhibited metabolisms were beta-alanie, inositol phosphate, glycerolipid, galactose, ether lipid and glutathione metabolism (figure 2D). The results above were verified by GSEA, which visualised the upregulation of epithelial mesenchymal transition, focal adhesion and TNF-α signalling and suppression of oxidative phosphorylation (figure 2E).
Figure 2.
(A) Differential expression genes in intestinal specimens of patients with NXP2+DM compared with healthy control. (B) Interferon type I genes upregulated in the intestinal of patients with NXP2+DM. (C) Upregulated and downregulated pathways indicated by functional enrichment analysis of differentially expressed genes. (D) Enrichment scores of metabolic genes revealed various metabolisms were inhibited in patients with NXP2+DM. (E) Gene sets enrichment analysis showed epithelial mesenchymal transition, focal adhesion and TNF-α signalling were upregulated while oxidative phosphorylation was downregulated. DM, dermatomyositis; NXP2, nuclear matrix protein 2. TNF, tumor necrosis factor. FDR, false discovery rate. NES, normalized enrichment score.
The expression profile of NXP2+DM was compared with that of Crohn’s disease and ulcerative colitis.16 The results showed a significant difference between NXP2+DM and inflammatory bowel disease (IBD) (online supplemental figure S2A). NXP2 shared upregulated TNF-α signalling and IL-2-STAT5 pathway with IBD but had its specific active pathways. The suppressed metabolism pathways of them also had their unique pattern (online supplemental figure S2B).
Treatment and prognosis
GI perforation in NXP2+DM was a catastrophic complication with half of the perforated patients (4/8) deceased. Eight surgical interventions in total, for example, perforation repair and enterectomy were performed in five of eight of the perforated patients. Two elderly patients (Case1 and Case2) who were non-operable soon died of haemorrhagic shock and diffuse intravascular coagulation. Among the five patients who underwent surgical intervention, two patients (Case3 and Case4) ultimately died from recurrent perforation and bleeding, despite Case4 having received a second, yet futile, salvage operation. Case9 who survived underwent three surgeries within 6 months due to recurrence of perforation (online supplemental table S1). Additional risks associated with perforations contributed to the high mortality rate: for example, intraperitoneal or bloodstream infections, especially by Enterobacteriaceae, can be very common.
Due to its rarity and the absence of evidence-based treatment protocol yet, the first-line empirical therapy for NXP2+DM with GI involvement was glucocorticoids (GC) with or without intravenous immunoglobulin, the response of which was unfortunately suboptimal. The investigator-dependent treatment protocol continues to evolve though as the case series and data accumulate. Two perforated patients received Janus kinase inhibitor post-surgery, considering the type I IFN-related vasculitis/vasculopathy. Case8 received baricitinib 2 mg one time a day and Case5 tofacitinib 5 mg two times a day. Both cases were ameliorated, achieving low disease activity status in DM and GI domain with prednisone tapering to lower than 10 mg per day within 2 years follow-up. However, Case8 still suffered from intermittent abdominal pain despite stabilised inflammatory markers and anaemia. Nevertheless, the acute abdominal phase often precluded Janus kinase inhibitors utility for the route of per os administration.
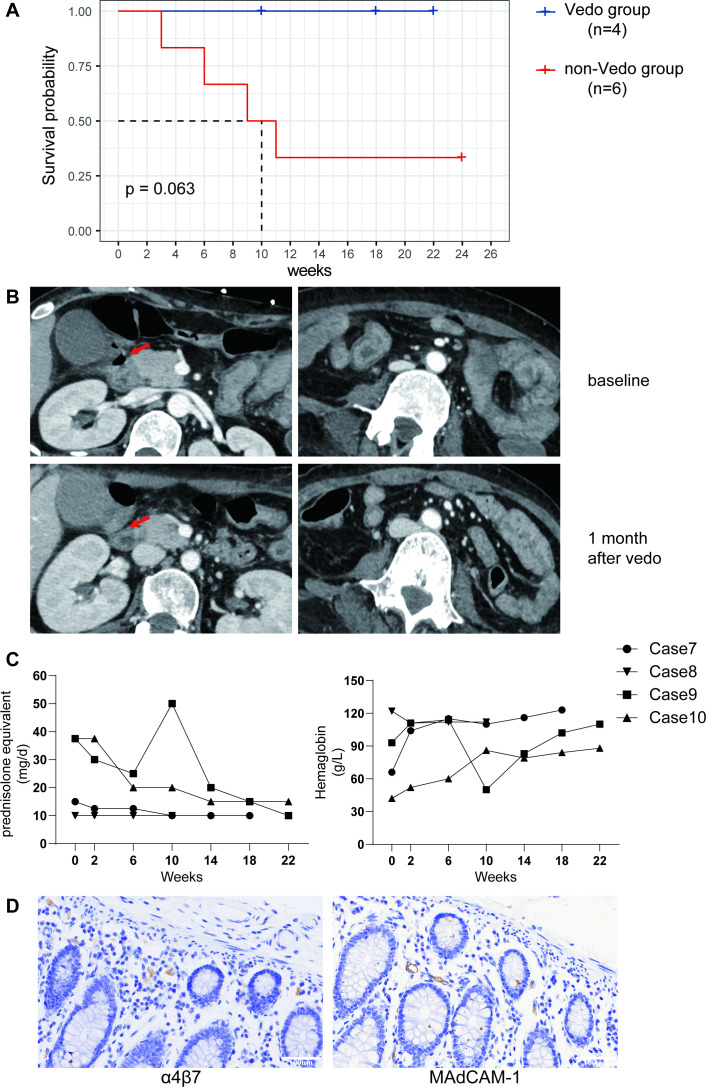
With the attempts to balance between GI immune-mediated processes and the vulnerability to infection, vedolizumab was introduced to block the migration of lymphocytes to the inflamed intestine. Vedolizumab is a monoclonal antibody that occludes the interaction between α4β7 integrins on T lymphocytes and mucosal vascular addressin cell adhesion molecule 1 (MAdCAM1) on gut endothelial cells. With its favourable efficacy and safety profile, it has been approved for treating IBD. Four patients received the treatment of vedolizumab with informed consent obtained in this case series, three of which (Case8, Case9 and Case10) were perforated patients. The fourth patient (Case7) reported of intractable abdominal pain resistant to various immunosuppressants (online supplemental table S1). All four patients receiving vedolizumab survived with a dosing of 300 mg for three to five infusions and a median drug exposure of 20 weeks (figure 3A). CT scan revealed improved perforation and intestinal oedema 1 month after the initiation of vedolizumab in Case10 who eventually unnecessitated surgical intervention (figure 3B). Moreover, along with relieved GI symptoms, the improvement of anaemia and GCs tapering can be appreciated. Case8 and Case9 had GI perforation and surgical intervention before vedolizumab application; after two doses of vedolizumab infusion, the GI symptoms have abated. However, Case9 and family turned to another institution seeking therapeutic trial of faecal microbiota transplantation, after which the abdominal pain and gastrointestinal perforation recurred. She was readmitted and received the third emergency surgeries. Re-initiation of vedolizumab immediately after the surgery resulted in gradually improved GI symptoms and anaemia. She had a full recovery, and her GCs was tapered to 10 mg prednisone after 2 months follow-up (figure 3C). Indeed, immunohistochemical staining proved α4β7+T cells were infiltrated in GI tracts as well as MAdCAM1 positivity in Case9 (figure 3D).
Figure 3.
(A) Kaplan-Meier survival curves of patients using vedolizumab compared with non-vedolizumab users. (B) CT images comparison of a patient pre-vedolizumab and post-vedolizumab treatment. Red arrow indicated the perforated site. (C) Follow-up (weeks) of patients using vedolizumab in glucocorticoids tapering (mg/day, prednisolone equivalent) and haemoglobin recovery (g/L). (D) Immunohistochemical staining showed α4β7 as well as MAdCAM1 positive in the intestinal of a perforated nuclear matrix protein 2+ patient. MAdCAM1, mucosal vascular addressin cell adhesion molecule 1.
Discussion
Although GI involvements in patients with juvenile DM were previously described,17–20 this is the first case series reporting in adult NXP2+DM. The referral bias likely existed, since patients who were more severely ill or had entangled complications were frequently transferred to our hospital which served as a tertiary referral centre; nevertheless, our data do suggest that GI involvement in adult NXP2+DM is a significant phenomenon with its own pattern. GI perforation and bleeding is the typical manifestation and emergency surgery is usually required. Further, recurrence of the disease is not uncommon and patients may require multiple operations. With its high mortality rate, this complication demands increased awareness and early recognition.
We confirm that abdominal pain and melena as the initial signs of GI involvements, usually several months after NXP2+DM diagnosis with myositis stepdown. NXP2+DM with GI involvements in adults is dominant in women and those with more systemic disease, for example, ILD and calcinosis. Unexplained anaemia may serve as a red herring for GI involvements. Imaging study and endoscopy can be helpful to establish the diagnosis. Both upper and lower GI tract, all their layers inclusive, can be affected in adult NXP2+DM. The three most typical pathological features include: profound vasculitis/vasculopathy, intestinal smooth muscle necrosis and serosal calcinosis. Recent studies reported acute endarteropathy and occlusion of small and medium-sized arteries by microthrombus in mucosa and submucosa in patients with juvenile DM, which led to intestinal ischaemia and necrosis. The authors indicated that vasculopathy but not vasculitis was responsible for the perforation.17 21 However, in our study, we have identified significant infiltration of lymphocytes in vascular walls and perivascular which is classic of vasculitis; along with vasculopathic narrowing or occlusion of small and medium arteries. Moreover, positive staining of MxA indicated that type-I IFN may be an important driving force. These histopathology findings are consistent to that by muscle biopsy investigations,22 23 where microinfarction and MxA positive inflammation are typical for NXP2+DM. In addition, subcutaneous calcinosis was one of the most representative features of NXP2+ DM, which we described for the first time also being identified in GI tracts.
To take a deeper look, transcriptomic analysis revealed that IFN-related genes, which are known to be upregulated in skin and muscle, were indeed significantly increased in GI samples. Other inflammatory pathway activation accompanied IFN, such as TNF-α and IL-2-STAT5. In addition, the epithelial mesenchymal transition (EMT) pathway was activated in NXP2+DM. EMT usually occurs in wound healing and fibrosis reaction to battle inflammation. EMT is described as the loss of epithelial functionality, impairment of the epithelial capacity to regenerate the damaged tissue and exacerbation of inflammation and recruitment of immune cells during the epithelial response to injury. EMT is eminently involved in intestinal fibrosis and fistulae formation in IBD.24 The EMT finding which reflects tissue repair and GI tract remodelling, was in line with the subacute course of GI involvement in NXP2+DM, that is, it took weeks and months to perforate and the inflammation tend to persist and recur after surgery. Also, subclinical GI involvement may happen way before symptom onset, which revives the consideration for increased screening sensitivity and punctuality, as it may facilitate early identification and intervention. Another important pathway revealed by RNA-seq was the focal adhesions (FAs) pathway. Integrin mediated FAs activation with enhanced cell migration and motility25 can orchestrate the interplay between stromal cells and immune cells. Finally, the oxidative phosphorylation and universal metabolic suppression in NXP2+DM reflect the downstream effects of tissue necrosis. All these molecular pathways may inspire potential treatments for this significant yet unresolved disease.
The treatments for NXP2+DM with GI involvements are largely empirical and supportive. Our data suggested, in line with other reports,26 that high dose GC might be a risk factor for GI perforation, especially in elderly patients. In addition, emergency surgical intervention for perforation is hardly a sustainable approach for this systemic disease. Targeting IFN pathway is a promising therapeutic choice for DM in general. An increasing27 amount of evidence now supports the inhibition of Janus kinase (JAK), which is downstream of the type I IFN pathway. However, whether JAK inhibitors could be an option in the early phase, for example, subclinical GI involvement, or as maintenance therapy afterwards, remains an open question. On the other hand, during the full-blown episode of GI event, anti-cell migration therapy by blocking integrin can be an attractive novel approach. Vedolizumab, an α4β7-integrin-specific antibody, achieved success in augmenting remission rates and in reducing GC use in IBD with an excellent safety profile.28 Considering shared mechanisms, vedolizumab was introduced into NXP2+DM GI involvements therapy. Although only four patients received this therapy, a signal of improved prognosis can be appreciated since all four patients survived the GI event. However, the efficacy and safety of vedolizumab still need to be testified by further clinical trials.
There are still some limitations in our study: (1) All patients included in our study were Chinese Han people. These results should be examined in other various ethnicities. (2) The screening of NXP2+DM was performed by line-blot and all the positive results were validated by ELISA. However, immunoprecipitation (IP) was a more sensitive method in detection of anti-NXP2 antibodies.29 Therefore, the estimation of incident rate may be improved by IP method.
Taken together, GI involvement is a relatively rare but grave complication of adult NXP2+DM, usually rising months after myositis starting to stepdown. Vigilance with early recognition is critical in the regard of its poor prognosis leaving unchecked. Type I IFN-related vasculitis/vasculopathy might play a central role in the pathophysiology. Some novel targeted treatments such as vedolizumab or JAK inhibitors deserve further investigations.
Acknowledgments
The authors appreciated the contributions of all the participants in this study. We thank Professor Wenhua Zhu from Shanghai Huashan Hospital and Claire Ye from Imperial College London, for their critical assistance in pathological works and manuscript preparation, respectively.
Footnotes
YF, LG and JC contributed equally.
Correction notice: This article has been corrected since it was first published online. In the original article, Dr Qiong Fu was not listed as a corresponding author. This has now been amended. In addition to this, the affiliation for Yakai Fu and Xiaodong Wang has been updated to Department of Rheumatology, Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital, Shanghai, China.
Contributors: YF, QFu and SY contributed to design the study and conception as well as draft the manuscript. YF, LG and JC contributed to collection, analysis and interpretation of the data. YD contributed to analyse the transcriptomic data. QFe contributed to analysis of the CT images. JF and MG contributed to analysis of the pathology. ZC and XW contributed to analysis of clinical data. All the authors reviewed and approved the final submitted manuscript. SY is responsible for the overall content as guarantor.
Funding: This research is supported by grant from the Clinical Research Plan of Shanghai Hospital Development Center (Project No. SHDC2020CR1015B).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All sequencing data included in this study are available at the National Omics Data Encyclopedia (NODE) database (OEP004694).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The retrospective study was approved by the ethics committees of Renji Hospital, Shanghai Jiao Tong University School of Medicine (RA-2020-516).
References
- 1.Mammen AL, Allenbach Y, Stenzel W, et al. Group Etws. 239Th ENMC International workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14-16 December 2018. Neuromuscular Disorders 2020;30:70–92. 10.1016/j.nmd.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, Uruha A, Suzuki N, et al. Integrated diagnosis project for inflammatory Myopathies: an association between Autoantibodies and muscle pathology. Autoimmun Rev 2017;16:693–700. 10.1016/j.autrev.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 3.DeWane ME, Waldman R, Lu J. Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol 2020;82:267–81. 10.1016/j.jaad.2019.06.1309 [DOI] [PubMed] [Google Scholar]
- 4.Gunawardena H, Wedderburn LR, Chinoy H, et al. Autoantibodies to a 140-Kd protein in juvenile dermatomyositis are associated with Calcinosis. Arthritis Rheum 2009;60:1807–14. 10.1002/art.24547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimoto M, Watanabe R, Ishitsuka Y, et al. Recent advances in dermatomyositis-specific Autoantibodies. Curr Opin Rheumatol 2016;28:636–44. 10.1097/BOR.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 6.Yan T-T, Zhang X, Yang H-H, et al. Association of anti-Nxp2 antibody with clinical characteristics and outcomes in adult dermatomyositis: results from clinical applications based on a Myositis-specific antibody. Clin Rheumatol 2021;40:3695–702. 10.1007/s10067-021-05667-x [DOI] [PubMed] [Google Scholar]
- 7.Ichimura Y, Konishi R, Shobo M, et al. Anti-nuclear matrix protein 2 antibody-positive inflammatory Myopathies represent extensive Myositis without dermatomyositis-specific rash. Rheumatology (Oxford) 2022;61:1222–7. 10.1093/rheumatology/keab518 [DOI] [PubMed] [Google Scholar]
- 8.Matas-Garcia A, Milisenda JC, Espinosa G, et al. Gastrointestinal involvement in dermatomyositis. Diagnostics (Basel) 2022;12:1200. 10.3390/diagnostics12051200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Sun C, Zhang L, et al. Clinical heterogeneity of patients with Antinuclear matrix protein 2 antibody-positive Myositis: A retrospective cohort study in China. J Rheumatol 2022;49:922–8. 10.3899/jrheum.211234 [DOI] [PubMed] [Google Scholar]
- 10.Lundberg IE, Tjärnlund A, Bottai M, et al. European League against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory Myopathies and their major subgroups. Arthritis & Rheumatology 2017;69:2271–82. 10.1002/art.40320 Available: https://acrjournals.onlinelibrary.wiley.com/toc/23265205/69/12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chen Y, Shi C, et al. Soapnuke: a Mapreduce acceleration-supported software for integrated quality control and Preprocessing of high-throughput sequencing data. Gigascience 2018;7:1–6. 10.1093/gigascience/gix120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strohmeier V, Andrieux G, Unger S, et al. Interferon-driven immune dysregulation in common variable immunodeficiency-associated Villous atrophy and Norovirus infection. J Clin Immunol 2023;43:371–90. 10.1007/s10875-022-01379-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-Seq data with Deseq2. Genome Biol 2014;15:550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, et al. Machine learning Algorithms reveal unique gene expression profiles in muscle biopsies from patients with different types of Myositis. Ann Rheum Dis 2020;79:1234–42. 10.1136/annrheumdis-2019-216599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido-Trigo A, Corraliza AM, Veny M, et al. Macrophage and neutrophil heterogeneity at single-cell spatial resolution in human inflammatory bowel disease. Nat Commun 2023;14:4506. 10.1038/s41467-023-40156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamyrova G, Kleiner DE, James-Newton L, et al. Late-onset gastrointestinal pain in juvenile dermatomyositis as a manifestation of ischemic ulceration from chronic Endarteropathy. Arthritis Rheum 2007;57:881–4. 10.1002/art.22782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gitiaux C, De Antonio M, Aouizerate J, et al. Vasculopathy-related clinical and pathological features are associated with severe juvenile dermatomyositis. Rheumatology (Oxford) 2016;55:470–9. 10.1093/rheumatology/kev359 [DOI] [PubMed] [Google Scholar]
- 19.Gadiparthi C, Hans A, Potts K, et al. Gastrointestinal and hepatic disease in the inflammatory Myopathies. Rheum Dis Clin North Am 2018;44:113–29. 10.1016/j.rdc.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Ma X, Zhou Z, et al. Gastrointestinal Perforation in anti-Nxp2 antibody-associated juvenile dermatomyositis: case reports and a review of the literature. Pediatr Rheumatol Online J 2021;19:2. 10.1186/s12969-020-00486-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebert EC. Review article: the gastrointestinal complications of Myositis. Aliment Pharmacol Ther 2010;31:359–65. 10.1111/j.1365-2036.2009.04190.x [DOI] [PubMed] [Google Scholar]
- 22.Tanboon J, Inoue M, Saito Y, et al. Dermatomyositis: muscle pathology according to antibody subtypes. Neurology 2022;98:e739–49. 10.1212/WNL.0000000000013176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q, Li QX, Bi FF, et al. The association between Myositis-specific Autoantibodies and muscle Pathologies in idiopathic inflammatory Myopathies. Clin Rheumatol 2021;40:613–24. 10.1007/s10067-020-05274-2 [DOI] [PubMed] [Google Scholar]
- 24.Lovisa S, Genovese G, Danese S. Role of epithelial-to-Mesenchymal transition in inflammatory bowel disease. J Crohns Colitis 2019;13:659–68. 10.1093/ecco-jcc/jjy201 [DOI] [PubMed] [Google Scholar]
- 25.Chen S, He T, Zhong Y, et al. Roles of focal adhesion proteins in skeleton and diseases. Acta Pharm Sin B 2023;13:998–1013. 10.1016/j.apsb.2022.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller LC, Michael AF, Kim Y. Childhood dermatomyositis. clinical course and long-term follow-up. Clin Pediatr (Phila) 1987;26:561–6. 10.1177/000992288702601101 [DOI] [PubMed] [Google Scholar]
- 27.Le Voyer T, Gitiaux C, Authier F-J, et al. JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis: a Monocentric retrospective study. Rheumatology (Oxford) 2021;60:5801–8. 10.1093/rheumatology/keab116 [DOI] [PubMed] [Google Scholar]
- 28.Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 2017;14:269–78. 10.1038/nrgastro.2016.208 [DOI] [PubMed] [Google Scholar]
- 29.Ichimura Y, Konishi R, Shobo M, et al. Reliability of Antinuclear matrix protein 2 antibody assays in idiopathic inflammatory Myopathies is dependent on target protein properties. J Dermatol 2022;49:441–7. 10.1111/1346-8138.16295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003901supp001.pdf (1.6MB, pdf)
Data Availability Statement
Data are available upon reasonable request. All sequencing data included in this study are available at the National Omics Data Encyclopedia (NODE) database (OEP004694).