Abstract
We have shown previously that intravenous injection of Candida albicans mannan (MAN) into naive mice induced CD8+ effector downregulatory cells and that such cells were not produced if mice were deficient in CD4+ or I-A+ cells during the early interval (≤30 h) following the introduction of MAN. Moreover, the nonspecific biological response modifier monophosphoryl lipid A (MPL), given in vivo or incubated with cells in vitro, can abrogate the MAN-specific immunomodulatory activity. The mechanism by which the abrogation is mediated is unknown, but it is hypothesized to involve cytokines. Therefore, we measured the number of cytokine-secreting cells for the Th1 cytokine interleukin-2 (IL-2) and the Th2 cytokine IL-4, as well as for gamma interferon (IFN-γ), in splenocyte populations from MAN and/or MPL-treated mice, using an enzyme-linked immunospot assay designed to detect individual cytokine-secreting cells (spot-forming cells [SFC]). Cytokine-secreting cells were demonstrated in cell suspensions enriched for CD4+ cells, but no SFC could be demonstrated in populations enriched for CD8+ cells. Both MAN and MPL, when administered to separate groups of animals, stimulated the production of increased numbers of cytokine-producing cells for each of the three cytokines tested. The response with respect to IL-4-secreting cells, however, was the most striking. Despite the fact that MAN and MPL independently caused increases in SFC to all three cytokines, when both MAN and MPL were administered to the same animal, all increases were reversed, and the numbers of SFC detected were at or below those detected in saline control animals. These data support the hypothesis that IL-4 is involved in MAN-specific immunoregulatory activities. The data also emphasize the fact that two immunomodulators, i.e., MAN and MPL, having similar effects when given in vivo independently, may be antagonistic when administered sequentially to the same animal.
In previous studies from our laboratory, we illustrated the presence in mice of MAN-specific CD8+ cells capable of downregulating delayed hypersensitivity (DH) in immunized animals (18). We have also demonstrated that effector downregulatory cells were genetically restricted and that CD4+ and I-A+ cells were required for the development of the CD8+ cells at an early stage after exposure to MAN (30). However, little is known regarding the mechanism by which CD4+ cells induce CD8+ effector cells, or regarding the mechanism by which downregulation itself is affected, although both events or series of events likely involve cytokines.
Cytokine involvement has been implicated in a wide range of downregulated immunologic phenomena (15, 23, 57). Sher et al. (50) stressed the importance of interleukin-4 (IL-4), IL-10, and transforming growth factor β as the best-characterized inhibitory lymphokines, their activity contributing at least in part to the downregulation of cell-mediated immunity in both parasitic (45) and retroviral (21) infections. Others have implicated IL-2 (57) and gamma interferon (IFN-γ) (11, 22, 25). For fungal models, few data are available to implicate a particular cytokine in a specific inhibitory phenomenon. Buchanan and Murphy (7), however, reported decreased quantities of IL-2 and IFN-γ in antigen-soaked sponges implanted in animals given cryptococcal antigen-specific suppressor-inducer (Ts1) cells, but whether the decreased production of these two cytokines resulted from the lack of stimulation of T cells involved in the normal DH response or from a more direct effect of a third factor and/or cell downregulating the production of the two cytokines is unknown. IL-5 was detected in the sponges as well, but there were no differences between those from immune and downregulated mice. Despite attempts to do so, no IL-4 was detected in the cryptococcal model.
In addition to demonstrating the phenotype of the inducer and effector cells in the MAN-specific pathways, we have determined that the downregulatory activity of the effector cell could be abrogated by in vivo treatment of animals with monophosphoryl lipid A (MPL) (14) or by in vitro treatment of effector cell suspensions with MPL prior to transfer to immunized recipients (12). The in vitro incubation requires very small amounts of MPL to be effective, and the incubation time is short and at low temperature, 30 min at 4°C. Baker et al. (4, 5) were the first to show that MPL was an effective modulator of downregulatory activity associated with Ts lymphocytes. They showed that the antigen-specific unresponsiveness induced by a single injection of a marginally immunogenic dose of pneumococcal polysaccharide type III was inactivated both in vivo and in vitro by MPL.
MPL is a derivative of bacterial lipopolysaccharide (LPS) which retains the adjuvant properties of LPS but loses most of the toxicity and pyrogenicity associated with the parent molecule, even when administered at high doses (40, 41). There have been a few reports in which selected cytokines, such as IL-1, IL-2, IL-6, tumor necrosis factor alpha (TNF-α), and IFN-γ, have been implicated in the activity of LPS or MPL (2, 24, 54), but none have involved investigations of the role of lymphokines and LPS or MPL on downregulatory activity attributable to T lymphocytes.
Since we have a well-defined model for the induction of CD8+ effector downregulatory cells, and since we have established the conditions under which the activity of these CD8+ cells can be abrogated, we decided to use this model to begin our investigations of the potential role of various cytokines in the MAN-specific immunoregulatory phenomena that we have described. Moreover, since much has been written about the Th1 and Th2 divisions of lymphocytes in the mouse (34, 35), especially from the point of view of their having opposing effects, we decided to concentrate on one cytokine from each of those classes, viz., IL-2 (Th1) and IL-4 (Th2), in addition to the cytokine IFN-γ, the latter of which is produced by multiple cell types, including macrophages, NK cells, and Th1 cells. IL-4 was selected in particular because of its known negative effect on experimentally induced candidiasis (10, 42, 53) and because of its critical role in contact sensitivity (1, 46, 58), one manifestation of DH.
MATERIALS AND METHODS
Mice.
Male CBA/J mice were obtained from Jackson Laboratory (Bar Harbor, Maine). Animals were 2 to 4 months of age at the time of use. They were provided food and water ad libitum and maintained in hoods under laminar flow conditions.
Culture and fractionation procedures.
Candida albicans 20A, a serotype A isolate originally obtained from E. Reiss (Centers for Disease Control and Prevention, Atlanta, Ga.) was used to extract MAN. It was maintained at 4°C by regular transfer on Sabouraud dextrose agar (Emmons modified; Adam Scientific Inc., West Warwick, R.I.). Viable blastoconidia were harvested from cultures in Trypticase soy dialysate broth (39) incubated for 18 h at 37°C with constant aeration and agitation. Following centrifugation, the blastoconidia were washed with 0.15 M phosphate-buffered saline (PBS; pH 7.2) containing 2 mM phenylmethylsulfonyl fluoride. Blastoconidia were stored frozen in PBS containing the inhibitor. MAN was extracted from whole blastoconidia as previously described in detail (13) by the method of Peat et al. (37) as modified by Kocourek and Ballou (27). It was dialyzed for 4 days against sterile distilled water and lyophilized. MAN extracted in this manner typically consists of approximately 95% mannose and 5% protein.
MPL, MAN, and animal inoculations.
MPL from Salmonella typhimurium was a gift from Ribi ImmunoChem Research, Inc. (Hamilton, Mont.). It was prepared for injection into animals as described previously (14). Fifty micrograms of MPL was injected intraperitoneally (i.p.) at selected times prior to sacrifice. The quantity of MPL and the timing of the injections were based on previous studies (14). To induce the production of MAN-specific downregulatory cells, 500 μg of MAN dissolved in nonpyrogenic saline (NPS; McGaw, Inc., Irvine, Calif.) was administered intravenously (i.v.) to mice via the lateral tail vein at various times prior to sacrifice. The quantity of MAN and the timing of its administration with respect to MPL and the development of MAN-specific downregulatory cells were based on previous studies (14, 18).
Preparation, treatment, and analysis of lymphoid cells.
Single-cell suspensions were prepared from murine spleens by using Hanks’ balanced salt solution (HBSS; Life Technologies, Inc., Grand Island, N.Y.) supplemented with 0.2% bovine serum albumin (BSA; Sigma Chemical Co., St. Louis, Mo.) by gently macerating the tissue between the frosted ends of two glass slides. Erythrocytes were removed by treating cell suspensions with ACK lysing buffer (28). T-enriched cell suspensions were obtained by depleting splenocytes of surface immunoglobulin-positive cells by panning on petri dishes coated with affinity-purified anti-mouse immunoglobulin M (μ-chain specific; Sigma) (30).
CD4+ and CD8+ T-enriched cells were obtained by magnetic cell sorting (33) of populations which had first been T enriched by panning on anti-μ-coated petri dishes. The equipment and reagents were purchased from Miltenyi Biotec Inc. (Auburn, Calif.). Briefly, T-enriched splenocytes were suspended in cold PBS-BSA (PBS [pH 7.2], 5 mM EDTA, 1% BSA) and incubated on ice with anti-Ly2 microbeads (20 μl/107 cells) for 15 min. After washing with medium, the cells were passed through the separation column which had been placed in the magnetic field. This resulted in the removal of CD8+ cells and enrichment for CD4+ cells. The cells remaining on the column, CD8+, were eluted after removing the column from the magnetic field.
The purities of various cell populations were determined by flow cytometry in the following manner: 105 cells were suspended in 0.2 ml of staining buffer (36) and incubated for 20 min on ice with a 1:40 dilution of fluorescein isothiocyanate-conjugated anti-CD4 and a 1:80 dilution of phycoerythrin-conjugated anti-CD8 (Gibco BRL, Grand Island, N.Y.). After washing three times with staining buffer and fixing with 1% paraformaldehyde, analysis was performed using a FACScan cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Cytokine ELISPOT.
Individual cytokine-producing cells in lymphoid cell suspensions were detected by enzyme-linked immunospot (ELISPOT) methodology as originally described by Taguchi et al. (52) and Fujihashi et al. (17) and detailed in Current Protocols in Immunology (26). We followed the suggestions of the manufacturer (PharMingen, San Diego, Calif.) when determining the concentration of capture and detecting antibodies in preliminary experiments. The conditions used included the following. Multiscreen-HA Filtration Plates (Millipore, Bedford, Mass.) were coated with capture antibodies specific for IL-2 (ES6-1A12), IL-4 (BVD4-1D11), and IFN-γ (P4-6A2), using 4, 2, and 8 μg/ml, respectively, by incubating the plates overnight at room temperature. Plates were blocked by the addition of culture medium (31), incubated at 37°C for 30 min, and then washed with HBSS-BSA before the addition of 100 μl of a cell suspension containing 6 × 105 cells. The cultures were incubated at 37°C in 5% CO2 for 24 h, after which cells were removed by washing with PBS containing 0.05% Tween 20 (Sigma) (washing buffer). This was followed by the addition of biotinylated antibodies, all purchased from PharMingen, to the appropriate wells; biotinylated anti-IL-2 (JES-6-5 H4), anti-IL-4 (BVD6-24G2), and anti-IFN-γ (XMG1.2) antibodies, diluted with PBS containing 1% BSA (diluting buffer), were added at 0.5, 2, and 1 μg/ml, respectively. Following incubation at 4°C overnight and washing with washing buffer, peroxidase-labeled goat anti-biotin antibodies (Vector Laboratories, Burlingame, Calif.), diluted 1:1,500 with diluting buffer, were added. The plates were incubated for an additional 3 h at 37°C. The unbound peroxidase-labeled antibodies were removed by extensive washings with washing buffer, and aminoethylcarbazole (Sigma), prepared according to the manufacturer’s recommendation, was added to detect reactants with the peroxidase-labeled antibody. Brown spots, representing areas on the surface of the plates where individual cells secreting the cytokine under test had rested, were counted with the aid of a dissecting microscope. Results were expressed as mean spot-forming cells (SFC) per 6 × 105 cells, using duplicates or triplicates for each condition. When groups of three or more mice were used, each spleen was assayed independently. When two mice constituted a group, the splenocytes were pooled for analysis.
Statistics.
For most experiments in which ELISPOT analyses were done, groups consisted of three to six mice wherein each mouse was assayed independently. In such cases, the nonparametric Mann-Whitney test was used to determine statistically significant differences. No statistical analyses could be done with groups where the splenocytes were pooled prior to assay.
RESULTS
Influence of MAN on the frequency of IL-2-, IFN-γ-, and IL-4-secreting splenocytes.
As we had shown previously that MAN injected i.v. into naive mice resulted in the induction of fully functional MAN-specific downregulatory CD8+ splenocytes 72 to 96 h following the introduction of MAN (18), we decided to assay splenocyte populations for the numbers of cells secreting selected Th1 and Th2 cytokines at intervals ranging from 0 to 96 h after the injection of MAN or saline. Most cell populations were analyzed by flow cytometry as well.
It was clear from the flow cytometric analyses of splenocyte populations that the total percentage of CD4+ and CD8+ in splenocyte populations decreased following the administration of MAN, with the maximum depression occurring at 48 h. There was a subsequent increase thereafter, so that by 96 h the total percentage approached that seen in suspensions derived from saline-treated animals. For example, in one experiment where MAN was given i.v. 24, 48, 72, or 96 h prior to sacrifice, the total percentages of CD4+ and CD8+ cells in the spleens were 31, 15, 20, and 26, respectively, compared to 31% in saline-treated controls. A decrease in the proportion of lymphocytes to other cells, however, did not result in notable alterations in the ratio of CD4+ to CD8+ cells. These results were confirmed in four other experiments. In saline-treated animals, the percentage of CD4 compared to CD8 was 64 to 36, while the percentages 24, 48, 72, and 96 h after giving MAN were 55 to 45, 57 to 43, 60 to 40, and 61 to 39, respectively. We have determined in the past (unpublished data) that the injection of MAN i.v. into mice causes an increase in the number of MAC-1+ cells in spleens. Thus, we assume, although we have not proven it in the studies presented here, that the proportion of T cells in the spleens of MAN-treated mice under these experimental conditions decreases because of a relative increase in macrophages.
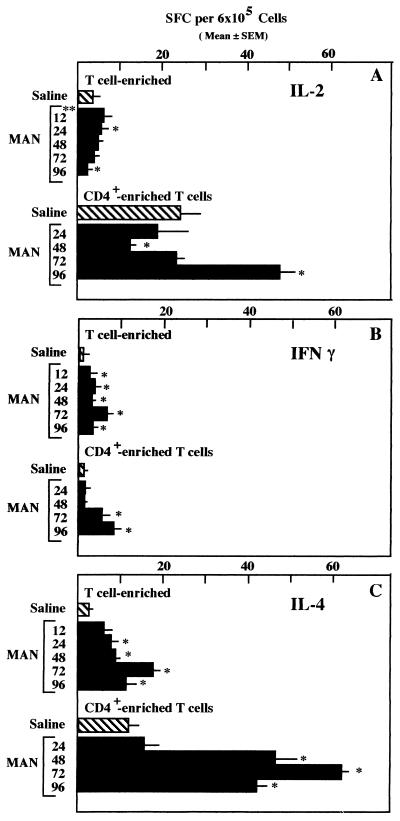
Data illustrating the development of cytokine-secreting cells over time, as well as the demonstration that cytokine-secreting activity was retained in the CD4+-enriched population, are illustrated in Fig. 1 and 2. The highest number of cytokine-secreting cells occurred in the CD4+-enriched subgroup secreting IL-4. As can be seen in Fig. 1, significant increases in IL-4-secreting cell numbers were demonstrable beginning at 24 and 48 h when both T-enriched and CD4+-enriched cell populations were assayed, respectively. There were no detectable CD8+ cells in the CD4+ populations shown in Fig. 1. However, there were other cells, presumably macrophages, in those populations. Flow cytometric analysis showed that both the control and 96-h CD4+ populations contained 80% CD4+ cells, whereas the suspensions obtained at 24, 48, and 72 h were only 56, 50, and 53% CD4+ cells. The greatest increases in IL-4-secreting SFC occurred at 72 h regardless of the percentage of CD4+ cells in the population tested. As the cell populations at 48 h always had fewer T cells in them than those at other intervals tested, the data obtained at 48 h for the cell populations that were T enriched, but unseparated with respect to CD4+ and CD8+, may be skewed on the low side. Despite the differences in percentages of CD4+ cells in the various populations, however, if all cell populations being compared were normalized to 100%, there would be even larger differences between the control population and the populations from the MAN-treated animals. In other experiments, 0 to 13% of the CD4+-enriched cell suspensions were CD8+. Despite the presence of CD8+ cells in the CD4+-enriched populations, reproducible data were obtained in at least three additional experiments.
FIG. 1.
Enumeration of IL-2-, IFN-γ-, and IL-4-secreting cells (SFC) among T- or CD4+-enriched splenocytes prior to or at intervals up to 96 h after administration of 500 μg of MAN i.v. Control animals were given saline 24 h prior to sacrifice. n = 4; significance determined by the Mann-Whitney test; ∗, P ≤ 0.05; ∗∗, hours following administration of MAN.
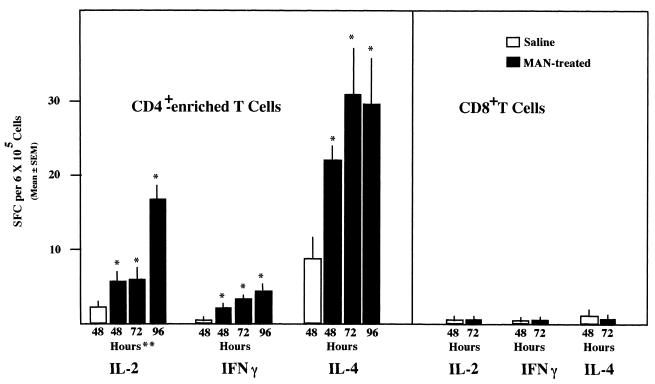
FIG. 2.
Enumeration of IL-2-, IFN-γ-, and IL-4-secreting cells (SFC) among CD4+- and CD8+-enriched splenocytes from mice treated with saline 48 h prior to sacrifice or 500 μg of MAN i.v. 48, 72, or 96 h prior to sacrifice. n = 4; significance determined by the Mann-Whitney test; ∗, P ≤ 0.05 compared to saline control; ∗∗, hours following administration of saline or MAN.
The increases in IL-2- and IFN-γ-secreting cells were not as dramatic as those for IL-4-secreting cells. In fact, the only time that increases in the number of IL-2-secreting cells were noted consistently was when CD4+-enriched cells from spleens of mice treated with MAN 96 h prior to sacrifice were assayed (Fig. 1A and 2). On the other hand, although the numbers of cytokine-secreting cells observed with IFN-γ were considerably fewer than those observed with IL-4, there was a consistent, statistically significant increase in the number of cells between 48 and 72 h in each of eight experiments, four experiments with T-enriched cells and four experiments with CD4+-enriched cells. With the T-enriched cells, there were significant increases detected at earlier time periods as well in several experiments.
To determine whether CD8+ cells were responsible for some of the increases in SFC following MAN treatment, naive mice were injected with either MAN or saline and sacrificed 48, 72 and 96 h later. CD4+ and CD8+ cells were isolated by magnetic cell sorting, using magnetic beads coated with anti-CD8, for the 72-h cell population, whereas the 48- and 96-h populations were enriched only for CD4+ cells. The data for the 48- and 96-h time points are shown to emphasize the reproducibility of the data shown in Fig. 1, reproducibility with respect to the kinetics of SPC development, not absolute numbers. Flow cytometric analysis indicated that the percentages of CD4+ in the suspensions at 48, 72, and 96 h were 85, 90, and 89, respectively, and that for the CD8+ cells was 98%. As is obvious in Fig. 2, CD8+ cells secreted no cytokines, whereas CD4+-enriched populations had SFC for all three cytokines. Since the CD4+-enriched populations were obtained by negative selection, it is not possible to determine whether the IFN-γ-producing cells were CD4+ cells or whether they were NK cells contaminating the preparation. As shown previously (Fig. 1), significant increases in SFC for all three cytokines were detected in MAN-treated mice when compared to saline controls.
As it is possible, but not probable, that the act of administering saline to control animals could alter the levels of SFC in the spleen, three groups of two mice each were given NPS i.v. 12, 48, or 96 h prior to sacrifice, and then the numbers of IFN-γ- and IL-4-secreting cells in T-enriched splenocyte populations from these animals were compared to those in T-enriched splenocyte populations from two additional groups of mice, viz., untreated mice and mice given MAN 96 h prior to sacrifice. Splenocytes were pooled for assay within each of the five groups prior to enriching for T cells. MAN, as has been illustrated above, stimulated an increase in SFC, demonstrable at 96 h for both IL-4-secreting SFC and IFN-γ-secreting SFC, 40 and 10 SPC, respectively, compared to ≤15 and ≤2 SFC, respectively, in saline-treated animals. Untreated animals had 14 SFC for IL-4 and 2 for IFN-γ. The IFN-γ portion of this experiment was repeated, with observations being made over an even broader range of time points, with similar results.
Changes in the frequency of IL-2-, IFN-γ-, and IL-4-secreting splenocytes from saline- and MAN-treated mice in response to treatment with MPL.
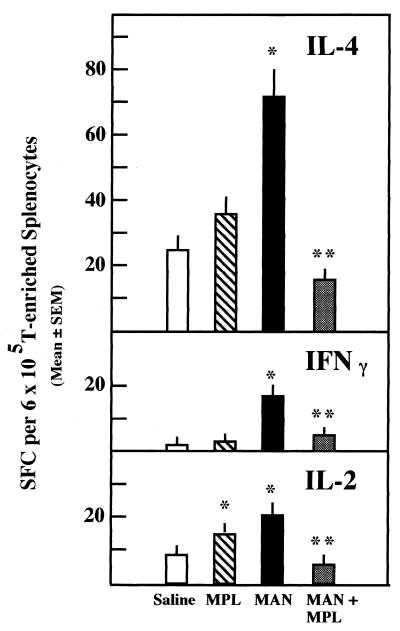
In the past we demonstrated that MPL abrogated the downregulatory activity exerted by CD8+ cells if donor MAN-treated mice were given MPL in vivo 48 to 72 h prior to sacrifice (14). Moreover, if splenocytes taken from MAN-treated mice were incubated with MPL in vitro prior to transfer of such cells to immunized mice, the ability to downregulate DH was lost as well (12). Therefore, in the initial experiments involving the effects of MPL on SFC, we chose to compare four groups of mice, with the group size ranging from three to six. Group I mice were given MAN i.v. at time zero; group II mice were given MPL i.p. at 48 h; group III mice were given MAN at time zero and MPL at 48 h; group IV mice were given saline i.v. at time zero and i.p. at 48 h. All mice were sacrificed at 72 h, and the numbers of cytokine-secreting cells were determined for IL-2, IFN-γ, and IL-4.
The results of a representative experiment are illustrated in Fig. 3. As shown previously, MAN induced significant increases in SFC, detectable 72 h after the introduction of MAN, for all three cytokines. The increases in MAN-treated animals were noted even in the presence of decreased numbers of T cells in the suspensions. As mentioned above, MAN treatment appears to increase the production of macrophages while the number of T cells appears to remain the same. Thus, the percentage of T cells in the populations derived from MAN-treated mice is lower because there are proportionally fewer in the population. For the experiment shown, the percentage of T cells in the splenocyte populations from saline-treated animals was 33.5, whereas the percentage in MAN-treated animals was 14.8. Animals treated with both MAN and MPL had similarly low values, 17.1%, while those treated with only MPL had 28% T cells. MPL alone, given 24 h prior to sacrifice, stimulated minimal increases in IL-2- and IL-4-secreting cells. Surprisingly, 24 h after MPL was given to MAN-treated mice, the number of cells secreting IL-2, IFN-γ, or IL-4 was reduced to control levels or below. In fact, the number of IL-2-secreting SFC was significantly (P ≤ 0.01) reduced below control (saline) levels in all experiments in which animals were given both MAN and MPL. While there were significant reductions well below MAN-only levels in SFC for IFN-γ and IL-4 in animals given MAN and MPL, the reductions were seldom below saline control values. These data were confirmed in three additional experiments. Moreover, an experiment similar to that illustrated in Fig. 2 was performed to determine whether CD8+ cells secreted cytokines after stimulation with MPL. SFC were not detected in any CD8+-enriched suspensions, despite the fact that the cell suspensions from the MAN- and MPL-treated groups were contaminated with 23 and 27% CD4+ cells. The saline- and MAN-MPL-treated groups were 97 and 93% pure CD8+ cells.
FIG. 3.
Enumeration of IL-2-, IFN-γ-, and IL-4-secreting cells in mice treated with MAN (500 μg, i.v.) 72 h and/or MPL (50 μg, i.p.) 24 h prior to sacrifice. n = 6; significance determined by the Mann-Whitney test; ∗, P ≤ 0.05 compared to saline control; ∗∗, P ≤ 0.05 compared to MAN.
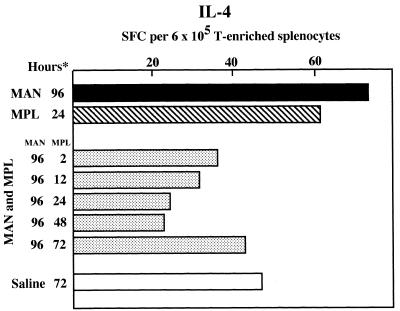
To examine the kinetics of the interaction between MPL and MAN, groups of two mice each were given MAN i.v. and then at intervals thereafter, beginning with 24 and ending with 94 h, mice were given MPL i.p. All mice were sacrificed at 96 h, and T-enriched splenocyte suspensions were assayed for IL-4-secreting SFC. As mice given saline only, regardless of the interval between the administration of saline and sacrifice, had SFC at or below those of untreated mice (described above), 24 h after the administration of MAN was arbitrarily selected for the administration of saline to control mice. The results of this experiment are shown in Fig. 4. In this particular experiment, the difference between IL-4-secreting SFC in splenocyte populations of mice given only MAN or MPL was not as great as in most other experiments. Nevertheless, the administration of MPL to MAN-treated mice at all intervals, even only 2 h prior to sacrifice, reduced the number of detectable IL-4-secreting SFC to levels that were at or below those detected in saline-treated animals.
FIG. 4.
Enumeration of IL-4-secreting cells in mice treated with MAN (500 μg, i.v.) 96 h prior to sacrifice, some of which were also treated with MPL (50 μg, i.p.) 2 to 72 h prior to sacrifice. n = 2; splenocytes pooled for each condition; ∗, hours following administration of MAN, MPL, or saline.
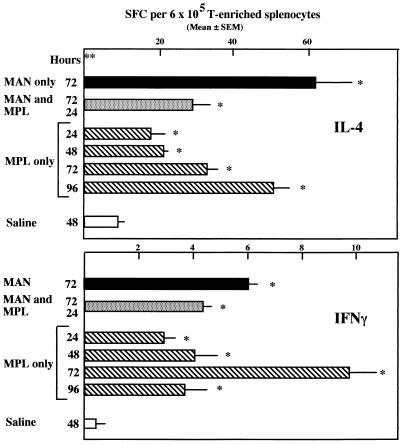
During the course of experimentation with MAN and MPL, it became clear that MPL, in the absence of MAN, was capable of modulating the number of splenic cytokine-secreting cells. To be able to interpret the MAN-MPL data adequately, therefore, it was necessary to determine the kinetics of the effect of MPL alone on the number of splenic SFC. Thus, groups of three mice each were injected i.p. with MPL 24, 48, 72, or 96 h prior to sacrifice, and splenic SFC specific for IL-4 and IFN-γ were determined. For comparison, additional groups of three mice each were given MAN i.v. 72 h prior to sacrifice, MAN i.v. at 72 h and MPL at 24 h, or saline at 48 h. Each spleen was assayed independently so that statistical comparisons could be made among the groups. The data from this experiment are summarized in Fig. 5. MPL alone stimulated an increase in the number of IL-4-secreting cells that was statistically significant compared to saline controls, with the greatest increases occurring 3 to 4 days after the introduction of MPL. The number of IFN-γ-secreting splenocytes also increased significantly above the saline value, but the peak increase was detected 72 h after MPL treatment, and the number was reduced to the 24- and 48-h averages at 96 h. The MAN-only and MAN-MPL data were consistent with those shown previously in Fig. 3 as well as in similar experiments for which data are not shown. A second study in which the MPL-only portion of this experiment was repeated resulted in similar data to those shown; viz., IL-4-secreting SFC increased steadily through 96 h after MPL administration, whereas IFN-γ-secreting SFC peaked 72 h after MPL was given and were detected at lower levels at 96 h.
FIG. 5.
Enumeration of IFN-γ- and IL-4-secreting cells in mice treated with MAN (500 μg, i.v.) 72 h prior to sacrifice, with MAN 72 h and MPL (50 μg, i.p.) 24 h prior to sacrifice, or with MPL only 24 to 96 h prior to sacrifice. n = 3; significance determined by the Mann-Whitney test; ∗, P ≤ 0.05 compared with saline control; ∗∗, hours following administration of MAN, MPL, or saline.
DISCUSSION
We report here changes in the frequency of cytokine-producing cells during the 4 days required to induce and produce C. albicans MAN-specific downregulatory cells. Two pieces of evidence presented here are consistent with the hypothesis that a Th2-driven response involving elevated numbers of CD4+ splenocytes secreting IL-4 contributed to the development and/or activity of CD8+ effector cells. First, significantly increased numbers of IL-4-secreting cells, with little increase in IL-2- or IFN-γ-secreting cells, were detected 24 h after the introduction of MAN. This is within the time frame wherein CD4+ cells are required for the induction of CD8+ effectors (30). Second, we demonstrated that in the presence of MPL, an immunomodulator known to abrogate the activity of the CD8+ effectors, the number of IL-4-secreting cells that could be detected was significantly reduced. While both pieces of evidence are suggestive, they are admittedly circumstantial. Moreover, it is important to recognize that these conclusions are based on the number of cytokine-secreting cells and not on the quantitation of free cytokine in tissue or serum. It is assumed, however, that if there is an increase in the number of cytokine-secreting cells, there would be a concomitant increase in free cytokine in vivo. Others have demonstrated in an in vitro system that the amount of IL-4 secreted increased in parallel with the number of cytokine-secreting cells (16). A third piece of evidence supporting a role for IL-4 in the induction of CD8+ effector cells (56) involved treatment of animals with anti-IL-4. DH responses in immunized animals treated with anti-IL-4 at the time of CD8+ effector induction by MAN were considerably greater than those in animals treated with rat immunoglobulin G.
The importance of cytokines in candidal disease and the ability of C. albicans or its components, predominantly MAN or MAN-containing compounds, to stimulate the synthesis of cytokine-specific mRNA or the secretion of cytokine into body fluids have been topics of considerable interest over the last decade. In general, the data provide evidence for the involvement of Th1 cytokines in protective responses and Th2 cytokines in nonprotective responses. Both IL-4 and IL-10, Th2 cytokines, have been reported to exacerbate candidiasis in mice (53); the effect can be reversed by treatment with anti-IL-4 (42), anti-IL-10 (43), or soluble IL-4 receptor (38). Th1 and Th2 cytokine responses were both detected in a murine model involving gastrointestinal challenge, but protection correlated with the Th1 responses (9).
The i.p. injection of nonviable C. albicans or fractions thereof also upregulated primarily Th1 responses, in that mRNA for IL-2 (44, 48, 49) and IFN-γ, as well as for an acute-phase cytokine, IL-1β, increased following treatment, whereas IL-4 and IL-5 mRNAs were not detectable (44). Despite the upregulation of mRNAs for cytokines associated with Th1, mRNA for IL-12p40, a cytokine associated with the induction of Th1 responses, was not detectable (44).
In vitro studies using human peripheral blood leukocytes have confirmed the in vivo studies with regard to the ability of viable or nonviable C. albicans, mannoproteins, or glucomannoproteins to upregulate mRNA for IL-2 and/or IFN-γ (3, 29, 51), but the relationship of the in vitro findings to in vivo observations is not clear. Complicating the issue even further is that mannoprotein, both in vivo and in vitro, is known to upregulate mRNA and/or promote increased secretion of several acute-phase cytokines, viz., TNF-α, IL-1β, and IL-6 (3, 20, 55). The interrelationship of the latter cytokines with Th1 and Th2 cytokines in experimental candidal models is largely unexplored, although there is some evidence that TNF-α has a protective role following i.v. challenge of naive animals with C. albicans (32).
We reported previously that MPL abrogated the downregulatory effects induced by C. albicans MAN (12, 14), and we show here that MPL significantly reduced the number of demonstrable IL-4-secreting cells, as well as those secreting IFN-γ or IL-2. These reductions occurred despite the fact that when given alone, MPL induced progressive increases during the 4-day incubation period. Others have shown that MPL alone, given i.v. to mice, caused a rapid accumulation of IFN-γ in serum (24) and that leukocytes taken from uremic patients, when incubated in vitro with MPL, produced IFN-γ as well as IL-2 (8). Recently, Salkowski et al. (47) reported on cytokine mRNA induction in macrophages in response to LPS and MPL. LPS was more potent in inducing IL-12p35, IL-12p40, and IFN-γ mRNAs than MPL, but MPL induced higher levels of IL-10 mRNA. It was suggested that IL-10 contributes to the decreased production of IL-12 when macrophages were stimulated with MPL instead of LPS. It remains to be determined if IL-10 plays a role in the modulatory events described in our system with MPL.
Our data suggest that IL-4 may be a participatory cytokine for the development of downregulatory activity and that modest increases in cells secreting IFN-γ or IL-2 cannot overcome the influence of IL-4. We have no data to address the mechanism by which IL-4 might mediate the early events in the induction of the downregulatory activity, but report of reactive nitrogen oxides being inhibited by IL-4, IL-10, and transforming growth factor β (50) and the inhibition of both candidacidal activity and nitric oxide production of IFN-γ-activated macrophages by IL-4 and/or IL-10 (10) may provide clues for further research in this area.
It is not clear how the changes in frequency of detection of SFC for all three cytokines in animals treated with both MAN and MPL might be involved in abrogating the inhibitory activity of CD8+ cells. Rather than an actual decrease in numbers of cells producing cytokines, however, the prevention of secretion may be the key factor. MPL may simply bind to the surface of antigen-activated cytokine-secreting cells in such a way as to prevent secretion of cytokine, whereas its binding to resting cells could actually stimulate them. If IL-4 cannot be secreted, it would not be available to promote and maintain anergy. Alternatively, MPL could be cytotoxic to cells that are actively secreting cytokine, especially those secreting IL-4. We have, however, done vital staining of suspensions of splenocytes following incubation with MPL (data not shown) and could find no evidence for cytotoxicity. Despite a lack of evidence for cytotoxicity, it is possible that the cells become programmed for cell death during the incubation period with MPL but do not die immediately.
The role of the MAN-specific downregulatory cells in candidal disease is not entirely clear. It was shown that the presence of such cells in immunized mice did not prevent the demonstration of immunity when said animals were challenged systemically (19). A possible role for MAN as a modulator of immunologic events occurring at mucosal surfaces, however, has never been ruled out. Since there seems to be a clear indication that cellular immunity is more important to the defense of mucosal surfaces against C. albicans than to the defense of internal tissues (6), it might be more profitable to investigate the role of these regulatory cells in mucosal disease. Such investigations await the development of a suitable model in which to demonstrate this.
In summary, these data support the hypothesis that IL-4 participates in the induction of MAN-specific downregulatory cells. Since CD8+ effector cells did not secrete IL-2, IFN-γ, or IL-4 in this system, and since CD4+ cells must be present during the first 30 to 40 h after the introduction of MAN (30), we postulate a role for Th2 cells, specifically IL-4-secreting CD4+ cells, in this phenomenon. In another study (56) wherein anti-IL-4 was administered to animals treated with MAN, IL-4 does appear to be involved in MAN-specific downregulatory activity. It does not appear to be the only factor, however.
ACKNOWLEDGMENTS
This investigation was supported by Public Health Service grant AI-12806 from the National Institute of Allergy and Infectious Diseases.
We are grateful to Ribi ImmunoChem for the gift of the MPL.
REFERENCES
- 1.Asherson G L, Dieli F, Sireci G, Salerno A. Role of IL-4 in delayed type hypersensitivity. Clin Exp Immunol. 1996;103:1–4. doi: 10.1046/j.1365-2249.1996.845537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astiz M E, Galera A, Saha D C, Capati C, Rackow E C. Monophosphoryl lipid A protects against gram-positive sepsis and tumor necrosis factor. Shock. 1994;2:271–274. doi: 10.1097/00024382-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello C, Urbani F, Gessani S, Spagnoli G C, Gomez M J, Cassone A. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents from Candida albicans. Infect Immun. 1993;61:4105–4111. doi: 10.1128/iai.61.10.4105-4111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker P J, Hiernaux J R, Fauntleroy M B, Prescott B, Cantrell J L, Rudbach J A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988;56:1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker P J, Taylor C E, Stashak P W, Fauntleroy M B, Haslov K, Qureshi N, Takayama K. Inactivation of suppressor T cell activity by the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides. Infect Immun. 1990;58:2862–2868. doi: 10.1128/iai.58.9.2862-2868.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balish E, Filutowicz H, Oberley R D. Correlates of cell-mediated immunity in Candida albicans-colonized gnotobiotic mice. Infect Immun. 1990;58:107–113. doi: 10.1128/iai.58.1.107-113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan R L, Murphy J W. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1994;62:2930–2939. doi: 10.1128/iai.62.7.2930-2939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carozzi S, Salit M, Cantaluppi A, Nasini M G, Barocci S, Cantarella S, Lamperi S. Effect of monophosphoryl lipid A on the in vitro function of peritoneal leukocytes from uremic patients on continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1989;27:1748–1753. doi: 10.1128/jcm.27.8.1748-1753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K-H, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 10.Cenci E, Romani L, Mencacci A, Spaccapelo R, Schiaffella E, Puccetti P, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 11.Coffman R L, Varkila K, Scott P, Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 12.Domer J E, Asherson G L, Li S P. Treatment of Candida albicans mannan-specific downregulatory cell populations with divergent concentrations of monophosphoryl lipid A and intact lipopolysaccharide in vitro abrogates their effect on delayed hypersensitivity. Cell Immunol. 1996;167:8–17. doi: 10.1006/cimm.1996.0002. [DOI] [PubMed] [Google Scholar]
- 13.Domer J E, Garner R E, Befidi-Mengue R N. Mannan as an antigen in cell-mediated immunity (CMI) assays and as a modulator of mannan-specific CMI. Infect Immun. 1989;57:693–700. doi: 10.1128/iai.57.3.693-700.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domer J E, Human L G, Andersen G B, Rudbach J A, Asherson G L. Abrogation of suppression of delayed hypersensitivity induced by Candida albicans-derived mannan by treatment with monophosphoryl lipid A. Infect Immun. 1993;61:2122–2130. doi: 10.1128/iai.61.5.2122-2130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorf M E, Kuchroo V K, Collins M. Suppressor T cells: some answers but more questions. Immunol Today. 1992;13:241–243. doi: 10.1016/0167-5699(92)90002-O. [DOI] [PubMed] [Google Scholar]
- 16.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997;204:57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 17.Fujihashi K, McGhee J R, Beagley K W, McPherson D T, McPherson S A, Huang C-M, Kiyono H. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4, and IL-6 producing cells. J Immunol Methods. 1993;160:181–189. doi: 10.1016/0022-1759(93)90176-8. [DOI] [PubMed] [Google Scholar]
- 18.Garner R E, Childress A M, Human L G, Domer J E. Characterization of Candida albicans mannan-induced, mannan-specific delayed hypersensitivity suppressor cells. Infect Immun. 1990;58:2613–2620. doi: 10.1128/iai.58.8.2613-2620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner R E, Domer J E. Lack of effect of Candida albicans mannan on development of protective immune responses in experimental murine candidiasis. Infect Immun. 1994;62:738–741. doi: 10.1128/iai.62.2.738-741.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garner R E, Hudson J A. Intravenous injection of Candida-derived mannan results in elevated tumor necrosis factor alpha levels in serum. Infect Immun. 1996;64:4561–4566. doi: 10.1128/iai.64.11.4561-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazzinelli R T, Makino M, Chattopadhyay S K, Snapper C M, Sher A, Hugin A W, Morse H C., III CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J Immunol. 1992;148:182–188. [PubMed] [Google Scholar]
- 22.Gosselin D, Turcotte R, Lemieux S. Cellular target of in vitro-induced suppressor cells derived from the spleen of Mycobacterium lepraemurium-infected mice and role of IFN-γ in their development. J Leukocyte Biol. 1995;57:122–128. doi: 10.1002/jlb.57.1.122. [DOI] [PubMed] [Google Scholar]
- 23.Green D R, Webb D R. Saying the “S” word in public. Immunol Today. 1993;14:523–525. doi: 10.1016/0167-5699(93)90180-S. [DOI] [PubMed] [Google Scholar]
- 24.Gustafson G L, Rhodes M J. Effects of tumor necrosis factor and dexamethasone on the regulation of interferon-γ induction by monophosphoryl lipid A. J Immunother. 1994;15:129–133. doi: 10.1097/00002371-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Holda J H, Maier T, Claman H N. Evidence that IFN-γ is responsible for natural suppressor activity in BFHD spleen and normal bone marrow. Transplantation. 1988;45:772–777. doi: 10.1097/00007890-198804000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Klinmam D M, Nutman T B. ELISPOT assay to detect cytokine-secreting murine and human cells. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1994. pp. 6.19.1–6–19.18. [Google Scholar]
- 27.Kocourek J, Ballou C E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969;100:1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruisbeek A M. Isolation of mouse mononuclear cells. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1994. pp. 3.1.1–3.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Levitz S M, North E A. Gamma interferon gene expression and release in human lymphocytes directly activated by Cryptococcus neoformans and Candida albicans. Infect Immun. 1996;64:1595–1599. doi: 10.1128/iai.64.5.1595-1599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S P, Lee S, Wang Y, Domer J E. Candida albicans mannan-specific, delayed hypersensitivity down-regulatory CD8+ cells are genetically restricted effectors and their production requires CD4 and I-A expression. Int Arch Allergy Immunol. 1996;109:334–343. doi: 10.1159/000237260. [DOI] [PubMed] [Google Scholar]
- 31.Li S P, Miller R A. Age-associated decline in IL-4 production by murine T lymphocytes in extended culture. Cell Immunol. 1993;151:187–195. doi: 10.1006/cimm.1993.1230. [DOI] [PubMed] [Google Scholar]
- 32.Louis A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 35.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 36.Otten G, Yokoyama W M, Holmes K L. Flow cytometry analysis using the Becton Dickinson FACScan. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1994. pp. 5.4.1–5.4–19. [Google Scholar]
- 37.Peat S, Whelan W J, Edwards T E. Polysaccharide of baker’s yeast. Part IV. Mannan. J Chem Soc (London) 1961;1:29–34. [Google Scholar]
- 38.Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Enssle K-H, Romani L, Bistoni F. Cure of murine candidiasis by recombinant soluble interleukin-4 receptor. J Infect Dis. 1994;169:1325–1531. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- 39.Restrepo-Moreno A, Schneidau J D. Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967;93:1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Modifiers. 1984;3:1–9. [PubMed] [Google Scholar]
- 41.Ribi E, Cantrell J L, Takayama K, Qureshi N, Peterson J, Ribi H O. Lipid A and immunotherapy. Rev Infect Dis. 1984;6:567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- 42.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 44.Rosati E, Scaringi L, Cornacchione P, Fettucciari K, Sabatini R, Mezzasoma L, Benedetti C, Cianetti S, Rossi R, Marconi P. Activation of cytokine genes during primary and anamnestic immune response to inactivated C. albicans. Immunol. 1996;89:142–151. doi: 10.1046/j.1365-2567.1996.d01-702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon γ-independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salerno A, Dieli F, Sireci G, Bellavia A, Asherson G L. Interleukin-4 is a critical cytokine in contact sensitivity. Immunology. 1995;84:404–409. [PMC free article] [PubMed] [Google Scholar]
- 47.Salkowski C A, Detore G R, Vogel S N. Lipopolysaccharide and monophosphoyl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;65:3239–3247. doi: 10.1128/iai.65.8.3239-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scaringi L, Rosati E, Cornacchione P, Rossi R, Marconi P. In vivo modulation of lymphokine-activated killer cell activity by cell wall components of Candida albicans. Cell Immunol. 1992;139:438–454. doi: 10.1016/0008-8749(92)90084-3. [DOI] [PubMed] [Google Scholar]
- 49.Scaringi L, Cornacchione P, Rosati E, Fettucciari K, Rossi R, Marconi P. Induction and persistence in vivo of NK/LAK activity by a mannoprotein component of Candida albicans cell wall. Cell Immunol. 1994;155:265–282. doi: 10.1006/cimm.1994.1121. [DOI] [PubMed] [Google Scholar]
- 50.Sher A, Gazzinelli R T, Oswald I P, Clerici M, Kullberg M, Pearce E J, Berzofsky J A, Mosmann T R, James S L, Morse III H C, Shearer G M. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 51.Spagnoli G, Ausiello C, Caslinuova I, Antonelli G, Dianzani F, Cassone A. Candida albicans and a phosphorylated glucomannan-protein fraction of its cell wall induce production of immune interferon by human peripheral blood mononuclear cells. IRCS Med Sci. 1985;13:1190–1191. [Google Scholar]
- 52.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Detection of individual mouse splenic T cells producing IFN-γ and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 53.Tonnetti L, Spaccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman R L, Bistoni F, Romani L. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 54.Ulrich J T, Myers K R. Monophosphoryl lipid A as an adjuvant. Pharm Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 55.Vecchiarelli A, Puliti M, Torosantucci A, Cassone A, Bistoni F. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell Immunol. 1991;134:65–76. doi: 10.1016/0008-8749(91)90331-5. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Li S P, Yuan L, Moser S A, Bost K L, Domer J E. Cytokine involvement in immunomodulatory activity affected by Candida albicans mannan. Infect Immun. 1998;66:1384–1391. doi: 10.1128/iai.66.4.1384-1391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb D R, Kraig E, Devens B H. Suppressor cells and immunity in mechanisms of immune regulation. Chem Immunol. 1994;58:146–192. doi: 10.1159/000319224. [DOI] [PubMed] [Google Scholar]
- 58.Weigmann B, Schwing J, Huber H, Ross R, Mossmann H, Knop J, Reske-Kunz A B. Diminished contact hypersensitivity response in IL-4 deficient mice at a late phase of the elicitation reaction. Scand J Immunol. 1997;45:308–314. doi: 10.1046/j.1365-3083.1997.d01-402.x. [DOI] [PubMed] [Google Scholar]