Abstract
Objective
Residual sequelae after surgical repair of tetralogy of Fallot (rTOF) affect clinical outcome. We investigated the prognostic impact of right ventricular (RV) dyssynchrony in adults with rTOF years after the surgical repair.
Methods
Patients from the Swiss Adult Congenital HEart disease Registry were included. NT-proBNP levels, echocardiography, exercise testing and MRI data were collected. An offline strain analysis to quantify RV-ventricular and interventricular dyssynchrony was performed. The standard deviation of the time-to-peak shortening (TTP) of six RV segments defined the RV Dyssynchrony Index (RVDI). Maximal difference of TTP between RV and left ventricular segments defined the interventricular shortening delay (IVSD). Predictors of a composite adverse event (arrhythmias, hospitalisation for heart failure and death) were identified by multivariate Cox regression analysis. Their median values were used to create a risk score.
Results
Out of 285 included patients (mean age 34±14 years), 33 patients (12%) experienced an adverse event during a mean follow-up of 48±21 months. No correlation was found between RVDI, IVSD and clinical events. NT-proBNP, right atrial area and peak heart rate were independent predictors of outcomes. After 4 years-follow-up, no adverse events occurred in patients at low risk (score=0 points), while an adverse event occurred in 62% of patients at high risk (score=3 points, p<0.001).
Conclusion
In our cohort of adults with rTOF, surrogates of RV dyssynchrony did not correlate with outcomes. A multimodality approach was effective in predicting the risk for adverse events.
Keywords: Tetralogy of Fallot, Echocardiography, Diagnostic Imaging
WHAT IS ALREADY KNOWN ON THIS TOPIC
Residual sequelae after surgical repair of tetralogy of Fallot (TOF) can impact long-term outcomes.
The impact of the ventricular dyssynchrony on biventricular function, exercise capacity and clinical outcome is unknown.
WHAT THIS STUDY ADDS
This study investigated the role of right ventricular dyssynchrony on the long-term prognosis in repair of TOF patients.
Multimodality approach might help to stratify the risk of outcomes in this population.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The stratification might help to identify patients with better prognosis as well as high-risk patients, which may require earlier follow-up.
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect (CHD) and is present in 7%–10% of children with CHD.1 Since surgical repair was introduced in 1955, long-term prognosis of patients with TOF has improved steadily. Today, more than 95% of children with repaired TOF (rTOF) reach adulthood, compared with less than 20% in historical cohorts without repair.2 However, residual sequelae after surgical repair (eg, atrial or ventricular scars, pulmonary valve or right ventricular (RV) outflow tract dysfunction) can impact long-term outcomes by affecting ventricular size and function, and predisposing patients to arrhythmias, sudden cardiac death and heart failure.3 So far, most attention has been directed to the negative consequences of residual pulmonary regurgitation on RV size and function and the occurrence of arrhythmias.4 Less attention has been paid on the consequences of right bundle branch block and intraventricular dyssynchrony on biventricular function, exercise capacity and clinical outcome.
Echocardiographic 2D-speckle tracking strain analysis allows the assessment of the electromechanical dyssynchrony within the left and right ventricle. Previous studies have shown the prognostic importance of RV myocardial strain for various conditions, such as in patients with idiopathic pulmonary hypertension,5 arrhythmogenic RV dysplasia6 or with a systemic right ventricle.7 With only a few studies performed in children with rTOF,8 the prognostic value of RV strain and dyssynchrony in adults with rTOF is still poorly understood. The aim of this study was to investigate the role of RV dyssynchrony surrogates and the risk stratification of patients with rTOF years after surgical repair with clinical parameters.
Methods
Study population and characteristics
Adults with rTOF from the multicentre Swiss Adult Congenital HEart disease Registry (SACHER)9 followed at the University Hospitals of Bern and Zürich were included. The registry aim was to establish a prospective database of clinical information in order to improve the knowledge base of outcomes in adults with CHD. The inclusion criteria for the actual study were: patients with a Fallot-like physiology such as pulmonary atresia and ventricular septum defect, double outlet RV of Fallot type and complete atrioventricular septal defect with pulmonary stenosis were also included. We defined as baseline visit the first clinical visit between 2012 and 2016, where cardiovascular MR (CMR) was performed, as well as a transthoracic echocardiography (TTE) study was done within a 1-year interval.
Baseline characteristics including age at baseline visit, age at repair, type of repair, gender, body mass index, New York Heart Association functional classification and N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels were derived from the patients’ chart. A 12-lead ECG was part of every clinical visit. The underlying rhythm and QRS durations at baseline were documented. From the cardiopulmonary exercise testing reports the following variables were collected: peak oxygen uptake (pVO2), peak heart rate and ventilation for the carbon dioxide production (VE/VCO2 slope). Data regarding dimension and function of the heart chambers and severity of tricuspid and pulmonary regurgitation were retrieved from the TTE and CMR charts. All data had to be collected within 6 months before or after the baseline visit.
Strain analysis and quantification of dyssynchrony
2D-Speckle tracking strain analysis was performed offline by one author (AP) using a dedicated software TomTec-Arena V.2.30 (TomTech Imaging Systems, Munich, Germany). Grey-scale images were previously acquired with optimised gain and contrast at frame rates of 50–90 frames/s. RV longitudinal strain was measured in the right ventricle focused four-chamber view. Considering the thin RV wall, measurements were limited to the endocardial strain. The endocardial borders of the right and left ventricle were traced manually excluding papillary muscles and trabeculations. The software-generated tracking was further manually adjusted. Endocardial tracking was accepted for analysis if adequate at visual inspection during the entire cardiac cycle. In case the endocardial border of a wall segment was not evaluable, this echocardiographic examination was excluded from the strain analysis. Strain curves were generated at the basal, mid and apical segments of the RV lateral wall and of the interventricular septum (see figure 1). For each of the six RV segments the time-to-peak myocardial shortening (TTP) was measured. According to Hui et al,10 maximal RV delay was defined as the difference between the longest and the shortest TTP segment, and the standard deviation (SD) of all segments defined the RV Dyssynchrony Index (RVDI). The median value of RVDI was used to classify the study population into patients with low and high dyssynchrony. Moreover, the strain of the three anterolateral left ventricular (LV) segments was analysed in the apical four-chamber view and interventricular shortening delay (IVSD) was calculated as the maximal TTP difference between any RV and LV lateral walls. Finally, the interventricular ejection delay was defined as the difference between the RV and LV pre-ejection time measured with pulsed-wave Doppler at each ventricular outflow tract. Table 1 summarises the definitions of dyssynchrony surrogates.
Figure 1.
Strain analysis of the right ventricle with measurement of time-to-peak shortening in six right ventricular segments.
Table 1.
Dyssynchrony variables
| TTP | Time-to-peak myocardial shortening: Time interval between the beginning of the QRS complex and peak of systolic shortening. |
| Max RV delay | Maximal right ventricular delay: Maximal time difference between longest and shortest TTP among the 6 RV segments. |
| RVDI | RV Dyssynchrony Index: SD of the 6 TTPs. |
| pET | Pre-ejection time: Time from Q-wave to the beginning of ejection, measured on pw-Doppler at ventricular outflow tract. |
| IVED | Interventricular ejection delay: Difference between LV-pET and RV-pET. |
| IVSD | Interventricular shortening delay: Maximal difference between longest and shortest TTP among the RV and LV free wall segments. |
LV, left ventricular; SD, standard deviation.
Clinical outcome
An adverse clinical event was defined as the composite endpoint consisting of all-cause mortality, major arrhythmias and hospital admission for heart failure. Ventricular fibrillation, sustained ventricular tachycardia, atrial flutter and atrial fibrillation requiring cardioversion or ablation therapy were considered major arrhythmias. The time to the first clinical event between baseline visit and 31st December 2019 was analysed.
Statistical analyses
Continuous variables were reported as mean and SD if normally distributed and compared using Student’s t-test or Welch’s t-test, depending on the equality of the variances. Non-normally distributed variables were reported as median with IQR and compared using the Mann-Whitney U test. Categorical variables were summarised as frequency or proportion and compared using χ2 or Fisher’s exact tests. Univariate or multivariate Cox proportional hazard survival models were used to investigate the association between variables of interest and clinical outcome. Harrel’s C-index, also known as concordance index, was calculated as a measure of goodness of fit for every univariate variable. In a second step, a multivariable model consisting of variables with the highest C-index among clinical characteristics, NT-proBNP levels, ECG, imaging or exercise testing data was constructed. The proportional hazards assumption for this model was tested by assessing the relationship between scaled Schoenfeld residuals and time to event. Significant independent predictors of an adverse clinical outcome were used to create a risk score, with the median values of each predictor as cut-off for a score 0 or 1.
To assess intraobserver variability, the same observer (AP) re-analysed strain parameters of 20 randomly selected subjects at the end of the data sampling. For interobserver variability, a second blinded observer (CN) analysed the strain of 20 randomly selected subjects. The interclass correlation coefficient (ICC) using a two-way random/absolute agreement model was used to assess interrater reliability, where a value >0.8 suggests an excellent agreement and value<0.4 indicates poor agreement. A p<0.05 was considered as statistically significant. All data were analysed using STATA software (V.15.1; Stata).
Results
Study population
Among the 285 patients, 44% were female with a mean age at baseline 34±14 years. Most of the patients were asymptomatic and in sinus rhythm at baseline visit. The number of patients with a diagnosis of TOF was 244 (86%). A total of 186 patients (65%) underwent a palliation procedure prior to definitive repair. Table 2 summarises the baseline characteristics. Further characteristics are shown in online supplemental table 1.
Table 2.
Baseline characteristics
| Clinical characteristics | Total cohort N=285 | No of observations |
| Age at baseline visit—years | 34±14 | 285 |
| Age at repair—years | 6 (1–6) | 285 |
| Type of repair | ||
| Non transannular patch | 118 (40%) | |
| Transannular patch | 110 (37%) | |
| RV-PA conduit | 56 (19%) | |
| Unknown | 10 (3%) | |
| Female | 126 (44%) | |
| BMI—kg/m2 | 24.2±4.8 | |
| NYHA functional class | ||
| I | 229 (80%) | |
| II or III | 56 (20%) | |
| NT-proBNP—pg/mL | 133 (66–262) | 217 |
| Sinus rhythm | 259 (91%) | |
| Atrial fibrillation or flutter | 10 (4%) | |
| Pacemaker rhythm | 12 (4%) | |
| QRS duration—ms | 143±26 | 281 |
| Echocardiography data | ||
| LVEDD—mm | 47±7 | 275 |
| LVESD—mm | 32±7 | 273 |
| LAVI—mL/m2 | 24±15 | 280 |
| RAA—cm2 | 19±7 | 247 |
| LVEF—% | 59±8 | 280 |
| RV FAC—% | 39±8 | 272 |
| RV-RA gradient—mm Hg | 31±12 | 254 |
| Tricuspid regurgitation>mild | 260 (91%) | 275 |
| Pulmonary regurgitation<severe | 226 (79%) | 273 |
| Strain and dyssynchrony | ||
| RV GLS—% | −15.4±5 | 222 |
| RV Dyssynchrony Index—ms | 40 (28–52) | 222 |
| Max RV Delay—ms | 95 (62–127) | 222 |
| IVED—ms | −3±19 | 260 |
| IVSD—ms | 105±52 | 209 |
| CMR data | ||
| LV EDVI—mL | 88±21 | 185 |
| RV EDVI—mL | 126±35 | 201 |
| RVEF—% | 48±9 | 208 |
| LVEF—% | 57±8 | 206 |
| Exercise data | ||
| Peak heart rate—bpm | 158±27 | 235 |
| Peak VO2—mL/kg/min | 22±9 | 237 |
| VE/VCO2 slope | 27±5 | 232 |
Data are expressed as N (% of total), mean±SD or median (IQR) unless otherwise specified. The number of observations refers to the available data in the registry.
BMI, body mass index; CMR, cardiac MR; EDV, end-diastolic volume; EDVI, end-diastolic volume indexed; GLS, global longitudinal strain; IVED, interventricular ejection delay; IVSD, interventricular shortening delay; LAVI, left atrial volume indexed; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; RAA, right atrial area; RVEF, right ventricular ejection fraction; RV FAC, RV fractional area change; VE/VCO2, minute ventilation/carbon dioxide output; VO2, oxygen uptake.
openhrt-2023-002583supp001.pdf (87.2KB, pdf)
RV dyssynchrony
In 222 patients (78%), echocardiographic imaging quality was sufficient to measure the TTP in all 6 RV segments. On average, the median of maximal RV delay was 95 ms (62–127 ms) and the median RVDI was 40 ms (28–52 ms). Patients with above-median RVDI had a larger left atrium and ventricles, lower LV ejection fraction and lower RV global longitudinal strain (GLS). The proportion of patients with severe pulmonary regurgitation was higher in the group higher RV dyssynchrony (15% vs 25%, p=0.023). No significant differences were observed in NT-proBNP levels, cardiopulmonary exercise variables or outcomes (see table 3).
Table 3.
Comparison between patients with higher and lower RV Dyssynchrony Index (RVDI)
| Patients with RVDI<median N=113 |
Patients with RVDI≥median N=109 |
P value | |
| Clinical characteristics | |||
| Age at repair—years | 5.7 (1–9) | 4.7 (1–6) | 0.35 |
| Age at baseline—years | 33±12 | 34±13 | 0.45 |
| Primary repair | 73 (65%) | 71 (65%) | 0.9 |
| NYHA functional class I | 94 (83%) | 93 (85%) | 0.8 |
| NT-proBNP—pg/mL | 119 (57–983) | 153 (70–1666) | 0.3 |
| No device (ICD, PM, CRT) | 104 (92%) | 98 (89%) | 0.7 |
| QRS duration—ms | 143±25 | 144±27 | 0.91 |
| Adverse clinical events | 10 (9%) | 14 (13%) | 0.3 |
| Echocardiography data | |||
| LVEDD—mm | 46±6 | 48±7 | 0.008 |
| LVESD—mm | 30±5 | 33±7 | 0.001 |
| LVEF—% | 60±8 | 58±8 | 0.05 |
| LAVI—mL/m2 | 24±10 | 29±17 | 0.04 |
| RA area—cm2 | 19±7 | 20±7 | 0.12 |
| RV-RA gradient—mm Hg | 32±12 | 30±12 | 0.21 |
| Severe tricuspid regurgitation | 2 (1.8%) | 1 (0.6%) | 0.147 |
| Severe pulmonary regurgitation | 16 (14.5%) | 43 (24.5%) | 0.023 |
| RV FAC—% | 38±7 | 39±9 | 0.4 |
| RV GLS—% | −16±4.4 | −14.6±5 | 0.032 |
| IVSD—ms | 86±38 | 126±56 | <0.001 |
| IVED—ms | −4.5±15.9 | −1.6±21.9 | 0.3 |
| CMR data | |||
| LV EDVI—mL | 83±17 | 90±23 | 0.007 |
| RV EDVI—mL | 122±28 | 137±19 | 0.004 |
| RV EF—% | 47±8 | 47±8 | 0.8 |
| LV EF—% | 60±8 | 57±9 | 0.02 |
| Exercise data | |||
| Peak VO2—mL/kg/min | 21±9 | 21±9 | 0.9 |
| Peak heart rate—bpm | 159±30 | 158±26 | 0.7 |
| VE/VCO2 | 26±5 | 27±5 | 0.6 |
Data are expressed as N (% of total), mean±SD or median (IQR) unless otherwise specified.
CMR, cardiac MR; CRT, cardiac resynchronization therapy; EDVI, end-diastolic volume indexed; FAC, fractional area change; GLS, global longitudinal strain; ICD, Implantable cardioverter-defibrillator; IVED, interventricular ejection delay; IVSD, interventricular shortening delay; LAVI, left atrial volume indexed; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; NYHA, New York Heart Association; PM, pacemaker; RV EDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; VE/VCO2, minute ventilation/carbon dioxide output; VO2, oxygen uptake.
Clinical outcomes
During a mean follow-up of 48±21 months, 33 patients (12%) suffered an adverse clinical outcome. Overall, 13 (4.5%) patients suffered an atrial flutter or fibrillation, while 11 (3.9%) patients were hospitalised due to decompensated heart failure. Five patients (1.8%) experienced a ventricular arrhythmia and 4 patients (1.4%) died during the follow-up. Patients who experienced an adverse event during follow-up were older, had undergone repair later in life and had more often a palliative procedure prior to complete cardiac repair. These patients were more symptomatic, had higher NT-proBNP levels and longer QRS duration. In the event group the left ventricle and both atria were larger, the RV fractional area change was lower and the RV systolic pressure higher. The proportion of patients with severe tricuspid or pulmonary regurgitation was significantly higher in the event group. Their exercise capacity, peak heart rate and the ventilatory efficiency were lower compared with patients without event. No differences were found with respect to surrogate markers of RV dyssynchrony and myocardial deformation. Patient characteristics and clinical findings stratified by events are shown in table 4. The type of events and further details are summarised in online supplemental table 2.
Table 4.
Comparison of the baseline characteristics between patients with and without event
| Patients without event N=252 (88%) |
Patients with event N=33 (12%) |
P value | |
| Clinical characteristics | |||
| Age at repair—years | 2.6 (2.2–35.6) | 6.2 (2.2–29.9) | 0.023 |
| Age at baseline—years | 29.3 (22.2–65.8) | 47.5 (33.1–67.5) | <0.001 |
| Primary repair | 172 (68) | 14 (42) | 0.003 |
| NYHA functional class I | 210 (83) | 19 (58) | 0.001 |
| NT-proBNP—pg/mL | 120 (59–983) | 434 (191–1871) | <0.001 |
| No device (ICD, PM, CRT) | 236 (94) | 27 (82) | 0.011 |
| QRS duration—ms | 142±25 | 156±27 | 0.004 |
| Echocardiography data | |||
| LVEDD—mm | 46±6 | 50±8 | 0.016 |
| LVESD—mm | 31±6 | 35±9 | 0.012 |
| LVEF—% | 59±8 | 57±11 | 0.31 |
| LAVI—mL/m2 | 24±11 | 38±21 | 0.004 |
| RA area—cm2 | 18±6 | 25±6 | <0.001 |
| RV-RA gradient—mm Hg | 30±12 | 39±10 | <0.001 |
| RV FAC—% | 39±8 | 35±11 | 0.03 |
| Severe tricuspid regurgitation | 1 (0.4%) | 2 (6.3%) | 0.002 |
| Severe pulmonary regurgitation | 47 (18%) | 12 (36%) | 0.014 |
| RV GLS—% | −16±5 | −15±6 | 0.38 |
| RV Dyssynchrony Index—ms | 49±31 | 41±18 | 0.21 |
| IVSD—ms | 103±50 | 130±65 | 0.09 |
| IVED—ms | −3.3±19 | 2.5±19 | 0.12 |
| CMR data | |||
| LV EDVI—mL | 86±19 | 99±31 | 0.99 |
| RV EDVI—mL | 124±31 | 139±56 | 0.95 |
| RV EF—% | 43±13 | 48±8 | 0.11 |
| LV EF—% | 57±7 | 53±11 | 0.02 |
| Exercise data | |||
| Peak VO2—mL/kg/min | 22±9 | 18±11 | 0.03 |
| Peak heart rate—bpm | 161±24 | 133±33 | 0.003 |
| VE/VCO2 | 26±5 | 30±5 | <0.001 |
Data are expressed as N (% of total), mean±SD or median (IQR) unless otherwise specified.
CMR, cardiac MR; CRT, cardiac resynchronization therapy; EDVI, end-diastolic volume indexed; RV FAC, right ventricular fractional area change; GLS, global longitudinal strain; ICD, Implantable cardioverter-defibrillator; IVED, interventricular ejection delay; IVSD, interventricular shortening delay; LAVI, left atrial volume indexed; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; PM, pacemaker; RV EDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; VE/VCO2, minute ventilation/carbon dioxide output; VO2, oxygen uptake.
Univariate and multivariate predictors of events
By univariate Cox regression analysis, age at repair and at baseline visit, NYHA class, NT-proBNP levels and QRS duration were significantly associated with adverse clinical events. Age at inclusion and NT-proBNP showed the highest association. Among all echo and CMR imaging data, the dimensions of left and right atrial area (RAA) showed the highest association with outcome and exceeded markers of LV and RV size and function, as well as all surrogates of ventricular dyssynchrony. Using a multivariate Cox-regression model, NT-proBNP, RAA and peak heart rate were independent predictors of outcomes after correction for age at baseline. Online supplemental table 3 and online supplemental figure 1 provide detailed results of the univariate and multivariate analysis.
openhrt-2023-002583supp002.pdf (370.7KB, pdf)
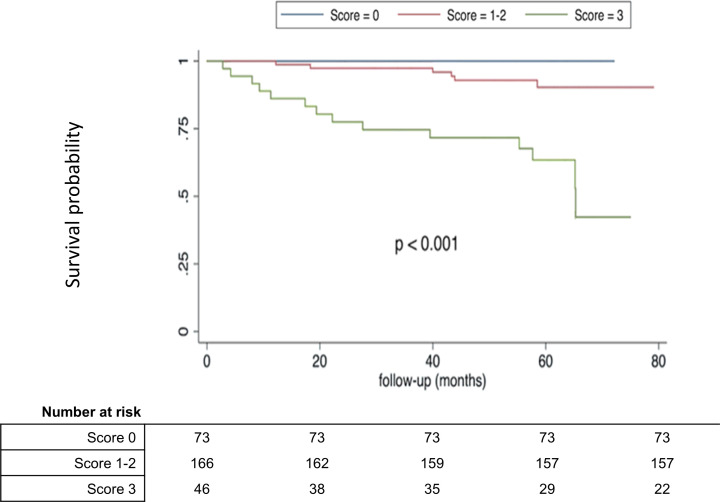
Using the median values of the variables as cut-off (NT-proBNP>133 pg/mL, RAA>18 cm2 and peak heart rate<164 bpm), a risk score was calculated. Patients with NT-proBNP values >133 pg/mL had a 5.9-fold increased risk (95% CI 2.1 to 17.1) of an adverse event compared with patients with values below median. Patients with RAA>18 cm2 and a peak heart rate <164 bpm had an HR of 5.1 (95% CI 1.9 to 13.5) and 5.3 (95% CI 1.8 to 15.3), respectively. The combined score accurately stratified patients into low-risk (score 0; n=39) and high-risk (score 3; n=38) for an adverse event, as depicted in the Kaplan-Meier curve (figure 2 and online supplemental figure 2). After a mean follow-up of 4 years, none of the patients with a score of 0 had an adverse event, while 62% of patients with score of 3 experienced adverse events (p<0.001).
Figure 2.
Kaplan-Maier event-free survival curve according to the risk score of 0, 1–2 and 3. 1 point is given if NT-proBNP>133 pg/mL, 1 point if right atrial area >18 cm2 and 1 point if peak heart rate <164 bpm. NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Intraobserver and interobserver variability
According to the ICC, the intraobserver and interobserver variability was 0.90 and 0.55 for RV GLS and 0.83 and 0.50 for the RVDI, respectively. Mean difference and SD for intraobserver comparison was 0.6±2 and – 2.8±3.0 for RV GLS and for RVDI, respectively. Similarly, mean difference and SD for interobserver comparison was −1.6±3 and −0.5±16 for RV GLS and for RVDI, respectively.
Discussion
The aim of this study was to investigate the role of RV dyssynchrony surrogates and the risk stratification of patients with rTOF years after surgical repair with clinical parameters. The main finding was that markers of RV dyssynchrony did not predict clinical events over a mean follow-up of 4 years. However, a multimodality approach including NT-proBNP levels, RAA and peak heart rate during exercise testing was very useful in identifying patients at low and high risk of an adverse event.
RV dyssynchrony
Almost all adults with rTOF have a right bundle branch block on ECG, as a result of either right ventriculotomy, infundibular muscular resection, patch closure of the ventricular septal defect or due to a combination of injuries to the right bundle branch.11 Delayed electrical activation of RV segments contributes to electromechanical dyssynchrony. Other factors, such as RV regional wall stress, thickness and shape can also affect RV mechanical dispersion.12 At present, a single widely accepted definition of RV dyssynchrony is still lacking.13 In this study, we focused on RVDI as defined by Hui et al,10 taking into account all six RV segments. In our cohort, we found no correlation between ventricular mechanical dispersion and clinical outcomes. Similar results have been reported by other groups using CMR to identify RV dyssynchrony. For instance, Moon et al14 showed that LV and RV CMR-derived strain parameters of ventricular function were associated with death or ventricular tachycardia in adults with rTOF, but no such correlation was found for ventricular dyssynchrony. Jing et al15 reported that CMR-derived RVDI was not predictive of changes in RV size and function over time in a large cohort of adults with rTOF. In a study with children after TOF-repair, RVDI by echocardiography was found to have a weak correlation with RV remodelling and function by univariate analysis.8 In this study, RV septal delay and prestretch duration were independent predictors of outcomes. These results suggest that RV dyssynchrony, in contrast to RV strain measures, is less associated with adverse clinical outcomes. This is in contrast with other conditions such as idiopathic pulmonary hypertension or in patients with systemic RV, where the predictive value of RV dyssynchrony has been well described.16 17 One could conclude that mechanical dispersion caused by surgical scars after repair might be less related to outcome than the RV mechanical dispersion due to a pressure-overloaded. This is supported by the fact that, until now, no prognostic risk scores proposed in the literature for rTOF employ dyssynchrony parameters.18 Further studies are needed to validate these hypotheses.
Multimodality risk stratification
Our study demonstrates that risk stratification with NT-proBNP levels, RAA and peak heart rate is highly predictive for identifying low-risk and high-risk patients for adverse event. Elevated NT-proBNP levels are well known to predict adverse events in patients with CHD.19 In our study, NT-proBNP levels of 133 pg/mL predicted the composite outcome of death, decompensated heart failure and major arrhythmias (AUC 0.83). These results are comparable with those reported by Westhoff-Bleck et al20 in a cohort of rTOF patients, where NT-proBNP levels of 126 pg/mL predicted a composite outcome with an AUC of 0.87.
A dilated right atrium is related to supraventricular arrhythmia in TOF patients,21 as the atrial stretch alters the electrical refractoriness enhancing the intrinsic propensity to reentry.22 In our analysis, an RAA>18 cm2 was a significant predictor of adverse outcomes. Diller et al23 showed that a RAA>22 cm² on CMR identified rTOF patients at increased risk of death or malignant arrhythmias during follow-up. Of note, the cut-off proposed in our study (RAA>18 cm2) is identical to the one used in patients with pulmonary arterial hypertension to assess their risk of adverse events.24
In addition to neurohormones and imaging data at rest, cardiopulmonary exercise testing variables reflect the patients’ haemodynamic reserve at exercise. In adults with rTOF, a correlation between mortality and peak heart rate, pVO2 and the VE/VCO2 slope has been demonstrated.25 In our cohort, these three variables were all predictors of a clinical adverse events at univariate analysis. However, in the multivariate Cox regression only peak heart rate remained an independent predictor. Peak heart rate is easily and reliably measurable and appears to be a crucial parameter of exercise performance in rTOF.26 Patients with surgically repaired CHD often experience chronotropic incompetence, resulting in reduced maximal heart rate and compromised exercise performance.27 The mechanisms underlying the diminished chronotropic response in rTOF remain inadequately understood. Although therapy with beta-blockers may also play a role, recent studies suggest that autonomic dysfunction, neurohormonal activation and cardiac arrhythmias may also be associated with chronotropic incompetence in the adult CHD populations.28 Overall, decreased peak heart rate may be a surrogate of advanced heart disease, and not simply the effect of beta-blockers.
Composite score for risk prediction
Combining three established predictors yielded an easily applicable risk stratification tool that was very useful to identify patients at low risk (score 0) and high risk (score 3) of adverse events. In our study, these two groups represented half of our cohort (25%, 25%). A score of 0 (no risk factor present) reliably identified patients at low risk, whereas a score of 3 (all risk factors present) was highly specific in identifying patients at risk of an adverse event. This reflects the independence of all three risk factors.
Study limitations
Our study has several limitations. First, this was a retrospective study with limitations related to such a design. Second, the number of patients who experienced adverse events was small, limiting the statistical power of the study. Third, not all measures could be obtained in all patients, further reducing the number of observations. Furthermore, despite growing evidence of the prognostic role of late-gadolinium-enhancement in CMR in rTOF patients, this feature was not investigated in the routine CMR of our cohort.
Another potential limitation of our study relates to the method of assessing RV longitudinal strain. We measured the strain of both the RV free wall and the septal segments for dyssynchrony analysis. There is an ongoing debate about whether to include the right side of the interventricular septum in the assessment of RV longitudinal strain or not. Current recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging do not provide clear guidance.29 Excluding the interventricular septum from RV strain analysis might have a superior prognostic value in some cardiovascular diseases, nonetheless, it would limit the ability to assess mechanical dispersion within the right ventricle.
Finally, we found only a moderate interobserver reproducibility of the RVDI, which is consistent with the findings of the EACVI-ASE Strain Standardisation Task Force.30 This may have implications for the application of RVDI in clinical practice, as it suggests that RVDI measurement may be operator-dependent.
Conclusion
In our study, we found no correlation between RV dyssynchrony markers and the occurrence of adverse events during a 4-year follow-up period in adults with rTOF. A risk score combining NT-proBNP levels, RAA and peak heart rate was highly predictive in stratifying patients at low and high risk. Further prospective validation studies are required to confirm the prognostic value of this score.
Footnotes
Contributors: AP contributed to the sampling of the data from the register, off-line strain analysis, statistical analysis and manuscript writing under the supervision of MS, who is responsible for the overall content as guarantor. CN contributed to the off-line strain analysis and data sampling. EG, FS, KW, DT and MG contributed substantially in the conception, design and supervision of the manuscript and its final review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. The Open Access fee was granted by the University of Bern
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The study complies with the Declaration of Helsinki. This study involves human participants. The research protocol has been approved by the local ethics committee (Ethikkommission beider Basel, Basel, 23 July 2013, Ref. Nr. EK 180/13) and informed consent has been obtained from subjects during the recruitment in SACHER.
References
- 1.Apitz C, Webb GD, Redington AN. Tetralogy of fallot. Lancet 2009;374:1462–71. 10.1016/S0140-6736(09)60657-7 [DOI] [PubMed] [Google Scholar]
- 2.Campbell M. Natural history of cyanotic malformations and comparison of all common cardiac malformations. Br Heart J 1972;34:3–8. 10.1136/hrt.34.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valente AM, Gauvreau K, Assenza GE, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of fallot enrolled in the INDICATOR cohort. Heart 2014;100:247–53. 10.1136/heartjnl-2013-304958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokma JP, Geva T, Sleeper LA, et al. Improved outcomes after pulmonary valve replacement in repaired tetralogy of fallot. J Am Coll Cardiol 2023;81:2075–85. 10.1016/j.jacc.2023.02.052 [DOI] [PubMed] [Google Scholar]
- 5.Badagliacca R, Pezzuto B, Papa S, et al. Right ventricular strain curve morphology and outcome in idiopathic pulmonary arterial hypertension. JACC Cardiovasc Imaging 2021;14:162–72. 10.1016/j.jcmg.2020.08.017 [DOI] [PubMed] [Google Scholar]
- 6.Kjaergaard J, Sogaard P, Hassager C. Right ventricular strain in pulmonary embolism by doppler tissue echocardiography. J Am Soc Echocardiogr 2004;17:1210–2. 10.1016/j.echo.2004.06.026 [DOI] [PubMed] [Google Scholar]
- 7.Chow PC, Liang XC, Cheung EWY, et al. New two-dimensional global longitudinal strain and strain rate imaging for assessment of systemic right ventricular function. Heart 2008;94:855–9. 10.1136/hrt.2007.131862 [DOI] [PubMed] [Google Scholar]
- 8.Yim D, Hui W, Larios G, et al. Quantification of right ventricular electromechanical dyssynchrony in relation to right ventricular function and clinical outcomes in children with repaired tetralogy of fallot. J Am Soc Echocardiogr 2018;31:822–30. 10.1016/j.echo.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 9.Tobler D, Schwerzmann M, Bouchardy J, et al. Swiss adult congenital heart disease registry (SACHER) - rationale, design and first results. Swiss Med Wkly 2017;147:w14519. 10.4414/smw.2017.14519 [DOI] [PubMed] [Google Scholar]
- 10.Hui W, Slorach C, Bradley TJ, et al. Measurement of right ventricular mechanical synchrony in children using tissue doppler velocity and two-dimensional strain imaging. J Am Soc Echocardiogr 2010;23:1289–96. 10.1016/j.echo.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 11.Hazan E, Bical O, Bex JP, et al. Is right bundle branch block aviodable in surgical correction of tetralogy of fallot Circulation 1980;62:852–4. 10.1161/01.cir.62.4.852 [DOI] [PubMed] [Google Scholar]
- 12.Fraser AG, Bijnens BH, Friedberg MK. Understanding right ventricular dyssynchrony: its myriad determinants and clinical relevance. Exp Physiol 2021;106:797–800. 10.1113/EP089366 [DOI] [PubMed] [Google Scholar]
- 13.Bowen D, Kauling M, Loff Barreto B, et al. Right ventricular electromechanical dyssynchrony in adults with repaired tetralogy of fallot. Front Pediatr 2023;11:1085730. 10.3389/fped.2023.1085730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon TJ, Choueiter N, Geva T, et al. Relation of biventricular strain and dyssynchrony in repaired tetralogy of fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. Am J Cardiol 2015;115:676–80. 10.1016/j.amjcard.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 15.Jing L, Wehner GJ, Suever JD, et al. Left and right ventricular dyssynchrony and strains from cardiovascular magnetic resonance feature tracking do not predict deterioration of ventricular function in patients with repaired tetralogy of fallot. J Cardiovasc Magn Reson 2016;18:49. 10.1186/s12968-016-0268-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiina Y, Inai K, Takahashi T, et al. Inter- and intra-ventricular dyssynchrony in the systemic right ventricle is a surrogate marker of major cardiac events in mildly symptomatic patients. Heart Vessels 2018;33:1086–93. 10.1007/s00380-018-1144-2 [DOI] [PubMed] [Google Scholar]
- 17.Badagliacca R, Papa S, Valli G, et al. Right ventricular dyssynchrony and exercise capacity in idiopathic pulmonary arterial hypertension. Eur Respir J 2017;49:1601419. 10.1183/13993003.01419-2016 [DOI] [PubMed] [Google Scholar]
- 18.Ghonim S, Gatzoulis MA, Ernst S, et al. Predicting survival in repaired tetralogy of fallot. JACC Cardiovasc Imaging 2022;15:257–68. 10.1016/j.jcmg.2021.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolger AP, Sharma R, Li W, et al. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 2002;106:92–9. 10.1161/01.cir.0000020009.30736.3f [DOI] [PubMed] [Google Scholar]
- 20.Westhoff-Bleck M, Kornau F, Haghikia A, et al. NT-proBNP indicates left ventricular impairment and adverse clinical outcome in patients with tetralogy of fallot and pulmonary regurgitation. Can J Cardiol 2016;32:1247. 10.1016/j.cjca.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 21.Bonello B, Kempny A, Uebing A, et al. Right atrial area and right ventricular outflow tract akinetic length predict sustained tachyarrhythmia in repaired tetralogy of fallot. Int J Cardiol 2013;168:3280–6. 10.1016/j.ijcard.2013.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojodjojo P, Peters NS, Davies DW, et al. Characterization of the electroanatomical substrate in human atrial fibrillation: the relationship between changes in atrial volume, refractoriness, wavefront propagation velocities, and AF burden. J Cardiovasc Electrophysiol 2007;18:269–75. 10.1111/j.1540-8167.2007.00723.x [DOI] [PubMed] [Google Scholar]
- 23.Diller GP, Orwat S, Vahle J, et al. Prediction of prognosis in patients with tetralogy of fallot based on deep learning imaging analysis. Heart 2020;106:1007–14. 10.1136/heartjnl-2019-315962 [DOI] [PubMed] [Google Scholar]
- 24.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023;61:2200879. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 25.Inuzuka R, Diller G-P, Borgia F, et al. Comprehensive use of cardiopulmonary exercise testing identifies adults with congenital heart disease at increased mortality risk in the medium term. Circulation 2012;125:250–9. 10.1161/CIRCULATIONAHA.111.058719 [DOI] [PubMed] [Google Scholar]
- 26.Bhatt SM, Elci OU, Wang Y, et al. Determinants of exercise performance in children and adolescents with repaired tetralogy of fallot using stress echocardiography. Pediatr Cardiol 2019;40:71–8. 10.1007/s00246-018-1962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wessel HU, Paul MH. Exercise studies in tetralogy of fallot: a review. Pediatr Cardiol 1999;20:39–47. 10.1007/s002469900393 [DOI] [PubMed] [Google Scholar]
- 28.Ohuchi H, Watanabe K, Kishiki K, et al. Heart rate dynamics during and after exercise in postoperative congenital heart disease patients. their relation to cardiac autonomic nervous activity and intrinsic sinus node dysfunction. Am Heart J 2007;154:165–71. 10.1016/j.ahj.2007.03.031 [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 30.Mirea O, Pagourelias ED, Duchenne J, et al. Variability and reproducibility of segmental longitudinal strain measurement. JACC Cardiovasc Imaging 2018;11:15–24. 10.1016/j.jcmg.2017.01.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002583supp001.pdf (87.2KB, pdf)
openhrt-2023-002583supp002.pdf (370.7KB, pdf)
Data Availability Statement
No data are available.