Abstract
Introduction
Tuberculosis (TB) is a leading infectious cause of death globally. It is the most common opportunistic infection in people living with HIV, and the most common cause of their morbidity and mortality. Following TB treatment, surviving individuals may be at risk for post-TB lung disease. The TB Sentinel Research Network (TB-SRN) provides a platform for coordinated observational TB research within the International epidemiology Databases to Evaluate AIDS (IeDEA) consortium.
Methods and analysis
This prospective, observational cohort study will assess treatment and post-treatment outcomes of pulmonary TB (microbiologically confirmed or clinically diagnosed) among 2600 people aged ≥15 years, with and without HIV coinfection, consecutively enrolled at 16 sites in 11 countries, across 6 of IeDEA’s global regions. Data regarding clinical and sociodemographic factors, mental health, health-related quality of life, pulmonary function, and laboratory and radiographic findings will be collected using standardised questionnaires and data collection tools, beginning from the initiation of TB treatment and through 12 months after the end of treatment. Data will be aggregated for proposed analyses.
Ethics and dissemination
Ethics approval was obtained at all implementing study sites, including the Vanderbilt University Medical Center Human Research Protections Programme. Participants will provide informed consent; for minors, this includes both adolescent assent and the consent of their parent or primary caregiver. Protections for vulnerable groups are included, in alignment with local standards and considerations at sites. Procedures for requesting use and analysis of TB-SRN data are publicly available. Findings from TB-SRN analyses will be shared with national TB programmes to inform TB programming and policy, and disseminated at regional and global conferences and other venues.
Keywords: Tuberculosis, HIV & AIDS, Observational Study
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Use of a diverse, global cohort of individuals with and without HIV to study pulmonary tuberculosis (TB) treatment and post-treatment outcomes, with harmonisation of procedures and variables across 16 sites in 11 countries, across 6 global International epidemiology Databases to Evaluate AIDS regions.
Comprehensive data collection, including sociodemographic, clinical, mental health, respiratory quality of life, spirometry, laboratory and radiographic data, across the TB treatment and post-treatment time periods.
Research follow-up through 12 months after the end of TB treatment, enabling investigations of longer-term outcomes after TB treatment, and correlation with factors ascertained at TB treatment initiation or during treatment.
STRENGTHS AND LIMITATIONS OF THIS STUDY.
An inclusive approach informing real-world contexts of TB treatment. Specifically, this study includes both clinically diagnosed and microbiologically confirmed TB, and includes specific data collection and procedures for youth (ages 15–24) and for pregnant and postpartum participants.
Limitations include some variations by region in TB management, available treatment support and access to testing for diagnosis and monitoring (eg, TB cultures). These will be noted and accounted for in planned analyses.
Introduction
Before the onset of the COVID-19 pandemic, tuberculosis (TB) was the leading infectious cause of death globally by a single pathogen.1 The COVID-19 pandemic has disrupted TB and HIV services, with attendant challenges for optimal diagnosis, control and care management.1–4 In the years since the onset of the COVID-19 pandemic, global estimates of TB disease, drug-resistant TB and TB deaths have increased for the first time in many years.1–3 In 2021, an estimated 10.6 million people developed TB disease and 1.6 million people died from TB.1 Further—despite increasing global access to antiretroviral treatment (ART) and to TB preventive therapy (TPT) among people living with HIV (PLHIV)—TB remains the leading cause of morbidity and mortality among PLHIV.5–7 In this context, data are urgently needed to inform global strategies to address the dual TB and HIV epidemics.
Critical evidence gaps exist with regard to drivers of unfavourable TB treatment outcomes, such as mortality, TB recurrence and post-treatment sequelae/complications, including among PLHIV.5 8 9 Addressing these gaps is particularly critical in light of recent acceleration towards shorter TB treatment regimens for both drug-susceptible and drug-resistant TB, and given the prospect of possible individualised approaches to treat both TB and post-TB lung disease (PTLD).10 Key areas of ongoing research gaps relate to TB-HIV coinfection, treatment and associated complications; consequences of drug-resistant TB; pulmonary complications and post-treatment outcomes; the impacts of psychosocial and life course factors on TB outcomes; and mental health outcomes of TB. A global prospective cohort of individuals with TB and with TB-HIV coinfection enables harmonised data collection and procedures to inform questions in these key areas.
The International epidemiology Databases to Evaluate AIDS (IeDEA) global research consortium—established by the US National Institutes of Health (NIH) in 2006—collects and analyses observational data in a clinical cohort of over 2.2 million PLHIV and people at risk for HIV, in 44 countries.11 Data are organised by seven geographical regions and coordinated by regional data centres.11 IeDEA provides diverse global data from HIV treatment programmes. The TB Sentinel Research Network (TB-SRN) is a global platform for coordinated observational TB research within the IeDEA consortium, which receives funding from multiple institutes and centres within the US NIH. The TB-SRN working group of IeDEA developed an observational cohort study protocol. To facilitate possible pooled global analyses, protocol development was based in part on the framework and original protocol of the Regional Prospective Observational Research in TB (RePORT) International Consortium.12–14 The TB-SRN uses a common set of standards and definitions for prospective observational TB research. These were developed in alignment with the study concepts and measurement time points defined by RePORT International.12–14 The TB-SRN will facilitate the use of pooled data to study pulmonary TB treatment and post-treatment outcomes among people with and without HIV at TB-SRN sites in six of IeDEA’s global regions. The resulting findings and study infrastructure may be used to inform policy and practice regarding TB treatment, and create a platform for additional regional and multiregional TB research within IeDEA.
Methods and analysis
Objectives of the IeDEA TB-SRN
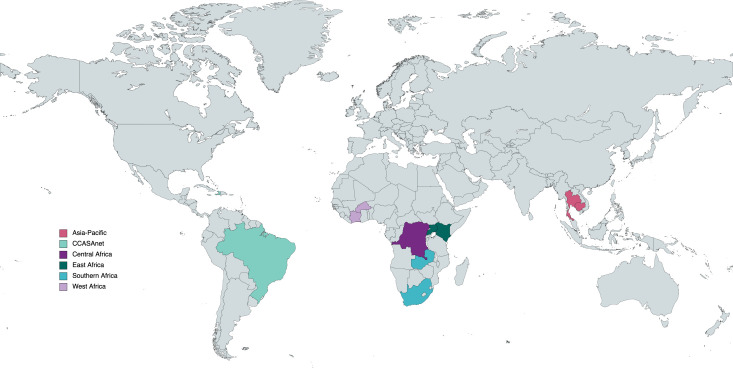
With its focus on HIV and associated coinfections and comorbidities, the global IeDEA research consortium is ideally positioned to study TB outcomes among people with and without HIV. To accomplish this, the TB-SRN will study outcomes of people diagnosed with pulmonary TB through a network of 16 sentinel sites (table 1) located in 11 low-income and middle-income countries in 6 IeDEA regions: Asia-Pacific, CCASAnet (Caribbean, Central and South America), Central Africa, East Africa, Southern Africa and West Africa (figure 1).15
Table 1.
Planned initial study sites in the tuberculosis sentinel research network of the International epidemiology databases to evaluate AIDS (IeDEA), by IeDEA region and target sample size
| IeDEA region | Site name | Sample size |
| Asia-Pacific | National Centre for HIV, AIDS, dermatology and STDs, Cambodia | 300 |
| HIV Netherlands-Australia-Thailand Research Collaboration and Chulalongkorn Hospital, Thailand | ||
| Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand | ||
| CCASAnet | GHESKIO, Haiti | 100 |
| Instituto Nacional de Infectologia, Fiocruz-RJ, Brazil | 250 | |
| Centro Municipal de Saude Duque de Caxias, Brazil | 250 | |
| Instituto Brasileiro de Investigação da Tuberculous/Fiocruz-BA, Brazil | 250 | |
| Fundação de Medicina Tropical, Brazil | 250 | |
| Central Africa | Centre Hospitalier Kabinda, the Democratic Republic of the Congo | 300 |
| Bondeko Health Centre, Kinshasa, the Democratic Republic of the Congo | ||
| East Africa | Academic Model Providing Access to Healthcare—Moi Teaching and Referral Hospital, Kenya | 200 |
| Mbarara Regional Referral Hospital, Uganda | 100 | |
| Southern Africa | Kanyama and Chawama at CIDRZ, Lusaka, Zambia | 150 |
| Themba Lethu, Johannesburg, South Africa | 150 | |
| West Africa | CePReF, Abidjan, Côte d’Ivoire | 100 |
| Centre Hospitalier Universitaire Sourou Sanon, Bobo Dioulasso, Burkina Faso | 200 |
CCASAnet, the Caribbean, Central and South America network for HIV epidemiology; CePReF, Centre de Prise en charge, de Recherche, et de Formation; CIDRZ, Centre for Infectious Disease Research in Zambia; GHESKIO, Groupe Haïtien d’Étude du Sarcome de Kaposi et des Infections Opportunistes; STDs, Sexually transmitted infections.
Figure 1.
Country locations of planned initial study sites in the Tuberculosis Sentinel Research Network of the International epidemiology Databases to Evaluate AIDS. Map created using MapChart.net. CCASAnet, the Caribbean, Central and South America network for HIV epidemiology.
There are three specific objectives of the TB-SRN. First, the TB-SRN will collect and analyse clinical and treatment data among people treated for pulmonary TB with or without HIV coinfection, to improve our understanding of the prognosis of TB disease and its health-related outcomes, including quality of life and survival. Second, the TB-SRN will assess the individual-level effects of HIV and ART on TB symptomatology, diagnosis, treatment response and survival. As part of this aim, investigators will also explore the effect of site-level TB and HIV management and integration of TB and HIV services on pulmonary TB treatment and longer-term outcomes. Third, the TB-SRN will describe PTLD and associations with HIV infection, diabetes, chronic lung disease, mental health and tobacco, alcohol and substance use, including measuring physiological, structural, and functional impairment, health-related quality of life, and survival.
Study design
The TB-SRN is a prospective, observational study, with consecutive enrolment of PLHIV and HIV-negative individuals, ages 15 and above, with clinically diagnosed or microbiologically confirmed pulmonary TB disease. Microbiological confirmation of pulmonary TB is defined on the basis of either positive molecular diagnostic test (eg, GeneXpert), acid-fast bacilli smear and/or TB culture from sputum or other respiratory specimen. Microbiological confirmation of pulmonary TB may also be based on positive urine lipoarabinomannan assay in the presence of clinical signs, symptoms and/or radiographic findings of pulmonary TB. Clinically diagnosed pulmonary TB is defined by clinical diagnosis by medical providers through standard of care, in the absence of confirmatory testing, prompting initiation of TB treatment. Specific eligibility criteria that must be met as part of clinical diagnosis include having either (1) any signs or symptoms of active TB (eg, persistent cough, haemoptysis, fever, unintended weight loss, fatigue or lethargy, night sweats, pleuritic chest pain) together with chest X-ray findings consistent with pulmonary TB or (2) the presence of respiratory signs and symptoms (including chronic cough, haemoptysis or pleuritic chest pain) regardless of chest X-ray findings. Individuals who consent to participate will provide clinical, laboratory and radiographic data at study visits at specified time points from TB treatment initiation through 12 months after the end of treatment (table 2).
Table 2.
Study procedures in the Tuberculosis (TB) Sentinel Research Network of the International epidemiology Databases to Evaluate AIDS
| Form | Treatment phase* | Post-treatment phase | ||||||
| Screening | Baseline | Month 1 (weeks 3–7) |
Month 2 (weeks 8–12) |
End of TX (−4 to+6 weeks) |
6 M post-TX (−4 to +6 weeks) |
12 M post-TX (−4 to +6 weeks) |
Tx F/R/W | |
| Informed consent (and assent, if applicable)† | X | |||||||
| Demographics Including adolescent and young adult characteristics (if applicable)‡ |
X | |||||||
| Clinical history | ||||||||
| TB history and current diagnosis | X | X | ||||||
| HIV and other medical history | X | |||||||
| Pregnancy and postpartum history (female participants only) | X | X | X | X | X | |||
| Pregnancy and infant outcomes (if applicable) | X | X | X | X | X | |||
| Clinical evaluation visit information, vital signs including pulse oximetry, respiratory symptoms, physical signs |
X | X | X | X | X | X | X | |
| Substance use ASSIST and smoking history |
X | X | X | X | ||||
| Respiratory symptoms and health-related quality of life SGRQ |
X | X | X | X | X | |||
| Depression symptoms PHQ-9 and suicide risk assessment |
X | X | X | X | ||||
| Pulmonary testing§ | ||||||||
| Spirometry | X | X | X | |||||
| 1 min sit-to-stand test | X | X | X | X | ||||
| Performed if not already done as part of care: | ||||||||
| Chest X-ray (CXR)¶ | X | X | ||||||
| CD4 count (only for participants with HIV)** | X | X | ||||||
| Hemoglobin A1C and random blood glucose | X | X | ||||||
| Data collected from routine care, as available | ||||||||
| TB microbiology | X | X | X | X | X | |||
| HIV testing and other lab results†† | X | X | X | X | X | X | X | |
| TB treatment Anti-TB regimen, adherence to medications, use and type of directly observed therapy |
X | X | X | X | X | |||
| Antiretroviral treatment (if applicable) adherence to medications |
X | X | X | X | X | X | X | |
| Adverse events | X | X | X | X | ||||
| TB IRIS evaluation | X | X | ||||||
| TB treatment outcome | X | X | ||||||
| Death form (death during study, if applicable) | ||||||||
*Month 1 visit is optional, and not done at all sites.
†Adolescent minors who turn 18 years of age during the study will be reconsented on the first visit after turning age 18.
‡For all youth participants ages 15–24 on enrolment.
§For 12 sites performing pulmonary testing.
¶Digitised/digitisable CXR obtained, unless done within 4 weeks prior to the baseline or end of treatment as per standard of care. CXRs obtained at other time points through routine care will also be digitised/uploaded. Pregnant women are not required to have a CXR; regions may vary in approach in this population according to local standards.
**CD4 count will only be performed on participants who are HIV-positive and who have not had a CD4 count performed in the preceding 3 months.
††HIV testing of participants not known to be positive collected from routine data and not as part of the study. HIV viral load (if applicable), complet blood count, transaminases and TB microbiology data to be abstracted if available.
ASSIST, Alcohol, Smoking and Substance Involvement Screening Test; IRIS, immune reconstitution inflammatory syndrome; PHQ-9, Patient Health Questionnaire; SGRQ, Saint George’s Respiratory Questionnaire; Tx F/R/W, treatment failure, relapse, or withdrawal.
Most participants, if completing a standard duration of 6 months of TB treatment for drug-susceptible TB, will consequently be followed for a total of 18 months from TB treatment initiation. Time on study will be longer, however, if treatment duration is longer as determined by providers under standard of care. (Treatment duration may be longer than 6 months, eg, for some regimens for drug-resistant TB, or if treatment is interrupted, or if pulmonary disease coincides with infection at an extrapulmonary site warranting longer treatment duration.) In addition, some regions have longer follow-up periods after end of TB treatment, according to region-specific objectives. Some regions will also have data collection beyond this multiregional protocol, such as for laboratory biomarkers, pharmacokinetic data or biological specimens. Data will be aggregated for concept-driven analyses.
Sample selection
TB-SRN sites
Sixteen sites included in the TB-SRN are located in 11 countries: Brazil, Burkina Faso, Cambodia, Côte d’Ivoire, the Democratic Republic of the Congo, Haiti, Kenya, South Africa, Thailand, Uganda and Zambia. These sites represent a range of contexts for the dual TB-HIV epidemics, including differing prevalence of TB and HIV, care models and resources. Analyses in this study will include comparisons by site-level variables or by region.
Eligibility and exclusion criteria
The IeDEA TB-SRN will enrol participants ages 15 and older with clinically diagnosed or microbiologically confirmed active pulmonary TB who are initiating TB treatment at IeDEA TB-SRN sites. Participants must have either documentation of recent HIV testing or of HIV infection or willingness to be tested for HIV, as routinely indicated under TB treatment guidelines.16 Informed consent will be required for all participants, including parental/caregiver consent and minor assent for individuals younger than age 18 (or the legal age of majority). There will be no restrictions based on sex, gender identity, HIV status, pregnancy, ethnicity or nationality. Participants may be coenrolled in other research with the exception of clinical trials of novel TB treatment regimens.
An individual will be excluded if they meet any of the following criteria: have received >7 days of TB treatment within the prior 30 days, excluding TPT; have imminent plans to follow-up for TB care or relocate/return to a site distant from the enrolment site, which would interfere with the participant’s ability to complete all study visits; have substantial cognitive impairment that may interfere with the ability to give reliable informed consent; are currently imprisoned.
Enrolment began in September 2022 and is projected to continue through October 2024. Data collection is ongoing with a projected end date of April 2026.
Participant considerations
The inclusion of participants with and without HIV will facilitate analyses in which HIV status is evaluated as a factor potentially contributing to TB treatment or post-treatment outcomes. The proportion of participants with HIV coinfection is anticipated to vary across sites.
The inclusion of participants ages 15 and above will allow for dedicated analyses of participants in the age group of 15–24 years of youth with TB. While youth have specific needs that must be addressed in quality health services, TB programmes globally have not adopted youth-centred care models.17–20 Further, adolescents and youth have been neglected in TB research, either by failure to include individuals younger than age 18, or by not examining research data within stratified adolescent or young adult age groups.19 20 Guidance from the WHO now advises that youth and their specific needs should be included in global TB research and care efforts.21–23 This study will assess clinical characteristics, TB outcomes,and post-treatment outcomes in a subcohort of youth with TB across 5-year age strata (ie, older adolescents aged 15–19 and young adults aged 20–24 years).
TB during pregnancy can cause poor outcomes for both the mother and for the developing fetus (or infant, after delivery), including maternal complications, miscarriage, preterm birth, low birth weight or perinatal death.24–27 Existing data regarding clinical features and outcomes of TB in pregnancy are very limited. Pregnant and postpartum individuals with TB (who have been pregnant within the last 12 months) will be included in TB-SRN. Data collection will include specific variables related to pregnancy, receipt of TB and HIV medications during pregnancy, and maternal and infant outcomes. These will be collected over the course of the study period, including for individuals who become pregnant or give birth during the study.
Patient and public involvement
Key research questions of this study were informed by previous participatory research and advocacy from individuals with TB; in particular, calling for research in PTLD and other post-treatment outcomes,28 and for inclusion of adolescent minors in research.23 Individuals with TB were not involved in the study’s design. Draft case report forms (CRFs) were revised through iterative rounds of review and preliminary piloting at the study sites. In particular, clinical programmes at the sites were involved in revisions at this stage to ensure the feasibility of CRFs and to minimise burdens on individuals with TB participating in the study. Clinical programmes at the sites were also consulted related to study planning. This included preparations for referral for immediate and urgent health needs, such as for symptoms of depression and suicidal thinking (assessed by Patient Health Questionnaire (PHQ-9) and suicide risk assessment). Findings from this research will be shared with national TB programmes and HIV treatment programmes at the study sites, and disseminated to individuals with TB.
Assessments and data collection
Data (eg, chest X-rays, laboratory results, health-related outcome measures; table 2) will be collected according to a common schedule and methodology across sites, so that they can be harmonised and aggregated for analysis.
Participants who consent to the study will be followed during TB treatment and for 12 months after the end of their primary treatment course (ie, the treatment course initiated at study enrolment). For most participants, this will be approximately 18 months after provisional enrolment/treatment start if they have drug-susceptible TB and receive a 6-month TB treatment regimen, but it may be longer if they have drug-resistant TB or require a longer treatment regimen for other reasons (eg, if there is associated extrapulmonary TB disease). At the time of this study, TB programmes at the study sites are primarily using treatment regimens of 6 months’ duration as routine standard for drug-susceptible pulmonary TB.
Participants will be requested to provide data during visits at key time points: at baseline (at initiation of TB treatment), month 1 (optional; performed at some but not all sites), month 2, end of TB treatment, 6 months post-treatment, 12 months post-treatment and at the time of suspected or apparent treatment failure, TB recurrence or study withdrawal if it occurs during treatment or the 12-month post-treatment follow-up phase. The baseline visit will include detailed clinical history, including course of TB symptoms and diagnosis, previous TB, HIV diagnosis and treatment (as applicable), history regarding recent or current pregnancy, and history regarding non-communicable comorbidities and their treatment (eg, diabetes mellitus, hypertension, pulmonary or cardiovascular disease, cancer, immune suppression, mental health diagnoses). HIV and TB clinical data will be extracted from medical records and TB registers, while current symptoms, pregnancy history and history of other conditions will be collected via patient interview. Sociodemographic information will be collected including specific information relevant to youth ages 15–24 at enrolment (eg, orphan status, caregiver characteristics, school attendance). Data collected at multiple visits during the study will include TB symptoms; ascertainment of immune reconstitution inflammatory syndrome, TB treatment failure or TB recurrence; clinical, radiographic and microbiological data related to TB; assessments for symptoms of depression (by the PHQ-9)29 and substance use (by the Alcohol, Smoking, and Substance Involvement Screening Test; ASSIST)30 31; and pulmonary investigations including repeated pulse oximetry, spirometry, functional test (1 min sit-to-stand test), and respiratory symptoms and health-related quality of life (by the Saint George’s Respiratory Questionnaire, SGRQ).32 33 These validated questionnaires have been used previously in the respective regions, with adaptations as appropriate (eg, PHQ-9, ASSIST and SGRQ).
Data will be collected on paper or electronic CRFs according to local capacity and regulatory requirements. A copy of the paper CRFs is provided (online supplemental material 1). Paper forms will be subsequently entered into the electronic system by trained personnel. All sites will use the secure, web-based REDCap data collection platform and/or the REDCap Mobile App for data collection.34 35 Common data management processes and procedures will be developed in collaboration with the Harmonist team at Vanderbilt University Medical Center, which provides informatics resources for IeDEA.36 37 For sites using film-based chest X-rays, films will be scanned and digitised using standard procedures defined by the NIH TB Portals platform.38
bmjopen-2023-079138supp001.pdf (1.1MB, pdf)
Sites will work with the IeDEA regional data centres to enter, prepare and clean data using either the Vanderbilt REDCap server or a regional REDCap server to adhere to country regulations on research data storage. All sites will use the same REDCap project template to ensure variables and study events use the same names and code lists to facilitate subsequent data merging. Research staff will administer questionnaires using relevant local translations (in Bemba, French, Haitian Creole, Khmer, Lingala, Nyanja, Portuguese, Runyankole, Swahili and Thai) and adaptations as appropriate, implemented in paper CRFs and in the REDCap data collection platform. Regional data centres will conduct quality control or assurance activities for their sites based on guidance developed by the TB-SRN study team and the Harmonist team.36 37
Outcome measures
Multiple outcomes will be assessed in the TB-SRN (table 3). These include TB treatment outcomes, TB recurrence, mortality, other pulmonary, health-related quality of life and mental health outcomes.
Table 3.
Select study outcomes in the Tuberculosis (TB) Sentinel Research Network of the International epidemiology Databases to Evaluate AIDS
| Outcome | Definition |
| TB treatment outcomes39 | As defined by WHO39:
|
| TB recurrence57 | Any new TB diagnosis after TB treatment completion or cure57 |
| Post-TB mortality | Death from any cause after end of TB treatment* |
| Sustained treatment success39 | An individual assessed at 6 months (for DR-TB and DS-TB) and at 12 months (for DR-TB only) after successful TB treatment, who is alive and free of TB.39 |
| Post-TB lung disease | Characterised by any of the following, after completion of TB treatment and in the absence of TB recurrence40:
|
| Functional status | 1 min sit-to-stand testing |
| Health-related quality of life | St. George’s Respiratory Questionnaire32 33 score |
| Depression symptoms | Patient Health Questionnaire29 scores for none or minimal (0–4), mild,5–9 moderate,10–14 moderately severe15–19 or severe20–27 |
*Information about cause of death will be recorded if available.
DR-TB, drug-resistant TB; DS-TB, drug-susceptible TB; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GLI, Global Lung Function Initiative; LLN, lower limit of normal.
TB treatment outcomes for both drug-resistant and drug-susceptible TB will follow the current definitions set by the WHO and will be in alignment with RePORT International outcomes.12 39 These include both clinical and biological criteria for TB treatment outcomes. TB recurrence (inclusive of TB relapse and new TB infection) will be defined as any new TB diagnosis after the end of TB treatment for the primary course. Mortality will include deaths from any cause, and will be assessed during the treatment and post-treatment periods. Information about cause of death will be recorded if available.
Post-treatment outcomes to be ascertained are informed by emerging research surrounding post-TB sequelae.28 40 41 PTLD will be defined by new, recurrent or persistent respiratory symptoms or signs that occur post-treatment; hypoxaemia (oxygen saturation <90%); pulmonary function impairment or chest X-ray abnormalities.40 Spirometry definitions for pulmonary function impairment include the following: forced expiratory volume in 1 S (FEV1)/forced vital capacity (FVC) ratio <lower limit of normal (LLN), FEV1<LLN and/or FVC<LLN. Global Lung Function Initiative (GLI) standard reference equations will be used to calculate LLN (fifth centiles) for each participant; these will be compared with observed values.42 43 Functional status will be assessed with 1 min sit-to-stand testing. Further measures of well-being after TB treatment will include symptoms of depression (by PHQ-9)29 and health-related quality of life (by SGRQ).32 33 The SGRQ has previously been validated32 and applied in studies of individuals treated for pulmonary TB.44–49 Both the PHQ-9 and SGRQ are among the assessments recommended by some experts for the evaluation of post-TB sequelae.28
Data management and harmonisation
TB-SRN data from multiple REDCap installations will be accessed via the secure REDCap Application Programming Interface (REDCap API) and automatically merged on a monthly basis to generate study enrolment and monitoring reports. These reports will allow tracking of study progress and ensure the distributed data collection remains aligned in variable formats and naming. Merged research datasets will be generated on demand for analyses associated with approved research concepts. Study data procedures include methods for ensuring the privacy and confidentiality of participant data, including using codes in place of names, implementing password-protected and encrypted data collection systems, training of site personnel on data management best practices, and applying data pseudonymisation where required for compliance with national data protection regulations. Analyses will be conducted by the designated regional data centre. Data-sharing agreements and management procedures will be overseen by the IeDEA Executive Committee.
The NIH, which funds both the IeDEA consortium and RePORT International, provides guidance for the coordination and linkage of these parallel streams of research. In addition, the Harmonist project, which supports IeDEA through development of data standards and software to support research operations, will coordinate with RePORT regions to streamline their existing data structures and identify points of data alignment with IeDEA to enable future cross-consortium data harmonisation and research.
Data analysis plan
While data may be used by the individual TB-SRN sites or regions, they are primarily being collected and harmonised for multiregional research, following IeDEA’s standard operating procedures governing research collaboration.50 The TB-SRN observational cohort study is designed to inform multiple analyses. Analyses of global TB-SRN data will be proposed through concept sheets, for detailed review and feedback from collaborators in the TB-SRN and other IeDEA working groups relevant to the study, with subsequent final review and approval by the IeDEA Executive Committee.50 Concepts will centre on major research questions in TB and HIV clinical epidemiology.51 These will include analyses of TB severity, TB treatment and post-treatment outcomes including PTLD, health-related quality of life and associated clinical, mental health and life course factors. Youth with TB (ages 15–24) will be assessed as a subset of this cohort, with attention to their clinical, psychosocial and lung health findings. The subset of pregnant and postpartum participants will also be described, to include specific variables and outcomes in this group.
Current, initial TB-SRN concepts delineate analyses in the following areas: baseline TB severity and associated factors; baseline depressive symptoms and substance use; chronic hypoxaemia and respiratory symptoms; and PTLD in youth.
Sample size considerations
The IeDEA TB-SRN will enrol 2600 participants across all study sites, including 300 participants in each of 5 IeDEA regions (Asia-Pacific, Central Africa, East Africa, Southern Africa and West Africa) and 1100 participants in CCASAnet. It is estimated that between 5% and 10% of treated TB cases will result in TB treatment failure or TB recurrence. Thus, if 2600 participants with active TB are enrolled, it is expected that between 130 and 260 episodes of treatment failure or recurrence will occur, with 200 being an approximate midpoint estimate. Furthermore, the majority of recurrent episodes are estimated to occur within 6 months of treatment completion, and thus >90% of all such episodes are expected to be detected during the follow-up period. Pulmonary function impairment may be anticipated in approximately 50%–60% of participants after completion of TB treatment.52 The overall cohort sample size of 2600 participants will enable precise estimates of key treatment and post-treatment outcomes. Given the anticipated HIV prevalence of 20%–30% across the global cohort, this sample size will also allow for multivariable analyses, including on HIV coinfection and treatment-related factors, in addition to sex, age and additional demographic or clinical factors.
As the TB-SRN is a descriptive study encompassing multiple planned outcomes and analyses, statistical considerations and power will vary by the research question proposed within the concept process of IeDEA. For analyses of youth with TB, this age group is estimated to make up 17% of new TB cases globally.53 In a cohort of 2600 individuals with TB, we anticipate including several hundred in this age group; representing a valuable contribution to the evidence base for this group.19 20 For pregnant/postpartum participants with TB, while low numbers are anticipated, relevant variables collected in this study for maternal and infant outcomes will be described. Given the very limited existing data on this population, these data will add to the existing literature.24 25 27 Aggregation with data from other cohorts may be considered for pooled analyses of priority questions for TB in these subgroups.19 20 51
Ethics and dissemination
Ethical and safety considerations
Ethical and regulatory approvals have been obtained at all implementing study sites and affiliated institutions, from the following Ethics Committees or Institutional Review Boards, by IeDEA region:
CCASANet
Vanderbilt University Medical Center, Nashville, USA: Human Research Protections Programme—Health Sciences Committee 3 IRB, #141 049. (Site responsible for centralised forms development.)
GHESKIO, Haiti: Comité des Droites Humains des Centres GHESKIO—approval for ‘Nouveau Protocole—Complications après traitement TB—Cohorte Prospective’. (No IRB number assigned).
Instituto Nacional de Infectologia, FIOCRUZ, Brazil: Instituto Nacional de Infectologia Evandro Chagas, INI /FIOCRUZ IRB, #5.955.761.
Centro Municipal de Saúde (CMS) de Duque de Caxias, Brazil: Universidade do Grande Rio Professor José de Souza Herdy—UNIGRANRIO IRB, #6.063.843.
Instituto Brasileiro para Investigação da Tuberculose (IBIT), Brazil: Maternidade Climério de Oliveira—UFBA IRB, #5.998.764.
Fundação de Medicina Tropical (FMT), Brazil: Fundação de Medicina Tropical ‘Doutor Heitor Vieira Dourado’ IRB, #5.997.824.
Asia-Pacific
TREAT Asia, Thailand, Advarra IRB #1, IRB00000971, #Pro00060405.
The Kirby Institute, University New South Wales, Australia, IRB #1, IRB00001145, #HC220713.
NCHADS, Cambodia: National Ethics Committee Health Research (NECHR) IRB #1, IRB00003143, #321NECHR.
Chulalongkorn University, Thailand, IRB #1, IRB00001607, #0491/66.
Chiangrai Prachanukroh Hospital, Thailand, IRB #1, IRB00005481, #087/66 Ex.
Central Africa
Albert Einstein College of Medicine Institutional Review Board, Bronx, USA, #2022–13862.
Kinshasa School of Public Health Ethic Committee Board, Kinshasa, the Democratic Republic of the Congo, #ESP/CE/050/2023.
East Africa
Indiana University Institutional Review Board, Indianapolis, USA, #15525.
Moi Teaching and Referral Hospital/Moi University Institutional Research and Ethics Committee (IREC), Eldoret, Kenya, #IREC/347/2022.
National Commission for Science, Technology and Innovation, Kenya, #NACOSTI/P/23/23903.
Mbarara University of Science and Technology Research Ethics Committee, Mbarara, Uganda, #MUST-2022-618.
Uganda National Council of Science and Technology, #HS2619ES.
Southern Africa
Cantonal Ethics Committee of Bern, Switzerland, #PB_2016-00273.
Thembu Lethu and Crosby Clinic, Johannesburg, South Africa, #GP_202207_033.
University of Zambia Biomedical Research Ethics Committee, Lusaka, Zambia, #2538-2022.
University of the Witwatersrand, South Africa, Human Research Ethics Committee (Medical), ref. no. M220141.
West Africa
CePReF, Côte d’Ivoire: National Ethics Committee for Life Sciences and Health, US DHHS Registration #2: IRB00011917.
Centre Hospitalier Universitaire Sourou Sanon, Burkina Faso: Ethics Committee for Health Research (ECHR), #2022-01-009.
Overarching ethical considerations have included the general low risk of this observational study; assurances that the decision whether or not to participate will have no bearing on clinical care received at the sites; protocols to ensure privacy and confidentiality of participant data; adherence to infection prevention protocols at the study sites; compensation for time/travel to participate; and specific considerations for inclusion of minors and pregnant individuals (as described below).
Standardised procedures are in place to ensure appropriate linkage to care and further evaluation when study assessments identify a possible physical or mental health condition. This includes procedures for direct linkage to care when symptoms of depression or suicidal ideation are identified.
In terms of safety considerations, this study is intended to ascertain detailed data collection for individuals with pulmonary TB followed in routine TB care and management. The inclusion of minors and of individuals who are pregnant is to ensure that these groups are not excluded from TB research. This is particularly important given that these groups have largely been excluded from TB research, or specific data have not been collected that are relevant to their clinical or social factors or outcomes. Sites follow locally approved protocols with respect to use of chest X-ray in pregnancy.
While chest X-rays are recommended as part of routine TB care16 and the amount of radiation exposure from an X-ray procedure is considered safe in pregnancy when clinically indicated,54–56 chest X-rays are not required for pregnant participants with TB in this study. Further, ethical approvals followed local standards and approval processes for consideration of chest X-rays in this population.
Similarly, sites follow local standards and approvals for inclusion of minors. General approaches include requiring the consent of a parent or primary caregiver, along with assent of minors. Procedures are in place in recruitment and study activities to avoid inadvertent HIV disclosure to youth who have perinatally acquired HIV or to caregivers who may not be aware of a youth’s status.
Dissemination plan
Findings from TB-SRN analyses will be disseminated across the IeDEA consortium, and at site level, regional and global venues. Policy briefs will be developed summarising key study findings. These will be provided in direct communication with national TB programmes and with national HIV programmes to share and disseminate findings across these systems. Findings will be disseminated to study participants, and to TB care providers and individuals affected by TB, following setting-specific approaches at respective study sites.
Research findings from global and regional analyses will be presented at national and international meetings, and published in international peer-reviewed journals for a wide audience of clinicians, researchers and public health practitioners in the areas of TB and HIV care and lung health. Publications will be disseminated to global TB networks, including to WHO Global TB Programme working group leads as appropriate, and to relevant sections of the International Union Against Tuberculosis and Lung Disease.
TB-SRN data can be leveraged towards future research. Researchers from beyond the IeDEA research consortium may request IeDEA data for dedicated analyses. Procedures for requesting use of TB-SRN data are publicly available.50
In conclusion, the TB-SRN provides a unique platform for global observational research in TB and TB-HIV coinfection. Through harmonised procedures and comprehensive prospective data collection across TB treatment and post-treatment periods, the TB-SRN will generate key epidemiology data for drivers and correlates of TB treatment and post-treatment outcomes, across a diverse global cohort. Findings from this project will inform policy and practice regarding TB treatment, and further research efforts.
Supplementary Material
Acknowledgments
We thank the individual participants, their care providers and the study teams implementing the TB-SRN study across the clinical research sites for their support. We also acknowledge the contributions of members of the IeDEA Executive Committee and the IeDEA TB-SRN and TB and Lung Health Working Groups to the development and finalisation of the study protocol; the Harmonist team for their work on the data management and harmonisation framework and tools; and the RePORT-Brazil Network (Drs. Bruno Andrade, Valeria Rolla, Afranio Kritski and Marcelo Cordeiro-Santos).
Footnotes
Twitter: @leslie_enane
Collaborators: The International epidemiology Databases to Evaluate AIDS.
Contributors: LAE, OM, AHS, SND, AF, KW-K and MY designed this study and drafted the study protocol. TRS, MCF and TC contributed to revisions and refinements to the protocol. LAE, OM, SND and LRM led the development and refinement of data collection tools. TC, MB, LF, FM, KW-K and NN and others provided input on study procedures and data collection tools. SND and LRM developed the REDCap database for data collection under the multiregional protocol. LAE drafted the manuscript. All authors (LAE, SND, TC, CB-M, NN, EM, NM, LRM, MCF, JR, DE, LD, RA, NZ, AF, MFP, DR, MB, HB, NdC, MT, TRS, AHS, LF, KW-K, AP, MY, RH and OM) participated in manuscript revisions. All authors have read and approved the final manuscript.
Funding: LAE is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) under Award Number K23HD095778. NN is supported by the Francis Family Foundation under the Parker B. Francis Fellowship. The International Epidemiology Databases to Evaluate AIDS (IeDEA) is supported by the US National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center: Asia-Pacific, U01AI069907; CCASAnet and RePORT-Brazil, U01AI069923; Central Africa, U01AI096299; East Africa, U01AI069911; NA-ACCORD, U01AI069918; Southern Africa, U01AI069924; West Africa, U01AI069919; RePORT-Brazil, U01AI172064, CRDF Global R-202208-69014, Departamento de Ciência e Tecnologia (DECIT)—Secretaria de Ciência e Tecnologia (SCTIE)—Ministério da Saúde (MS), Brazil (25029.000507/2013-07). Informatics resources are supported by the Harmonist project, R24AI24872.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: AHS receives grants to her institution from ViiV Healthcare and Gilead Sciences.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization . Global tuberculosis report 2022. Geneva, 2022. [Google Scholar]
- 2.McQuaid CF, Vassall A, Cohen T, et al. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis 2021;25:436–46. 10.5588/ijtld.21.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visca D, Ong CWM, Tiberi S, et al. Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology 2021;27:151–65. 10.1016/j.pulmoe.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marti M, Zürcher K, Enane LA, et al. Impact of the COVID-19 pandemic on TB services at ART programmes in low- and middle-income countries: a multi-cohort survey. J Int AIDS Soc 2022;25:e26018. 10.1002/jia2.26018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan A, Nathavitharana RR. Addressing TB-related mortality in adults living with HIV: a review of the challenges and potential solutions. Ther Adv Infect Dis 2022;9:20499361221084163. 10.1177/20499361221084163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nliwasa M, MacPherson P, Gupta-Wright A, et al. High HIV and active tuberculosis prevalence and increased mortality risk in adults with symptoms of TB: a systematic review and meta-analyses. J Int AIDS Soc 2018;21:e25162. 10.1002/jia2.25162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016;19:20714. doi:20714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tornheim JA, Dooley KE. Challenges of TB and HIV Co-treatment: updates and insights. Curr Opin HIV AIDS 2018;13:486–91. 10.1097/COH.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 9.Weld ED, Dooley KE. State-of-the-art review of HIV-TB coinfection in special populations. Clin Pharmacol Ther 2018;104:1098–109. 10.1002/cpt.1221 [DOI] [PubMed] [Google Scholar]
- 10.Adjobimey M, Behr MA, Menzies D. Individualized treatment duration in tuberculosis treatment: precision versus simplicity. Am J Respir Crit Care Med 2021;204:1013–4. 10.1164/rccm.202107-1744ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International epidemiology Databases to Evaluate AIDS (IeDEA) . International epidemiology databases to evaluate AIDS (Iedea). n.d. Available: https://www.iedea.org [DOI] [PMC free article] [PubMed]
- 12.Hamilton CD, Swaminathan S, Christopher DJ, et al. Report International: advancing tuberculosis biomarker research through global collaboration. Clin Infect Dis 2015;61Suppl 3:S155–9. 10.1093/cid/civ611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geadas C, Stoszek SK, Sherman D, et al. Advances in basic and translational tuberculosis research. Tuberculosis 2017;102:55–67. 10.1016/j.tube.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Heijden YF, Abdullah F, Andrade BB, et al. Building capacity for advances in tuberculosis research; proceedings of the third report international meeting. Tuberculosis (Edinb) 2018;113:153–62. 10.1016/j.tube.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mapchart. 2023. Available: https://www.mapchart.net
- 16.World Health Organization . WHO consolidated guidelines on tuberculosis. Module 4: drug-susceptible tuberculosis treatment. Geneva: World Health Organization, 2022. [PubMed] [Google Scholar]
- 17.Enane LA, Eby J, Arscott-Mills T, et al. TB and TB-HIV care for adolescents and young adults. Int J Tuberc Lung Dis 2020;24:240–9. 10.5588/ijtld.19.0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laycock KM, Eby J, Arscott-Mills T, et al. Towards quality adolescent-friendly services in TB care. Int J Tuberc Lung Dis 2021;25:579–83. 10.5588/ijtld.21.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscibrodzki P, Enane LA, Hoddinott G, et al. The impact of tuberculosis on the well-being of adolescents and young adults. Pathogens 2021;10:1591. 10.3390/pathogens10121591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snow KJ, Cruz AT, Seddon JA, et al. Adolescent tuberculosis. Lancet Child Adolesc Health 2020;4:68–79. 10.1016/S2352-4642(19)30337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO operational handbook on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization, 2022. [PubMed] [Google Scholar]
- 22.World Health Organization . WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization, 2022. [PubMed] [Google Scholar]
- 23.Chiang SS, Waterous PM, Atieno VF, et al. Caring for adolescents and young adults with tuberculosis or at risk of tuberculosis: consensus statement from an international expert panel. J Adolesc Health 2023;72:323–31. 10.1016/j.jadohealth.2022.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miele K, Bamrah Morris S, Tepper NK. Tuberculosis in pregnancy. Obstet Gynecol 2020;135:1444–53. 10.1097/AOG.0000000000003890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phoswa WN, Eche S, Khaliq OP. The Association of tuberculosis mono-infection and tuberculosis-human immunodeficiency virus (TB-HIV) Co-infection in the pathogenesis of hypertensive disorders of pregnancy. Curr Hypertens Rep 2020;22:104. 10.1007/s11906-020-01114-5 [DOI] [PubMed] [Google Scholar]
- 26.Sobhy S, Babiker Z, Zamora J, et al. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG 2017;124:727–33. 10.1111/1471-0528.14408 [DOI] [PubMed] [Google Scholar]
- 27.Sugarman J, Colvin C, Moran AC, et al. Tuberculosis in pregnancy: an estimate of the Global Burden of Disease. Lancet Glob Health 2014;2:e710–6. 10.1016/S2214-109X(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 28.Nightingale R, Carlin F, Meghji J, et al. Post-TB health and wellbeing. Int J Tuberc Lung Dis 2023;27:248–83. 10.5588/ijtld.22.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negeri ZF, Levis B, Sun Y, et al. Accuracy of the patient health questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis. BMJ 2021;375:n2183. 10.1136/bmj.n2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humeniuk R, Henry-Edwards S, Ali R, et al. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): manual for use in primary care. Geneva: World Health Organization, 2010. [Google Scholar]
- 31.Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008;103:1039–47. 10.1111/j.1360-0443.2007.02114.x [DOI] [PubMed] [Google Scholar]
- 32.Pasipanodya JG, Miller TL, Vecino M, et al. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest 2007;132:1591–8. 10.1378/chest.07-0755 [DOI] [PubMed] [Google Scholar]
- 33.Stringer B, Lowton K, James N, et al. Capturing patient-reported and quality of life outcomes with use of shorter regimens for drug-resistant tuberculosis: mixed-methods Substudy protocol, TB PRACTECAL-PRO. BMJ Open 2021;11:e043954. 10.1136/bmjopen-2020-043954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Delacqua G, Taylor R, et al. The REDCap mobile application: a data collection platform for research in regions or situations with Internet scarcity. JAMIA Open 2021;4:ooab078. 10.1093/jamiaopen/ooab078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis JT, Stephens J, Musick B, et al. The Iedea Harmonist data Toolkit: a data quality and data sharing solution for a global HIV research consortium. J Biomed Inform 2022;131:104110. 10.1016/j.jbi.2022.104110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stover J, Glaubius R, Kassanjee R, et al. Updates to the spectrum/AIM model for the UNAIDS 2020 HIV estimates. J Int AIDS Soc 2021;24 Suppl 5(Suppl 5):e25778. 10.1002/jia2.25778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenthal A, Gabrielian A, Engle E, et al. The TB portals: an open-access, web-based platform for global drug-resistant-tuberculosis data sharing and analysis. J Clin Microbiol 2017;55:3267–82. 10.1128/JCM.01013-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . Meeting report of the WHO expert consultation on drug-resistant tuberculosis treatment outcome definitions. Geneva, 2020. [Google Scholar]
- 40.Allwood BW, van der Zalm MM, Amaral AFS, et al. Post-tuberculosis lung health: perspectives from the first International symposium. Int J Tuberc Lung Dis 2020;24:820–8. 10.5588/ijtld.20.0067 [DOI] [PubMed] [Google Scholar]
- 41.Migliori GB, Marx FM, Ambrosino N, et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis 2021;25:797–813. 10.5588/ijtld.21.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowerman C, Bhakta NR, Brazzale D, et al. A race-neutral approach to the interpretation of lung function measurements. Am J Respir Crit Care Med 2023;207:768–74. 10.1164/rccm.202205-0963OC [DOI] [PubMed] [Google Scholar]
- 43.Moffett AT, Bowerman C, Stanojevic S, et al. Race-neutral reference equations and pulmonary function test interpretation. JAMA Netw Open 2023;6:e2316174. 10.1001/jamanetworkopen.2023.16174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupte AN, Selvaraju S, Paradkar M, et al. Respiratory health status is associated with treatment outcomes in pulmonary tuberculosis. Int J Tuberc Lung Dis 2019;23:450–7. 10.5588/ijtld.18.0551 [DOI] [PubMed] [Google Scholar]
- 45.Kastien-Hilka T, Rosenkranz B, Schwenkglenks M, et al. Association between health-related quality of life and medication adherence in pulmonary tuberculosis in South Africa. Front Pharmacol 2017;8:919. 10.3389/fphar.2017.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuwagira E, Stadelman A, Baluku JB, et al. Obstructive lung disease and quality of life after cure of multi-drug-resistant tuberculosis in Uganda: a cross-sectional study. Trop Med Health 2020;48:34. 10.1186/s41182-020-00221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ralph AP, Kenangalem E, Waramori G, et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS ONE 2013;8:e80302. 10.1371/journal.pone.0080302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suyanto S, Geater A, Chongsuvivatwong V. The effect of treatment during A haze/post-haze year on subsequent respiratory morbidity status among successful treatment tuberculosis cases. Int J Environ Res Public Health 2019;16:4669. 10.3390/ijerph16234669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vashakidze SA, Kempker JA, Jakobia NA, et al. Pulmonary function and respiratory health after successful treatment of drug-resistant tuberculosis. Int J Infect Dis 2019;82:66–72. 10.1016/j.ijid.2019.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.International epidemiology Databases to Evaluate AIDS (IeDEA) . Multiregional research SOPs, templates: International Epidemiology Databases to Evaluate AIDS (IeDEA). 2022. Available: https://www.iedea.org/resources/multiregional-research-sops-templates/
- 51.NIAID Tuberculosis Research Strategic Plan Working Group . NIAID Strategic Plan for Tuberculosis Research. National Institute of Allergy and Infectious Diseases, 2018. [Google Scholar]
- 52.Taylor J, Bastos ML, Lachapelle-Chisholm S, et al. Residual respiratory disability after successful treatment of pulmonary tuberculosis: a systematic review and meta-analysis. EClinicalMedicine 2023;59:101979. 10.1016/j.eclinm.2023.101979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snow KJ, Sismanidis C, Denholm J, et al. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J 2018;51:1702352. 10.1183/13993003.02352-2017 [DOI] [PubMed] [Google Scholar]
- 54.Cunningham FG. Williams obstetrics. Twenty-sixth edition ed. McGraw Hill, 2022. [Google Scholar]
- 55.Hall EJ. Scientific view of low-level radiation risks. Radiographics 1991;11:509–18. 10.1148/radiographics.11.3.1852943 [DOI] [PubMed] [Google Scholar]
- 56.National Council on Radiation Protection and Measurements . Medical radiation exposure of pregnant and potentially pregnant women. Bethesda, MD: National Council on Radiation Protection and Measurements, 1977. [Google Scholar]
- 57.World Health Organization . Definitions and reporting framework for tuberculosis - 2013 revision. Geneva, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-079138supp001.pdf (1.1MB, pdf)