Abstract
Purpose
The National Congenital Anomaly and Rare Disease Registration Service (NCARDRS), part of National Disease Registration Service in National Health Service England, quality assures, curates and analyses individual data on the pregnancies, fetuses, babies, children and adults with congenital anomalies and rare diseases across England. The congenital anomaly (CA) register provides a resource for patients and their families, clinicians, researchers and public health professionals in furthering the understanding of CAs.
Participants
NCARDRS registers CAs occurring in babies born alive and stillborn, fetal losses and terminations in England. NCARDRS collects data from secondary and tertiary healthcare providers, private providers and laboratories covering fetal medicine, maternity or paediatric services. Data describe the pregnancy, mother, baby and anomaly. Established in 2015, NCARDRS expanded CA registration coverage from 22% of total births in England in 2015 to national coverage, which was achieved in 2018. Prior to 2015, data collection was performed independently by regional registers in England; these data are also held by NCARDRS.
Findings to date
NCARDRS registers approximately 21 000 babies with CAs per year with surveillance covering around 600 000 total births, the largest birth coverage for a CA register globally. Data on prevalence, risk factors and survival for children with CAs are available. Data have been used in several peer-reviewed publications. Birth prevalence statistics, including public health indicators such as the association with maternal age, infant and perinatal mortality, are published annually. NCARDRS supports clinical audit for screening programmes and service evaluation.
Future plans
NCARDRS provides a valuable resource for the understanding of the epidemiology, surveillance, prevention and treatment of CAs. Currently, approximately 21 000 new registrations of babies or fetuses with suspected or confirmed CAs are added each year. Identifiers are collected, enabling linkage to routinely collected healthcare and population statistics, further enhancing the value of the data.
Keywords: EPIDEMIOLOGY, PUBLIC HEALTH, REGISTRIES, NEONATOLOGY, PAEDIATRICS, Electronic Health Records
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Congenital anomaly registration coverage has been national across England since 2018 (approximately 21 000 anomalies registered from 600 000 total births per year), enabling the calculation of accurate estimates of prevalence, even for rare congenital anomalies.
National Congenital Anomaly and Rare Disease Registration Service (NCARDRS) collects personal identifiers and data are linked to other routinely collected administrative and healthcare data, allowing assessment of long-term outcome and survival over the life course of the baby, as well as associations with health inequalities and other risk factors.
NCARDRS provides data on all birth outcomes, including live births, stillbirths, fetal losses and terminations.
Case ascertainment is good for severe conditions, or those that are more frequently diagnosed antenatally or in the neonatal period.
Registration and case ascertainment in NCARDRS regions that initiated congenital anomaly registration from 2018 continues to develop and is progressing well.
Introduction
Congenital anomalies are a significant source of morbidity, mortality and long-term care needs in children. Approximately 2%–3% of children born in Europe have a congenital anomaly,1 which is defined as conditions present at birth and include structural, chromosomal, genetic and biochemical conditions. Some congenital anomalies are detected during pregnancy, some are found at birth, while others are diagnosed only as a baby grows older. In England and Wales, congenital anomalies were the most common cause of death in the post neonatal period in 2020, accounting for 36.3% of deaths.2 Globally, it is estimated that 240 000 newborns die within the neonatal period as a result of congenital anomalies.3
Registration of congenital anomalies became established in many countries from the 1960s and 70s as a consequence of the thalidomide tragedy and serves multiple purposes supporting epidemiology and public health.4 The National Congenital Anomaly and Rare Disease Registration Service (NCARDRS) is part of the National Disease Registration Service (NDRS) of National Health Service (NHS) England which collects, quality assures and analyses data on people living in England with cancer,5 congenital anomalies and rare diseases. NCARDRS curates a population-based congenital anomaly registry, collecting data on the pregnancies, fetuses, babies, children and adults with congenital anomalies across the whole of England. Data are collected to further the understanding of the causes of congenital anomalies, to inform the commissioning of public services, to audit health and social care and to provide information for patients, their carers and clinicians on their condition. To achieve this NCARDRS collects data from heath care settings across England. In England, healthcare is publicly funded and delivered in a centralised and universal way by the NHS.
The UK Rare Disease Strategy, developed in 2013, aimed to improve the lives of those with rare diseases, focusing on patient empowerment, identification and prevention, diagnosis and treatment, and the role of research and recommended the expansion of existing data collections.6 7 Established in 2015 in response, NCARDRS assumed responsibility for congenital anomaly registration in regions with an existing register and expanded geographically to provide congenital anomaly registration across the whole country8 (figure 1). Prior to 2015, data collection was performed independently by regional registers operating across some areas of England, covering up to 32% of births. NCARDRS continues to host the regional registers’ legacy registration data. A national congenital anomaly surveillance system was attempted by the UK Office for National Statistics (ONS) but this was closed in 2010 following concerns about data quality and completeness.9 NCARDRS national coverage for registration and reporting has been in place for babies born since 2018.10
Figure 1.
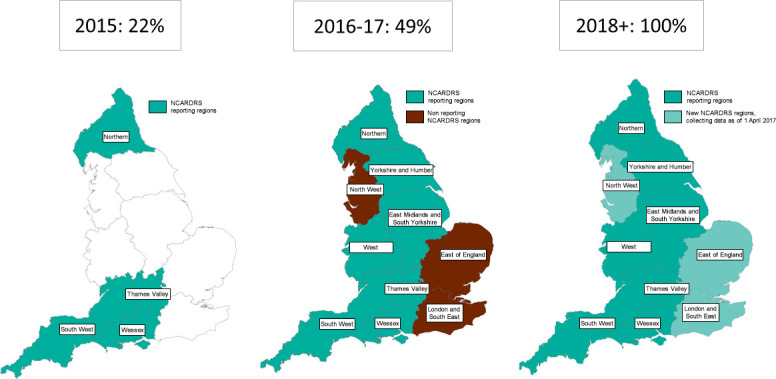
The regional structure of NCARDRS and the proportion of the birth population of England that was covered by congenital anomaly registration. NCARDRS, National Congenital Anomaly and Rare Disease Registration Service.
Cohort description
Study population
NCARDRS registers congenital anomalies that occur in babies that are live born and stillborn, fetal losses and terminations at any gestation delivered in England. NCARDRS registers all fetal losses reported, although in line with international standards11 only anomalies that occur in live births, stillbirths, terminations at any gestation and fetal losses between 20 and 24 completed weeks of gestation are included in prevalence reporting.12 13 There are approximately 600 000 live births and stillbirths in England every year. There is no upper age limit and information can be added for children as they grow older. Information on survival and vital status is updated at least annually.
Inclusion and exclusion criteria for registration of congenital anomalies in NCARDRS follow internationally recognised formats.11 Anomalies are clinically coded to international standards using the WHO’s International Classification of Diseases 10th revision (ICD-10)14 with the British Paediatric Association (BPA) Adaptation, which gives supplementary one-digit extensions to ICD-10 codes to allow greater specificity of coding.11 Inclusion criteria are based on international guidance11 predominantly covering the Q chapter in ICD-10. A detailed summary of the current inclusion and exclusion criteria is presented in online supplemental table S1. NCARDRS excludes cases with an isolated minor anomaly as specified by the European network of population-based registries for the epidemiological surveillance of congenital anomalies (EUROCAT).11 However, if minor anomalies occur in association with other anomalies, then these are registered.
bmjopen-2023-077743supp001.pdf (117.4KB, pdf)
Denominator data are obtained from the UK ONS. Individual-level birth data are available and are aggregated according to requirements.
Registration model and source data
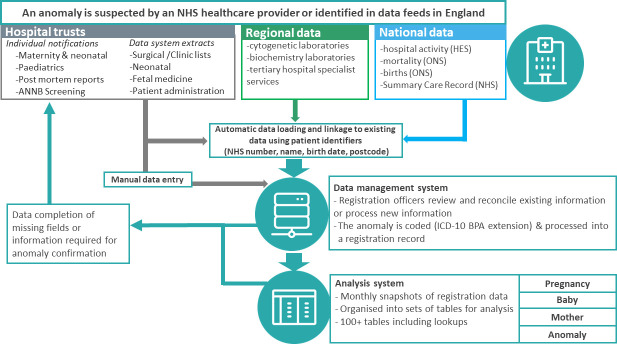
NCARDRS employs a multisource, event-based registration model. Over the life course of a patient, NCARDRS can be notified antenatally, at birth or in the neonatal period and beyond as the child is treated by various paediatric specialist services. Registration data are processed, held on a custom-built live data management application and regularly cloned to a separate PostgreSQL database, which creates regular snapshots of data for analysis, reporting and data release (figure 2).
Figure 2.
Schematic describing the multisource registration process used for congenital anomalies in England. BPA, British Paediatric Association; HES, Hospital Episode Statistics; ICD-10, International Classification of Diseases 10th revision; NHS, National Health Service; ONS, Office for National Statistics. ANNB, Antenatal and Newborn
The data collected by NCARDRS come from a range of sources including maternity units, multidisciplinary team meetings, postmortem reports, molecular testing results, treatment records, hospital patient administration systems, clinical data systems, national data sets describing hospital activity, clinical biochemistry and genetics laboratories. Hospital trusts, including all trusts with a maternity or paediatric service, submit data which are processed and combined by trained registration officers into a comprehensive clinical record of each baby and anomaly.
Data can be submitted at the individual case level or as large data extracts from clinical data management systems. Custom-built extracts from neonatal clinical data management systems (BadgerNet), including remote access to the record itself, are available for 94% of the trusts with a neonatal unit in England. Extracts of relevant data from fetal medicine software systems including fetal medicine (Viewpoint; Astraia) and specific services (HeartSuite) have been developed in conjunction with software suppliers; these extracts are produced and submitted to NCARDRS by the provider.
Information from providers is combined with routinely collected national data utilised for both data quality and case-ascertainment purposes. Linkage is conducted using NHS number or through date of birth, full name and address. Cases with defined ICD-10 codes that have been validated for accuracy are identified from Hospital Episode Statistics (HES) for ascertainment purposes. HES consists of routinely recorded, administrative data describing each hospital admission in the NHS.15 As well as demographic information on the patient, the primary reason for admission and any comorbidities are recorded using ICD-10 codes. Death certificate data from the ONS is provided to NCARDRS monthly for children born alive after 1 January 2018 and where a relevant ICD-10 coded condition (within a specified range) is listed as a cause of death. Information about babies that were born alive or stillborn after 24 weeks gestation (civilly registrable in England) is supplemented using birth registration information supplied by the ONS. Survival for all patients recorded is updated at least annually by linkage with the NHS Personal Demographics Service on NHS Spine, a collection of national databases that holds electronic records of important patient information and demographics.
Data processing
Once received, identifiable patient data are processed by trained registration officers. Processing involves manual extraction of information from clinical reports and letters, or free-text comments in clinical software systems. Registration officers require detailed knowledge of congenital anomalies, clinical coding and clinical pathways for the range of different conditions collected. As NCARDRS is a multisource register, patient identifiers are required to ensure that there is no duplication, and that incoming data are linked to the correct baby and pregnancy. Where cases are entered manually, patient identifiers are checked manually using the Summary Care Record on NHS Spine. Further information is requested from the relevant clinician or obtained by direct, manual interrogation of patient records by registration officers via secure remote access to a hospital’s clinical software systems or clinical documents, where this is available.
Data are input into the data management system in two ways: (1) data on individual patients submitted by providers are assessed by trained registration officers and manually entered or (2) data from electronic sources are loaded via a semiautomatic process known as the data waterfall (figure 2). The data waterfall is a process which loads data from electronic sources. Its purpose is to perform basic validations on the data, confirm the patient’s demographic information (via NHS Spine), match the patient to an existing patient (or create a new patient record) and create records, such as a screening or diagnostic test event which can be processed by registry staff. Most cases consist of information processed by both manual and automatic methods.
Anomalies are registered according to varying degrees of certainty depending on the clinical evidence available; confirmed, probable or suspected. Anomalies remain at the level of suspected until the evidence supporting the diagnosis of the anomaly attains agreed confirmation criteria. These criteria have been established with clinical input and consider the method by which the diagnosis is made, the specialism and confidence of the reporting clinician, the gestational or postnatal age at which the anomaly was identified, and the reliability of data sources. Criteria are different for each type of anomaly and a baby can have multiple anomalies, each with different statuses, depending on the level of evidence available for each one. Not all anomalies can be confirmed by the gold standard diagnostic test. Where there is a confident diagnosis by a relevant specialist in the field and the evidence is well-described, a probable confirmation status is used. Only data on probable and confirmed cases are used for routine reporting and analysis purposes and both are considered reportable.
Data structure
Registration is framed at the level of the anomaly, baby, pregnancy and birth mother. The data are organised into over 600 raw tables which are in turn further organised into a series of approximately 100 custom-built analytical views, tables and lookup tables reflecting five main thematic groups, mother, pregnancy, baby, anomaly and test, with one-to-many relationships across all. Each table contains a primary key that uniquely identifies records within that table and allows joins between tables. A baby and a mother are each assigned a unique identifier. Registration records are never closed and new events can be added if new information is submitted.
Key data fields
Detailed clinical and demographic information on the mother, baby, anomaly and pregnancy is recorded (table 1). Multiple anomalies can be registered against a baby, each with different evidence and confirmation status.
Table 1.
Summary of key data items currently available for each congenital anomaly registration in the NCARDRS congenital anomaly dataset
| Mother | Pregnancy | Baby | Anomaly | Test*‡ |
| Patient identifier | Pregnancy identifier | Patient identifier | Anomaly identifier | Test date |
| NHS no | Expected delivery date | NHS no. | Confirmation status of anomaly† | Test type |
| Date of birth | Pregnancy outcome | Sex | ICD-10 and BPA extension code | Test result(s) |
| Ethnicity | Delivery information | Date of birth | Description of the anomaly | Test provider |
| Country of birth | Screening details‡ | Gestational length at delivery | Gestation first suspected | Test requestor |
| Vital status | Body mass index | Birth weight (g) | Gestation at confirmation | Ultrasound markers |
| Previous births and pregnancies | Smoking status at booking, alcohol and substance use | Birth order if from a multiple pregnancy | Diagnostic method | Indication |
| Maternal illness status | Method of delivery | Aetiology of the anomaly/ies | ||
| Folic acid intake | Surgical status | |||
| Assisted conception status | Date of death | |||
| No. of fetuses | Postmortem status | |||
| Consanguinity | ||||
| Deprivation (derived from postcode of residence at delivery) | ||||
| Postcode at booking and at delivery |
Data are available for all years.
*Test information is only consistently registered for conditions with enhanced registration (ie, those conditions within the FASP audit).
†Some fields may require additional research ethics committee or other approvals on request.
‡Confirmation status: Anomalies are registered according to varying degrees of certainty depending on the clinical evidence available (confirmed, probable or suspected). Anomalies remain at the level of suspected until the evidence supporting the diagnosis of the anomaly attains agreed confirmation criteria.
BPA, British Paediatric Association; FASP, International Classification of Diseases 10th revision; ICD-10, International Classification of Diseases 10th revision; NCARDRS, National Congenital Anomaly and Rare Disease Registration Service; NHS, International Classification of Diseases 10th revision.
NCARDRS works closely with the NHS Fetal Anomaly Screening Programme (FASP) to audit the detection of 15 conditions groups and these conditions are subject to enhanced registration. To facilitate this audit, more extensive information on antenatal screening and the nature and timing of diagnostic testing is collected for these conditions and other closely-related or similar conditions. The conditions that are covered by this enhanced registration include severe cardiac anomalies, trisomy 13, trisomy 18 and trisomy 21, neural tube defects, severe skeletal dysplasia, cleft lip+/palate, bilateral renal agenesis, abdominal wall anomalies and congenital diaphragmatic hernia (see online supplemental table S1). These 15 condition groups reflected 35% (n=4501) of the 13 065 babies registered with a confirmed or probable congenital anomaly in 2020.
Data quality
Automatic and manual quality checks are embedded into the registration process at points of entry, at the level of the individual record and on the birth cohort as a whole prior to finalisation of the data for reporting. As well as internal data quality indicators (DQIs), the data are evaluated against DQIs for international bodies against known targets.16 For example, the prevalence of anencephaly is reported as an indicator of ascertainment of conditions detected at earlier gestations. Other DQIs focus on the accuracy of diagnosis, for example, the number of babies with more than one anomaly, or the prevalence of selected codes that have used the BPA extension code in addition to the ICD-10 code.
Patient and public involvement
Patient groups and third sector organisations representing patients were involved in the design of this register-based cohort and were members of an expert committee of stakeholders made up of academics, clinicians, third sector organisation and patient interest groups that oversaw the formation of NCARDRS.
Ethical approval and governance arrangements
NCARDRS has legal permission to collect patient-level data on those with a confirmed or suspected congenital anomaly or rare disease for specified purposes, without consent, to use it to protect the health of the population. Data are collected under legal instructions known as Directions, from the Secretary of State for Health and Social Care, made in accordance with section 254 of the Health and Social Care Act 2012 (2012 Act).17 Strict technical and contractual controls are put in place to prevent unauthorised access and use of the data, with staff undergoing regular training on data protection and information governance.
Findings to date
As of June 2023, NCARDRS held information on 117 682 mothers and 121 184 babies born in England since 2015. Table 2 shows the number of babies and other characteristics registered in regions with full congenital anomaly registration coverage by year of birth.
Table 2.
The number of pregnancies, babies and anomalies recorded registered since NCARDRS has been in operation until 2020 data extracted 13 June 2023
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| No. of regions reporting | 4 | 7 | 7 | 10 | 10 | 10 |
| No. of pregnancies with babies with a congenital anomaly of any status | 2915 | 9524 | 9882 | 20 036 | 19 636 | 18 440 |
| No. of babies with a congenital anomaly of any status | 2932 | 9574 | 9937 | 20 145 | 19 767 | 18 541 |
| No. of mothers | 2908 | 9506 | 9868 | 20 007 | 19 611 | 18 416 |
| Total no. of anomalies | 5902 | 18 839 | 18 803 | 35 483 | 34 500 | 33 344 |
| Total no. of confirmed and probable anomalies | 5432 | 15 819 | 15 316 | 28 282 | 25 988 | 25 617 |
| No. of live and still births in regions with active congenital anomaly registration (denominator) | 141 474 | 329 301 | 320 013 | 628 171 | 614 952 | 589 454 |
The numbers may differ from published estimates at point of reporting because of continued accumulation of data.
Birth population calculated using ONS row level birth information available via the UK Health Security Agency (UKHSA) DataLake.
NCARDRS, National Congenital Anomaly and Rare Disease Registration Service; ONS, Office for National Statistics.
NCARDRS currently collects data on more than 1000 different congenital anomalies, many of which are rare diseases, and provides expert analysis and interpretation of the data across a wide range of national and international functions. The data are available as a source of intelligence for clinicians, public health, healthcare performance, basic and applied research, patient groups, academics and commissioning and industry partners. A summary of the data is published each year describing congenital anomalies in England in the context of prevalence reported by anomaly group, timing of diagnosis and important public health indicators such as maternal age and infant mortality.10 13 18–21 In 2020, NCARDRS reported a total of 13 065 babies with one or more confirmed or probable congenital anomalies in 589 454 total births (live births and stillbirths), giving an overall birth prevalence of 221.7 per 10 000 total births (95% CI 217.9 to 225.5) or 1 for every 45 births.13 The rate of perinatal mortality associated with a congenital anomaly was highest for genetic disorders (3.1 per 10 000 total births, 95% CI 2.7 to 3.6), followed by congenital heart anomalies (2.8 per 10 000 total births, 95% CI 2.4 to 3.2). Infant mortality rate was highest for congenital heart anomalies (4.9 per 10 000 live births, 95% CI 4.4 to 5.5), followed by genetic conditions (3.0 per 10 000 live births, 95% CI 2.6 to 3.5). The rate of genetic conditions in babies born to women over 40 years old was almost seven times higher relative to babies born to mothers under 20 years old (risk ratio equal to 6.9, 95% CI 5.2 to 9.2).
Congenital anomaly registration data for England is submitted to international bodies to allow pooling of data across a wider geographical area to support analysis into causes of these rare conditions and how to prevent them. Data are submitted annually to EUROCAT and to the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Surveillance is performed annually using internationally recognised tools to identify potential clusters of anomalies and changes in trends.22
NCARDRS works closely with NHS Screening Programmes delivering service evaluation for antenatal and newborn screening services. NCARDRS audits the detection of the conditions included in the FASP (see online supplemental table S1) and, to enable this, these conditions are subject to enhanced registration and active ascertainment. By linking information at patient level, NCARDRS creates a longitudinal record of the screening and diagnostic pathway for each mother, fetus or baby, enabling analysis of the efficacy of the tests, the behavioural choices on the pathway and the operational standards of the service. NCARDRS has recently published the first national study of fetal anomaly ultrasound scan detection rates in England.23 The data are used to provide reliable information about the quality of screening services at local, regional and national level and contributes towards the safety and effectiveness of screening services. Each screening provider receives a report detailing hospital-level detection rates and also individual case-level detection status to allow further clinical audit and identify training requirements. NCARDRS is supporting the NHS evaluative roll-out of non-invasive prenatal testing (NIPT) for Edwards’ syndrome, Patau’s syndrome and Down’s syndrome in England.24 Routine laboratory surveillance is conducted on a monthly, quarterly and annual level, and NIPT performance will be evaluated by linking laboratory and registration data.
At the start of the COVID-19 pandemic, NCARDRS informed the production of the Shielded Patient List (SPL)25 by identifying individuals living with congenital anomalies and selected rare diseases that may have been at increased risk from COVID-19 infection and NCARDRS will support the continued evaluation of vaccines against COVID-19 in pregnancy.26
Many publications use datasets that predate NCARDRS including the legacy regional registers. NCARDRS data have been used to examine the epidemiology of congenital anomalies across Europe including Dandy-Walker syndrome,27 VACTERL association,28 29 neural tube defects,30 aplasia cutis31 achondroplasia32 and vascular disruption anomalies.33 Studies aim to improve outcomes of babies with a congenital anomaly and to inform policy so that some may be prevented, for example, to justify the fortification of flour with folic acid. Recently, the UK government announced plans for the fortification of flour with folic acid to reduce neural tube defects.34 NCARDRS will support the evaluation and monitoring of the impact of implementation of this policy.35
Strengths and limitations
National coverage
The key strength of the NCARDRS data set is its national coverage across a large birth population. NCARDRS is the largest register in Europe36 in both size of population and representativeness. With complete population coverage of pregnancies from 2018 onwards, the data are representative and comprehensive, capitalising on the centralised nature of English healthcare.
Multisource ascertainment
An NCARDRS congenital anomaly record can be made up of multiple difference sources, some automatically added and manually verified. Clinical information, often obtained by the treating clinician, is combined with cytogenetic laboratory data, data from routinely collected hospital activity, national statistics, and extracts from clinical systems to make a cohesive and comprehensive record detailing the phenotype. A registration record is never closed, allowing for the possibility of adding further genomic data as it becomes available with the wider use of whole genome sequencing. Data can continue to passively accumulate, enriching each record and facilitating the potential identification of future syndromes or providing more information on the phenotypic manifestations of genetic differences identified later in life.
Standardised disease coding and data entry
The development of NCARDRS has demonstrated that it is possible to conduct national registration on a large population using standardised approaches to data collection and management, disease coding, data classification, analysis and reporting. Data are coded consistently across the country and regions can be compared, allowing the identification of clusters and geographical disparities which may be a result of population demographics, social determinants of health or local exposure.
Ascertainment
National prevalence in England for 2018–2020 is consistent with European surveillance data for the same time period (excluding data for England) across most major congenital anomaly groups (figure 3). The prevalence of severe anomalies, such as severe congenital heart, abdominal wall, oro-facial cleft, respiratory and genetic conditions was higher than the European average. These anomaly subgroups include FASP-conditions which are subject to enhanced registration. Their higher prevalence reflects the integration of clinical audit in NCARDRS and demonstrates the impact of clinical engagement on data quality and ascertainment. The England national prevalence estimates for all cardiac conditions, limb anomalies and congenital anomalies of the kidney and urinary tract conditions are lower than the average for European registers. This likely reflects some underascertainment of anomalies that are predominantly confirmed postnatally in regions of NCARDRS new to reporting.10
Figure 3.
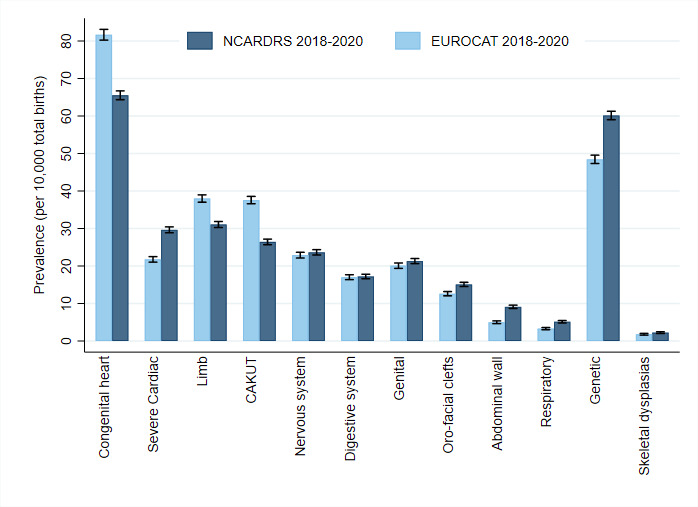
The prevalence of anomaly groups in England compared with EUROCAT registers excluding English registers (2018–2020) (data downloaded on 24 April 2023 from EUROCAT, first August 2022 NCARDRS). CAKUT, congenital anomalies of the kidney and urinary tract; EUROCAT, epidemiological surveillance of congenital anomalies; NCARDRS, National Congenital Anomaly and Rare Disease Registration Service.
Within England, there is some regional variation in the prevalence of different anomaly groups. This reflects developing ascertainment in regions new to congenital anomaly registration particularly for anomalies that are more frequently identified postnatally or for those anomalies that are less severe.10 21 As registration becomes embedded and ascertainment increases, differences in prevalence because of data collection should dissipate, revealing true regional differences, if they exist.
Risk factor information
Information on the demographics of the mother is collected for each pregnancy and include ethnicity, body mass index, illnesses or medications, folic acid intake and other lifestyle factors such as smoking. This information can be supplemented using data linkage to examine other factors, including social deprivation measured at the area level through deprivation scores for mother’s residential address at delivery.
Timeliness of data collection
Babies with a congenital anomaly are first registered by NCARDRS approximately 12 months after their expected date of delivery. This time lag allows for the notification of outcome of the pregnancy and a confirmatory diagnosis after delivery, along with the notification of other relevant postnatal information and follow-up if required. Finalised delivery-year cohorts are available approximately 18 months to 2 years following the end of a delivery year, for example, babies delivered in 2021 will be reported in early 2024. Recent advances in the automated processing of defined data feeds (eg, fetal medicine software system extracts) aim to improve the timeliness of data by reducing the time lag.
Future work
Planned improvements to the timeliness of data reporting and continued improvement to developing ascertainment for new regions and completeness of fields will further improve data quality. Proximity to the more established cancer registration service allows the register to build on synergies in data management, analytical infrastructure and data liaison.
As the service matures, new data sources will be added to improve data quality or ascertainment. Transition to NHS England has situated NCARDRS closer to clinical providers and commissioning services which should improve data access and facilitate linkage to a wider network of data. Linkage with the Maternity Services Dataset, a routinely collected national dataset describing maternity care in England,37 is underway and this aims to improve the completion of risk factor data items such as smoking, alcohol use as well as providing access to further information about the pregnancy. The inclusion of primary care data would be an obvious improvement to the ascertainment of postnatally diagnosed conditions, as would NHS England commissioned Highly Specialised Services data, and clinical audit data such as that provided by the Paediatric Intensive Care Audit (PICANET), the National Institute for Cardiovascular Outcomes Research (NICOR) and the Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRACE-UK).
Linkage to other datasets provides vital information on survival and health outcomes for these children throughout their life course. Cancer registration data collected by NDRS has been linked to the community prescriptions dataset38 and linkage with the congenital anomaly registration data is in progress. This could provide information on possible drug interactions and potential teratogens. Linkage to other disease registers, subject to adequate consenting materials and approvals, is possible and could provide valuable information on health outcomes for children with congenital anomalies. NCARDRS currently contains data that only relate to the health outcomes of the child and further work should also include linkage to social and educational data.
Conclusions
If the thalidomide scandal of the 1960s prompted the establishment of congenital anomaly registration to understand the causes of congenital anomalies, the COVID-19 pandemic amplified the need to be able to identify and protect individuals living with conditions that may put them at increased risk compared with the general population. NCARDRS’ congenital anomaly register collates information across the full patient pathway as the pregnancy progresses and the child grows. The value of this dataset in supporting clinical audits and evaluating service delivery is proven. This population-based national register—currently the largest data collection of its kind globally—has a critical role in supporting the epidemiology and monitoring of disease trends, investigating the causes of these conditions, evaluating the outcome and providing this crucial information to parents, patients, clinicians and service commissioners, so these children have what they need as they grow.
Supplementary Material
Acknowledgments
Thanks to the registration teams and associated functions in NDRS who register the data and the notifiers across the NHS and other healthcare organisations who send NDRS data. Thanks to Bhavisha Hirani for support with Figure 1. This work uses data that has been provided by patients, the NHS and other health care organisations as part of patient care and support. The data are collated, maintained and quality assured by the National Disease Registration Service, which is part of NHS England.
Footnotes
Collaborators: NDRS supports collaborations with academic and other institutions to use the data for a justified purpose. Enquiries, requests for statistical code used and requests for anonymised data should be directed to ndrs.enquiries@nhs.net.
Contributors: JMB led the drafting of the manuscript with advice from KMF, JB and SStevens as to the concept, structure and content. Additional sections were drafted by BW, DG, CJ, SStoianova, NA and KR. BW drafted the supplemental material. KR, CJ, BW, DG and SStoianova provided advice on registration and/or data management system practice. Data analysis was performed by JMB, DM, GM and EO. All authors reviewed and commented on the manuscript and gave approval for publication. JMB is the guarantor and accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but data are collected and analysed under the National Disease Registries Directions 2021, made in accordance with sections 254(1) and 254(6) of the 2012 Health and Social Care Act, meaning that individual informed consent is not required.
References
- 1.Boyle B, Addor M-C, Arriola L, et al. Estimating global burden of disease due to congenital anomaly: an analysis of European data. Arch Dis Child Fetal Neonatal Ed 2018;103:F22–8. 10.1136/archdischild-2016-311845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office for National Statistics . Child and infant mortality in England and Wales: 2020; 2022.
- 3.World Health Organisation . Congenital disorders. 2023. Available: https://www.who.int/health-topics/congenital-anomalies#tab=tab_3
- 4.Dolk H. EUROCAT: 25 years of European surveillance of congenital anomalies. Arch Dis Child Fetal Neonatal Ed 2005;90:F355–8. 10.1136/adc.2004.062810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henson KE, Elliss-Brookes L, Coupland VH, et al. Data resource profile: national cancer registration dataset in England. Int J Epidemiol 2020;49:16–16h. 10.1093/ije/dyz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GOV.UK: Department for Health . The UK Strategy for Rare diseases: Health UDo. 2013: 40. [Google Scholar]
- 7.CMO . Annual report of the chief medical officer 2011: on the state of the public’s health; 2011.
- 8.Stevens S, Miller N, Rashbass J. Development and progress of the national congenital anomaly and rare disease registration service. Arch Dis Child 2018;103:215–7. 10.1136/archdischild-2017-312833 [DOI] [PubMed] [Google Scholar]
- 9.Boyd PA, Armstrong B, Dolk H, et al. Congenital anomaly surveillance in England—ascertainment deficiencies in the National system. BMJ 2005;330:27. 10.1136/bmj.38300.665301.3A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCARDRS . National congenital anomaly and rare disease registration service congenital anomaly Statistics report 2018; 2020, Report No.: PHE publications gateway number: GW-1445. UK government: public health England
- 11.EUROCAT . EUROCAT guide 1.4: instruction for the registration of congenital anomalies. University of Ulster; 2013. [Google Scholar]
- 12.EUROCAT . 2021. Available: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en
- 13.NHS Digital . NCARDRS. National Congenital Anomaly and Rare Disease Registration Service Congenital Anomaly Official Statistics Report 2020. 2022.
- 14.World Health Organization . ICD-10: International Statistical Classification of Diseases and Related Health Problems. Geneva: World Health Organization, 2010. [Google Scholar]
- 15.Herbert A, Wijlaars L, Zylbersztejn A, et al. Data resource profile: hospital episode Statistics admitted patient care (HES APC). Int J Epidemiol 2017;46:1093–1093i. 10.1093/ije/dyx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loane M, Dolk H, Garne E, et al. EUROCAT data quality indicators for population-based registries for congenital anomalies. Birth Defects Res A Clin Mol Teratol 2011;91 Suppl 1:S23–30. 10.1002/bdra.20779 [DOI] [PubMed] [Google Scholar]
- 17.DHSC . National Disease Registries Directions 2021. Department of Health and Social Care, London, UK, 2021. [Google Scholar]
- 18.NCARDRS . National Congenital Anomaly and Rare Disease Registration Service Congenital Anomaly Statistics Report 2015; 2017, Report no.: PHE publications gateway number: GW-1445. UK government: public health England
- 19.NCARDRS . National Congenital Anomaly and Rare Disease Registration Service Congenital Anomaly Statistics Report 2016; 2018, Report no.: PHE publications gateway number: GW-1445. UK government: public health England
- 20.NCARDRS . National Congenital Anomaly and Rare Disease Registration Service Congenital Anomaly Statistics Report 2017; 2019, Report no.: PHE publications gateway number: GW-1445. UK government: public health England
- 21.NCARDRS . National Congenital Anomaly and Rare Disease Registration Service Congenital Anomaly Statistics Report 2019; 2021, Report no.: PHE publications gateway number: GOV-9201. UK government: public health England
- 22.Loane M, Dolk H, Kelly A, et al. Paper 4: EUROCAT statistical monitoring: identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 2011;91(Suppl 1):S31–43. 10.1002/bdra.20778 [DOI] [PubMed] [Google Scholar]
- 23.Aldridge N, Pandya P, Rankin J, et al. Detection rates of a national fetal anomaly screening programme: a national cohort study. BJOG 2023;130:51–8. 10.1111/1471-0528.17287 [DOI] [PubMed] [Google Scholar]
- 24.NIPT, Public Health England . Screening for Down’s syndrome, Edwards’ syndrome and Patau’s syndrome; 2021.
- 25.NHS Digital . Shielded patient list 2020. 2023. Available: https://digital.nhs.uk/coronavirus/shielded-patient-list
- 26.UK Health Security Agency . Immunisation and vaccine-preventable diseases division. protocol for the National surveillance and safety analysis of Coronavirus (COVID-19) vaccination in pregnancy. Published 25 November 2021; 2021.
- 27.Santoro M, Coi A, Barišić I, et al. Epidemiology of Dandy-Walker malformation in Europe: a EUROCAT population-based Registry study. Neuroepidemiology 2019;53:169–79. 10.1159/000501238 [DOI] [PubMed] [Google Scholar]
- 28.van de Putte R, van Rooij IALM, Haanappel CP, et al. Maternal risk factors for the VACTERL Association: a EUROCAT case-control study. Birth Defects Res 2020;112:688–98. 10.1002/bdr2.1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Putte R, van Rooij IALM, Marcelis CLM, et al. Spectrum of congenital anomalies among VACTERL cases: a EUROCAT population-based study. Pediatr Res 2020;87:541–9. 10.1038/s41390-019-0561-y [DOI] [PubMed] [Google Scholar]
- 30.Morris JK, Addor M-C, Ballardini E, et al. Prevention of neural tube defects in Europe: a public health failure. Front Pediatr 2021;9:647038. 10.3389/fped.2021.647038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coi A, Barisic I, Garne E, et al. Epidemiology of Aplasia Cutis congenita: a population-based study in Europe. J Eur Acad Dermatol Venereol 2023;37:581–9. 10.1111/jdv.18690 [DOI] [PubMed] [Google Scholar]
- 32.Coi A, Santoro M, Garne E, et al. Epidemiology of achondroplasia: a population-based study in Europe. Am J Med Genet A 2019;179:1791–8. 10.1002/ajmg.a.61289 [DOI] [PubMed] [Google Scholar]
- 33.Morris JK, Wellesley D, Limb E, et al. Prevalence of vascular disruption anomalies and association with young maternal age: A EUROCAT study to compare the United Kingdom with other European countries. Birth Defects Res 2022;114:1417–26. 10.1002/bdr2.2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GOV.UK . Folic acid added to flour to prevent spinal conditions in babies. Press release; 2021.
- 35.Broughan JM, Martin D, Higgins T, et al. Prevalence of neural tube defects in England prior to the mandatory fortification of non-wholemeal wheat flour with folic acid: a population-based cohort study. Arch Dis Child 2023. 10.1136/archdischild-2023-325856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EUROCAT . Coverage of the European live births, birth year 2015, by EUROCAT full or associate member registries (January 2020). 2020.
- 37.NHS Digital . MSDS, maternity services dataset; Available: https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-sets/maternity-services-data-set
- 38.Henson KE, Brock R, Shand B, et al. Cohort profile: prescriptions dispensed in the community linked to the national cancer registry in England. BMJ Open 2018;8:e020980. 10.1136/bmjopen-2017-020980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077743supp001.pdf (117.4KB, pdf)