Abstract
Introduction
Postoperative pancreatic fistula (POPF) remains one of the most severe complications of laparoscopic pancreaticoduodenectomy (LPD). Theoretically, transecting the pancreatic neck more distally has both advantages (more blood supply, and more central pancreatic duct) and disadvantages (maybe smaller the pancreatic duct) in preventing POPF. This theoretical contradiction pushed us to organise this trial to explore the impact of the level of pancreatic transection in clinical practice. We conduct this randomised trial with the hypothesis that extended pancreatic neck transection has superiority to conventional pancreatic neck transection.
Methods and analysis
The LPDEXCEPT (Extended pancreatic neck transection versus conventional pancreatic neck transection during laparoscopic pancreaticoduodenectomy) trial is a multicentre, randomised-controlled, open-label, superiority trial in 4 centres whose annual surgical volume for LPD is more than 25 cases with pancreatic surgeons who had completed their learning curve. A total of 154 patients who meet the inclusive and exclusive criteria are randomly allocated to the extended pancreatic neck transection group or conventional pancreatic neck transection group in a 1:1 ratio. The stratified randomised block design will be applied, with stratified factors are surgical centre and the diameter of the main pancreatic duct measured by preoperative CT scan (preMPD). The primary outcome is the incidence of the clinically relevant pancreatic fistula.
Ethics and dissemination
Ethics Committee on Biomedical Research of West China Hospital of Sichuan University has approved this trial in March 2023 (approval no. 2023-167). Results of this trial will be published in peer-reviewed journals and conference proceedings.
Trial registration number
Keywords: Pancreatic surgery, Pancreatic disease, Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study was designed as a multicentre, randomised, controlled, open-label, superiority trial with two parallel groups, and had been registered internationally.
The patients in the study group obtain extended pancreatic neck transection during laparoscopic pancreaticoduodenectomy, transecting the pancreatic neck at more than 5 mm and less than 10 mm beyond the left side of the portal vein. And the patients in the control group obtain conventional pancreatic neck transection, transecting the pancreatic neck above the mesenteric-portal axis.
This study applied stratified randomised block design, whose stratified factors are surgical centre and the diameter of the main pancreatic duct measured by the preoperative abdominal CT scan. This will balance possible bias among research centres and pancreatic features.
The main limitation is that this study is carried out by a large team of researchers, including surgeons, radiologists, pathologists, data collectors and statisticians. The coordination of this team is a big challenge.
LPDEXCEPT is an open-label trail; however, the primary and secondary outcomes are objective conditions which cannot be influenced by researchers.
Introduction
Pancreaticoduodenectomy is the standard procedure for patients with malignant or benign tumours of the pancreatic head, the lower common bile duct and the periampullary area of the duodenum. Since Gagner and his colleagues performed and introduced the first total laparoscopic pancreaticoduodenectomy (LPD) in 1994,1 LPD has become progressively acknowledged for its advantages such as less bleeding, less pain and faster recovery.2–4
Despite the advances in laparoscopic technology, postoperative pancreatic fistula (POPF) remains one of the most severe complications of LPD, which occurs in around 20% of patients.4 5 POPF is typically associated with secondary complications, such as post-pancreatectomy haemorrhage, intra-abdominal infection. These could lead to prolonged length of hospital stay, increased hospital cost and even death.6 7 Therefore, prevention of POPF has always been of high priority in pancreatic surgery.
Theoretically, the level of pancreatic transection can significantly affect the occurrence of POPF by influencing both the blood supply to the anastomosis and the location of the main pancreatic duct in the pancreatic transverse section, and maybe also the size of pancreatic duct. The head of the pancreas is supplied by the anterior and posterior pancreaticoduodenal arterial arcades which are formed by branches from the coeliac trunk and the superior mesenteric artery. The body and tail of the pancreas are supplied by branches from the splenic artery.8 And there is an intermediate zone lacking proper vascularisation in the neck of the pancreas, called ‘vascular watershed’.8 Therefore, the level of pancreatic neck transection might influence the pancreatic stump vascularisation. Strasberg et al have studied the impact of the defects of pancreatic stump vascularisation on POPF and showed there is a statistically significant correlation.9 10 The main pancreatic duct arises in the tail of the pancreas, and lies midway between the superior and inferior margins and slightly more posterior than anterior through the tail and body of the pancreas. Then, it turns caudad and posterior on reaching the head of the pancreas.8 Therefore, the level of pancreatic neck transection could influence the location of the main pancreatic duct in the pancreatic transverse section. Several studies had revealed the association between the location of the pancreatic duct and POPF.11 12 And they found the risk of POPF was reduced when the centre of pancreatic duct is far from the edge of pancreas. And the more distally the surgeon transect the pancreas, the smaller pancreatic duct he (or she) would get. As the small size of the pancreatic duct is the major risk factor for POPF, transecting the pancreatic neck more distally maybe has disadvantages in preventing POPF.
Bardol et al conducted a retrospective cohort study and consolidated that long remnant pancreatic neck could be an independent risk factor for POPF after pancreaticoduodenectomy.13 However, to date, there exists no randomised trial dedicated to answering whether patients could benefit from extended pancreatic neck transection. The above theoretical contradiction pushed us to organise a trial to explore the impact of the level of pancreatic transection in clinical practice. Thus, we conduct this multicentre randomised trial, LPDEXCEPT, with the hypothesis that extended pancreatic neck transection has superiority to conventional pancreatic neck transection.
Methods and analysis
We wrote this protocol in line with the Standard Protocol Item Recommendation for Interventional Trials 2013 guideline.14
Design
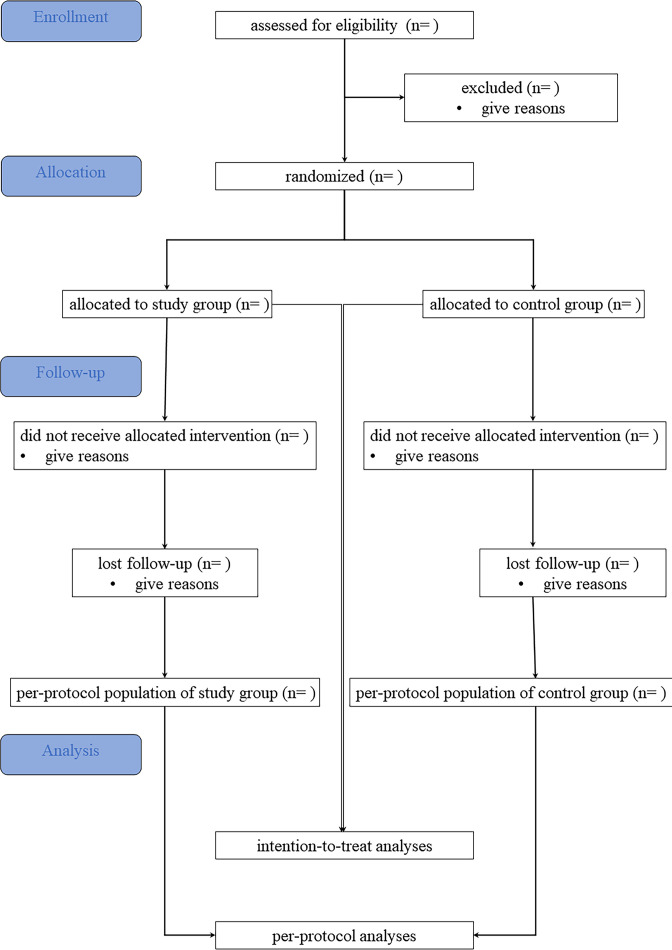
The LPDEXCEPT trial was designed as a multicentre, randomised, controlled, open-label, superiority trial with two parallel groups. The broad goal of this trial is to evaluate the superiority of extended pancreatic neck transection during LPD. The flow diagram for LPDEXCEPT was shown as figure 1.
Figure 1.
Flow diagram for LPDEXCEPT.
Patients and public involvement
Neither patients nor the public are involved in design, recruitment or conduct of this study.
Study population
All patients with an indication for elective LPD will be evaluated. The reasons for laparoscopic approach is the only choice for this trial but not open or robotic are as follows: there are many aspects that differ between open and minimally invasive (laparoscopic and robotic) pancreaticoduodenectomy, including some of the postoperative complications, duration of surgery, intraoperative bleeding, length of hospitalisation and so on.15–18 And it is still up for debate to choose the approach. Studies would inevitably introduce additional confounding factors once multiple approaches are included. The process of study design and study implementation would also become more complex to eliminate the bias introduced by these confounding factors. In order to control for these biases more simply and to obtain more accurate and trustworthy results, also because laparoscopic surgery is practised more in our research team, we chose only laparoscopic surgery for this trial. The inclusion and exclusion criteria for patients are as follows:
Participants inclusion criteria
Patients with benign or resectable malignant tumours of the lower common bile duct, Vater ampulla, head or uncinate process of the pancreas.
18 years old<age < 80 years old, no gender limit.
Patient is expected survival beyond 3 months.
No pregnancy or pregnancy plan within 3 months after surgery.
Nutrition risk score<3 according to the Nutritional Risk Screening for Inpatients 2002 standard score.19
No contraindication to surgery for anaesthetic evaluation.
The subjects voluntarily joined the study and signed an informed consent form, with good compliance and cooperation with follow-up.
Participants exclusion criteria
Patients with borderline resectable and unresectable malignancies according to the National Comprehensive Cancer Network and the General Office of National Health Commission clinical practice guidelines.20 21
Patients undergoing neoadjuvant chemotherapy or radiotherapy, because these patients routinely undergo open surgery in our research team.
Patients with tumours exceeding the level of the gastroduodenal artery as measured by preoperative radiography.
Intraoperative exploration reveals tumour adhesions with portal vein-superior mesenteric vein, requiring revascularisation and reconstruction.
Operation transfers to open.
Operation transfers to other procedure.
The duct-to-mucosa pancreaticojejunostomy is not performed due to the main pancreatic duct cannot be found intraoperatively.
Interventions
Study group: extended transection group
The patients in extended transection group obtain extended pancreatic neck transection during LPD. Surgeons will transect the pancreatic neck at more than 5 mm and less than 10 mm beyond the left side of the portal vein.
Control group: conventional transection group
The patients in conventional transection group obtain conventional pancreatic neck transection during LPD. Surgeons will transect the pancreatic neck above the mesenteric–portal axis.
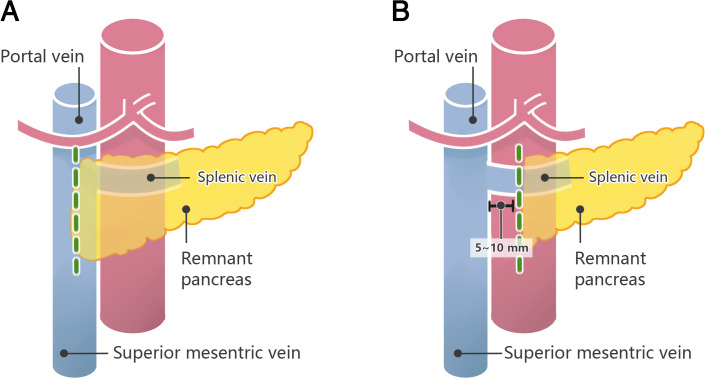
Figure 2 illustrates the level of the pancreatic neck transection of the two groups.
Figure 2.
The level of the pancreatic neck transection of the two groups. The green-dotted line illustrates the level of the pancreatic neck transection. (A) The level of the pancreatic neck transection of conventional transection group, in which surgeons will transect the pancreatic neck above the mesenteric–portal axis. (B) The level of the pancreatic neck transection of extended transection group, in which surgeons will transect the pancreatic neck at more than 5 mm and less than 10 mm beyond the left side of the mesenteric–portal axis.
Outcomes
Primary outcome measures
The primary outcome is the incidence of the CR-POPF according to the International Study Group of Pancreatic Surgery’s (ISGPS) definition and grading.22
Secondary outcome measures
The secondary objective of this trial is to compare the incidence of postoperative morbidity (Clavien-Dindo score≥3), the location of pancreatic duct, the surgical performance of pancreatojejunostomy and the short-term and long-term pancreatic endocrine and exocrine function between the two groups. Thus, the secondary outcomes include the location of the pancreatic duct in the pancreatic transverse section, the duration of pancreaticojejunostomy, postoperative morbidity, mortality within 3 months postoperatively, and the pancreatic endocrine and exocrine function of the participants at the third month postoperatively and at the first year postoperatively. The location of the pancreatic duct in the pancreatic transverse section will be measured by the way described as following: before performing the pancreaticojejunostomy, place the pancreatic transverse section in the central position of the lens. Measure the anterior–posterior diameter of the pancreas and the distance of the pancreatic duct from the back of the pancreas. The location of the pancreatic duct in the pancreatic transverse section is equal to the ratio of the distance of the pancreatic duct from the back of the pancreas to the anterior–posterior diameter of the pancreas. Postoperative morbidity will be classified according to the Clavien-Dindo score.23 For endocrine function, we detect diabetes mellitus development, and the diagnosis and classification of diabetes mellitus are according to the international criteria of diabetes.24 For pancreatic exocrine function, we defined pancreatic exocrine insufficiency as that stool evacuation was >3 times/day, and pasty or greasy stool was noted, associated with patient’s weight loss, and there was a need for enzyme supplementation resulting in recovery of bowel movements and cessation of steatorrhoea,25 considering that not all research centres can measure faecal elastase, N-benzol-L-tyrosyl-p-aminobenzoic acid, or faecal chymotrypsin.
Participating surgeons and hospital criteria
The trials will be conducted in tertiary care hospitals and academic hospitals. Participating hospitals must be high-volume medical centre whose annual surgical volume for LPD is more than 25 cases, according to the consensus on LPD.26 Participating surgeons must have completed their learning curve for LPD. We defined that a surgeon who had performed more than 104 cases of LPD is considered to have passed the learning curve, according to the study about practice patterns of LPD conducted by Wang et al.27
Surgical technique details
All study centres will perform the LPD using the optimisation of operative procedure. The specific operating procedures and details are reported in our previous articles.28 In this study, the surgical operation required attention to the following operational details: first, mark the level of the transection on the surface of the pancreas according to the group of participants before transecting the pancreatic neck, and after dissecting the upper and lower margins of the pancreas and revealing the superior mesenteric vein and portal vein. The level of transection in extended transection group is at more than 5 mm and less than 10 mm beyond the left side of the portal vein, while it in the conventional transection group is at the mesenteric–portal axis. Second, make sure not to pull on the pancreas and surrounding tissue, and make sure the pancreas is in situ when marking. Third, mark the pancreas from the superior margin to the inferior margin completely with an electrocoagulation hook, and transect the pancreas along the mark to prevent deviation.
Sample size
The sample size was determined based on the primary objective of comparing the incidence of CR-POPF between the two groups. According to the retrospective study,13 extended pancreatic neck transection (≥+7 mm) was associated with a lower incidence of CR-POPF than conventional pancreatic neck transection (15.4% vs 33.3%). Considering this study is a superiority trial, using the one-sided test with 80% power (1–β) at a significance level of 5% (α), the minimal sample size needed to detect a significant difference is calculated to be 70 patients in each group. Considering the loss of follow-up and washout, we enlarged the sample size by 10%. Then, there are 77 patients in each group, and the final sample size is 154 patients.
Participant timeline
The trial time schedule of enrolment is estimated to be a 3-year period, followed by a 1-year follow-up visit after discharge from the hospital. Once the eligibility of the patients is confirmed, randomisation will be applied. The intervention will be applied intraoperatively. The assessment and visits for patients will be mandatory in the first month, third month, and first year with either telephone or in-hospital follow-up. The participant timeline was shown in the table 1.
Table 1.
Participant timeline and data collection for LPDEXCEPT
| Study period | |||||||
| Time point Items |
Preoperative eligibility assessment | Allocation | Intraoperatively | Before discharge postoperatively | Follow-up | Close-out | |
| First month postoperatively | 1st month postoperatively | 3rd month postoperatively | 1st year postoperatively | ||||
| Patient demographics | ✓ | ||||||
| Informed consent | ✓ | ||||||
| Blood routine | ✓ | ✓ | |||||
| Coagulation routine | ✓ | ||||||
| Blood biochemistry | ✓ | ✓ | |||||
| Enhanced CT scan | ✓ | ✓ | |||||
| Allocation record | ✓ | ||||||
| Surgical videos | ✓ | ||||||
| Surgical record | ✓ | ||||||
| Postoperative records | ✓ | ✓ | ✓ | ✓ | |||
| Histopathological findings | ✓ | ✓ | |||||
| Other therapy (if necessary) | ✓ | ✓ | ✓ | ✓ | |||
Patient demographics includes date of admission, year of birth, sex, body mass index, previous surgical history, preoperative biliary drainage, Nutrition risk score, WHO-ECOG score, location of the tumour, diameter of the tumour, diameter of the main pancreatic duct, history of neoadjuvant therapy, and pancreatic thickness. Surgical record includes date of operation, ASA scores, location of the pancreatic neck transection (extended or conventional pancreatic neck transection), pancreatic texture, diameter of the main pancreatic duct, duration of pancreaticojejunostomy anastomosis, duration of the operation, estimated blood loss, whether to convert to open surgery or other procedures, whether to preserve the pylorus, whether to resect and reconstruct the main veins and variation of vessels. Postoperative records include blood transfusion, date of soft solid diet, date of drain removal, date of nasogastric tube removal, drain and production amylase, date of discharge, type of complication, reoperation and Clavien-Dindo grade, cost for hospitalisation, and short-term and long-term pancreatic exocrine and endocrine function. Histopathological findings include location of the tumour, size of the tumour, histological type, surgical margin status and the T&N classification and American Joint Committee on Cancer staging for malignant tumours. Other therapy includes readmission, treatment for any surgical complications, adjuvant therapy for malignant tumours and the cost for readmission.
Recruitment
The recruiters in each centre will screen eligible patients through the outpatient department or inpatient department. The duration of the recruitment period is estimated to be a 36-month interval depending on each centre’s recruiting rate. No financial incentives will be provided to trial investigators or patients for enrolment in the recruitment period.
Randomisation and allocation
Stratified randomised block design with a block number of four will be applied. The stratified factors are surgical centre and the diameter of the main pancreatic duct measured by the preoperative abdominal CT scan (preMPD). Due to pancreatic duct diameter is the main risk factor for pancreatic fistula,29 we included pancreatic duct diameter as a stratification factor. Also, because of the differences in healthcare delivery and quality, the study centre was included as another stratification factor, which could allow extrapolability of the study results to other hospitals. According to classification made by the ISGPS,29 the patients will be stratified into preMPD≤3 mm and preMPD >3 mm. Although pancreatic texture is also another major risk factor for pancreatic fistula, this study did not set it as a stratified factor for the following reasons: first, although there have been a few studies that have attempted to use CT values to represent pancreatic texture, there is a lack of a more recognised method to accurately assess pancreatic texture preoperatively.30 31 Second, pancreatic texture is not only determined by the type of pathologic diagnosis, but is also influenced by the site of the tumour, size of the tumour and so on. Besides, it is also difficult to accurately determine the pathologic diagnosis preoperatively, especially to differentiate the CT manifestations of chronic mass pancreatitis and pancreatic adenocarcinoma, both of whose pancreatic texture is firm. Third, too many stratification factors can add to the difficulties in the implementation of the study. Thus, we did not consider the pancreatic texture or pathological diagnosis as the stratification factor.
A data manager generated the randomisation lists by computer system. The randomisation lists will not be available to surgeons, recruiters and data collectors. And the randomisation lists will be embedded in a password-protected mobile application which was created to collect and manage data by our study team. The randomisation will be centralised through the mobile application. Allocation of each patient will be announced to the surgeon by the mobile application only after the assessment of baseline information of the patients and the upload of the signed informed consent.
Blinding
The patients, surgeons, data collectors, outcome assessors and data analysts are unblinded. The primary outcome of this study is the incidence of CR-POPF. The definition and the criteria of CR-POPF are objective condition and would not be influenced by the patients and surgeons even if they are unblinded. And the data collectors, outcomes assessors and the data analysts are not involved in perioperative management of the patients. Thus, they have no determination of the CR-POPF.
Data collection and management
Baseline characteristics will be recorded before randomisation. Intraoperative information, histopathological information, primary outcome and secondary outcomes will be collected after randomisation from hospitalisation up to 1 year postoperatively. The detailed data list was shown in the table 1.
We have created a special mobile application to collect and manage study data. The mobile application and database are password protected. The investigators and data collectors are to be qualified to the access the mobile application and the database. Data collection will be completed in accordance with standard specification processes. The investigators and data collectors enter the original data into the mobile application.
Data monitoring
Data Monitoring Committee (DMC) has been established. It is not competing interests. Through the combination of our internet-based and instantaneous mobile application, the DMC will conduct data monitoring to ensure that the reported clinical study data are accurate, complete and verifiable from source documents throughout the whole trial.
An interim analysis is performed on the primary endpoint when 50% of patients have been randomised and have completed the 3 months follow-up. The interim analysis is performed by an independent statistician. The statistician will report to the Ethics Committee on Biomedical Research of West China Hospital of Sichuan University. The ethics committee decides on the continuation of the trial.
Harms
An adverse event will be defined as any untoward medical occurrence in a subject without regard to the possibility of a causal relationship. All adverse events will be collected and recorded in detail according to the Common Terminology Criteria for Adverse Events (V.4.0) after the subject has provided consent and enrolled in the study. And the data will be collected by the Ethics Committee on Biomedical Research of West China Hospital of Sichuan University and the ClinicalTrials.gov Protocol Registration and Results System.
Protocol amendments
Any modifications to the protocol which may impact on the conduct of the study, potential benefit of the patient or may affect patient safety, including changes of study objectives, study design, patient population, sample sizes, study procedures, or significant administrative aspects will require a formal amendment to the protocol. Such amendment will be agreed on by the Ethics Committee on Biomedical Research of West China Hospital of Sichuan University. And the health authorities will be notified in accordance with local regulations.
Auditing
Auditing will be performed per year, at 50% of the inclusions, and at the end of the study by the Ethics Committee on Biomedical Research of West China Hospital of Sichuan University. The auditing will be independent from investigators.
Confidentiality
All study-related information will be stored securely at the study site. All participant information will be stored in locked file cabinets in areas with limited access. All databases will be secured with the password-protected data collection system.
Access to data
All participating investigators will be able to access the data of the registry, perform statistical analysis, discuss the results, and write the scientific manuscripts. Project principal investigators will have direct access to their own site’s data sets, and will have access to other sites data by request. Data dispersed to project team members will be blinded of any identifying participant information.
Statistical methods
Statistical analysis will be performed using IBM SPSS statistics V.25.0 (SPSS) and the R programme V.4.2.1 (R Foundation for Statistical Computing Platform). For continuous variables following a normal distribution, results were reported as the mean±SD for the data; otherwise, the median with IQR was reported. Categorical variables were reported as frequency and percentage. The two-side p value<0.05 was considered statistically significant. The χ2 test or Fisher’s exact test will be used to compare the categorical data between the study group and the control group as appropriate. The independent sample t test will be used to compare the continuous variables following a normal distribution between the two groups. And the continuous non-normally distributed variables will be compared using the Mann-Whitney U test. A logistic regression analysis will be performed to investigate predictors of CR-POPF. All variables with a p value<0.1 in a univariable analysis are included in the multivariable logistic regression analysis.
Bias due to missing data will be investigated by comparing the baseline characteristics of participants with and without missing values. Analysis in all randomly assigned patients (intention-to-treat analysis) will be conducted as sensitivity analyses. In addition, multiple imputations will be used to impute missing data, and the imputed data will also be analysed as part of the sensitivity analyses. The primary and secondary outcomes will also be analysed in all eligible patients who began the protocol treatment (per-protocol population), excluding ineligible patients and those not receiving the allocated treatment from all randomly assigned patients.
Ethics and dissemination
The ethics approval of the trial has been obtained from the Ethics Committee on Biomedical Research of West China Hospital of Sichuan University in March 2023 (approval no. 2023-167). The ethics Committee of each participating centres had accepted the decision of ethical review of the Ethics Committee on Biomedical Research of West China Hospital of Sichuan University. The English and Chinese versions of the informed consent materials were shown in online supplemental appendix 1. Trained research surgeons will introduce the trial to patients who have the indication for LPD. Patients will then be able to have an informed discussion with the participating consultant. Research surgeons will obtain written consent from patients willing to participate in the trial before entering the study.
bmjopen-2023-078092supp001.pdf (681.2KB, pdf)
The result of this study will be reported according to the CONSORT2010 guidelines.32 Any study results will be published in peer-reviewed journals and conference proceedings. The results will be released to the participating physicians, referring physicians, patients and the general medical community.
Supplementary Material
Acknowledgments
We thank Dr. Xinrui Zhu for the support of this study registration.
Footnotes
Contributors: BP obtained funding for this study. YC proposed the conceptualisation. JY, JZ, XW and HC designed the study. HW calculated the sample size. BP, YC, YL, CY, LW and XZ performed the operations. JY and JZ drafted the manuscript. YC and BP contributed to critical revision of the manuscript and approved the final version of the manuscript. All authors have read and approved the final manuscript.
Funding: This study was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (2018HXFH015).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Gagner M, Pomp A. Laparoscopic Pylorus-preserving Pancreatoduodenectomy. Surg Endosc 1994;8:408–10. 10.1007/BF00642443 [DOI] [PubMed] [Google Scholar]
- 2.de Rooij T, Lu MZ, Steen MW, et al. Minimally invasive versus open Pancreatoduodenectomy: systematic review and meta-analysis of comparative cohort and Registry studies. Ann Surg 2016;264:257–67. 10.1097/SLA.0000000000001660 [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Wu X, Zhu F, et al. Systematic review and meta-analysis of minimally invasive versus open approach for Pancreaticoduodenectomy. Surg Endosc 2016;30:5173–84. 10.1007/s00464-016-4864-3 [DOI] [PubMed] [Google Scholar]
- 4.Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic versus open Pancreaticoduodenectomy: A systematic review and meta-analysis of randomized controlled trials. Ann Surg 2020;271:54–66. 10.1097/SLA.0000000000003309 [DOI] [PubMed] [Google Scholar]
- 5.Klompmaker S, van Hilst J, Wellner UF, et al. Outcomes after minimally-invasive versus open Pancreatoduodenectomy: A pan-European propensity score matched study. Ann Surg 2020;271:356–63. 10.1097/SLA.0000000000002850 [DOI] [PubMed] [Google Scholar]
- 6.Smits FJ, Verweij ME, Daamen LA, et al. Impact of complications after Pancreatoduodenectomy on mortality, organ failure, hospital stay, and readmission: analysis of a nationwide audit. Ann Surg 2022;275:e222–8. 10.1097/SLA.0000000000003835 [DOI] [PubMed] [Google Scholar]
- 7.Abbott DE, Tzeng CWD, McMillan MT, et al. Pancreas Fistula risk prediction: implications for hospital costs and payments. HPB (Oxford) 2017;19:140–6. 10.1016/j.hpb.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 8.Skandalakis LJ, Rowe JS, Gray SW, et al. Surgical Embryology and anatomy of the Pancreas. Surg Clin North Am 1993;73:661–97. 10.1016/s0039-6109(16)46080-9 [DOI] [PubMed] [Google Scholar]
- 9.Strasberg SM, McNevin MS. Results of a technique of Pancreaticojejunostomy that Optimizes blood supply to the Pancreas. J Am Coll Surg 1998;187:591–6. 10.1016/s1072-7515(98)00243-9 [DOI] [PubMed] [Google Scholar]
- 10.Strasberg SM, Drebin JA, Mokadam NA, et al. Prospective trial of a blood supply-based technique of Pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg 2002;194:746–58; 10.1016/s1072-7515(02)01202-4 [DOI] [PubMed] [Google Scholar]
- 11.El Nakeeb A, Salah T, Sultan A, et al. Pancreatic anastomotic leakage after Pancreaticoduodenectomy. risk factors, clinical predictors, and management (single center experience). World J Surg 2013;37:1405–18. 10.1007/s00268-013-1998-5 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Sakamoto Y, Nara S, et al. A preoperative predictive scoring system for postoperative Pancreatic Fistula after Pancreaticoduodenectomy. World J Surg 2011;35:2747–55. 10.1007/s00268-011-1253-x [DOI] [PubMed] [Google Scholar]
- 13.Bardol T, Delicque J, Hermida M, et al. Neck Transection level and postoperative Pancreatic Fistula after Pancreaticoduodenectomy: A retrospective cohort study of 195 patients. Int J Surg 2020;82:43–50. 10.1016/j.ijsu.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 14.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uijterwijk BA, Kasai M, Lemmers DHL, et al. The clinical implication of minimally invasive versus open Pancreatoduodenectomy for non-pancreatic periampullary cancer: a systematic review and individual patient data meta-analysis. Langenbecks Arch Surg 2023;408:311. 10.1007/s00423-023-03047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uijterwijk BA, Wei K, Kasai M, et al. Minimally invasive versus open Pancreatoduodenectomy for Pancreatic Ductal adenocarcinoma: individual patient data meta-analysis of randomized trials. Eur J Surg Oncol 2023;49:1351–61. 10.1016/j.ejso.2023.03.227 [DOI] [PubMed] [Google Scholar]
- 17.Sattari SA, Sattari AR, Makary MA, et al. Laparoscopic versus open Pancreatoduodenectomy in patients with Periampullary tumors [published online ahead of print]. Annals of Surgery 2023;277:742–55. 10.1097/SLA.0000000000005785 [DOI] [PubMed] [Google Scholar]
- 18.Pfister M, Muller MK, Probst P. Minimally invasive versus open Pancreatic surgery: meta-analysis of randomized clinical trials. BJS Open 2023;7:zrad087. 10.1093/bjsopen/zrad087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budzyński J, Tojek K, Czerniak B, et al. Scores of nutritional risk and parameters of nutritional status assessment as predictors of in-hospital mortality and Readmissions in the general hospital population. Clin Nutr 2016;35:1464–71. 10.1016/j.clnu.2016.03.025 [DOI] [PubMed] [Google Scholar]
- 20.Chiorean EG, Chiaro MD, Tempero MA, et al. Ampullary adenocarcinoma, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2023;21:753–82. 10.6004/jnccn.2023.0034 [DOI] [PubMed] [Google Scholar]
- 21.General office of national health Commission . Standard for diagnosis and treatment of Pancreatic cancer in 2022. J Clin Hepatol 2022;38:1006–15. [Google Scholar]
- 22.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International study group (ISGPS) definition and grading of postoperative Pancreatic Fistula: 11 years after. Surgery 2017;161:584–91. 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36:S67–74. 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beger HG, Poch B, Mayer B, et al. New onset of diabetes and Pancreatic Exocrine insufficiency after Pancreaticoduodenectomy for benign and malignant tumors: A systematic review and meta-analysis of long-term results. Ann Surg 2018;267:259–70. 10.1097/SLA.0000000000002422 [DOI] [PubMed] [Google Scholar]
- 26.Qin R, Kendrick ML, Wolfgang CL, et al. International expert consensus on Laparoscopic Pancreaticoduodenectomy. Hepatobiliary Surg Nutr 2020;9:464–83. 10.21037/hbsn-20-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Peng B, Liu J, et al. Practice patterns and perioperative outcomes of Laparoscopic Pancreaticoduodenectomy in China: A retrospective multicenter analysis of 1029 patients. Ann Surg 2021;273:145–53. 10.1097/SLA.0000000000003190 [DOI] [PubMed] [Google Scholar]
- 28.Li Y-B, Cai Y-Q, Wang X, et al. Optimization of operative procedure in total Laparoscopic Pancreaticoduodenectomy (with Video. Sichuan Da Xue Xue Bao Yi Xue Ban 2020;51:446–52. 10.12182/20200760108 [DOI] [PubMed] [Google Scholar]
- 29.Schuh F, Mihaljevic AL, Probst P, et al. A simple classification of Pancreatic duct size and texture predicts postoperative Pancreatic Fistula: A classification of the International study group of Pancreatic surgery. Ann Surg 2023;277:e597–608. 10.1097/SLA.0000000000004855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Gao F, Qi Y, et al. Computed tomography-adjusted Fistula risk score for predicting clinically relevant postoperative Pancreatic Fistula after Pancreatoduodenectomy: training and external validation of model upgrade. EBioMedicine 2020;62:103096. 10.1016/j.ebiom.2020.103096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapshyn H, Petruch N, Thomaschewski M, et al. A simple preoperative stratification tool predicting the risk of postoperative Pancreatic Fistula after Pancreatoduodenectomy. Pancreatology 2021;21:957–64. 10.1016/j.pan.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078092supp001.pdf (681.2KB, pdf)