Abstract
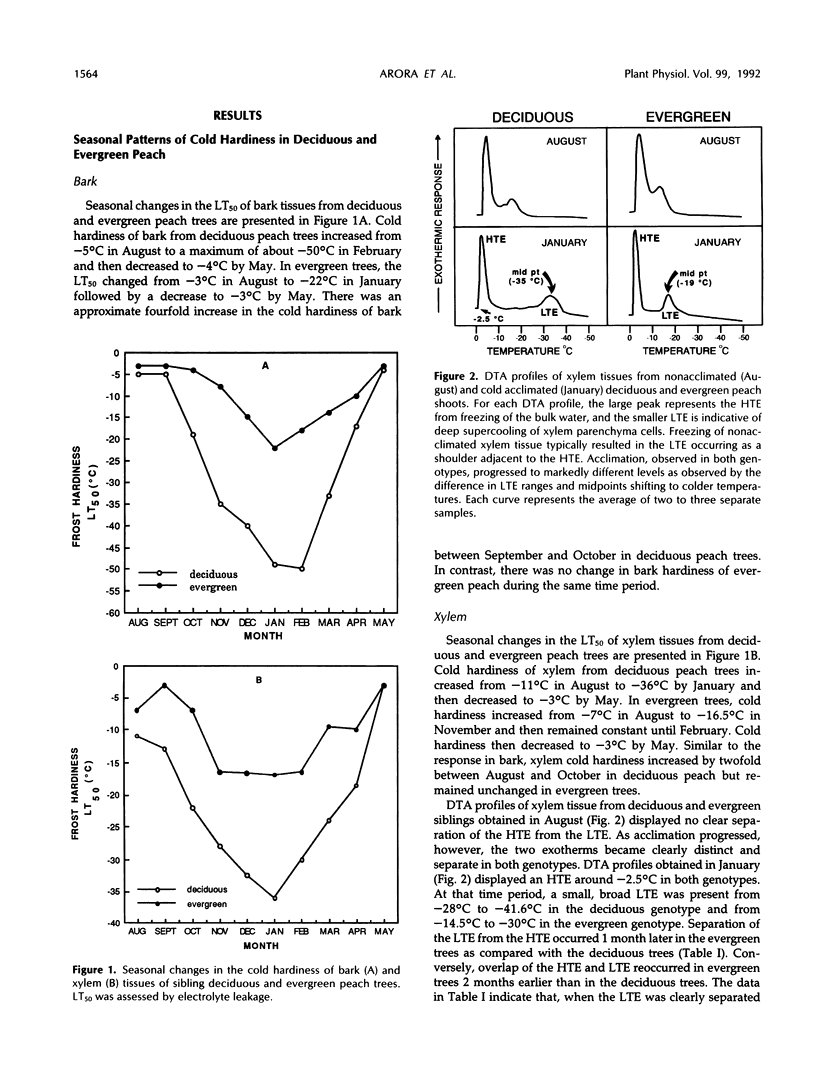
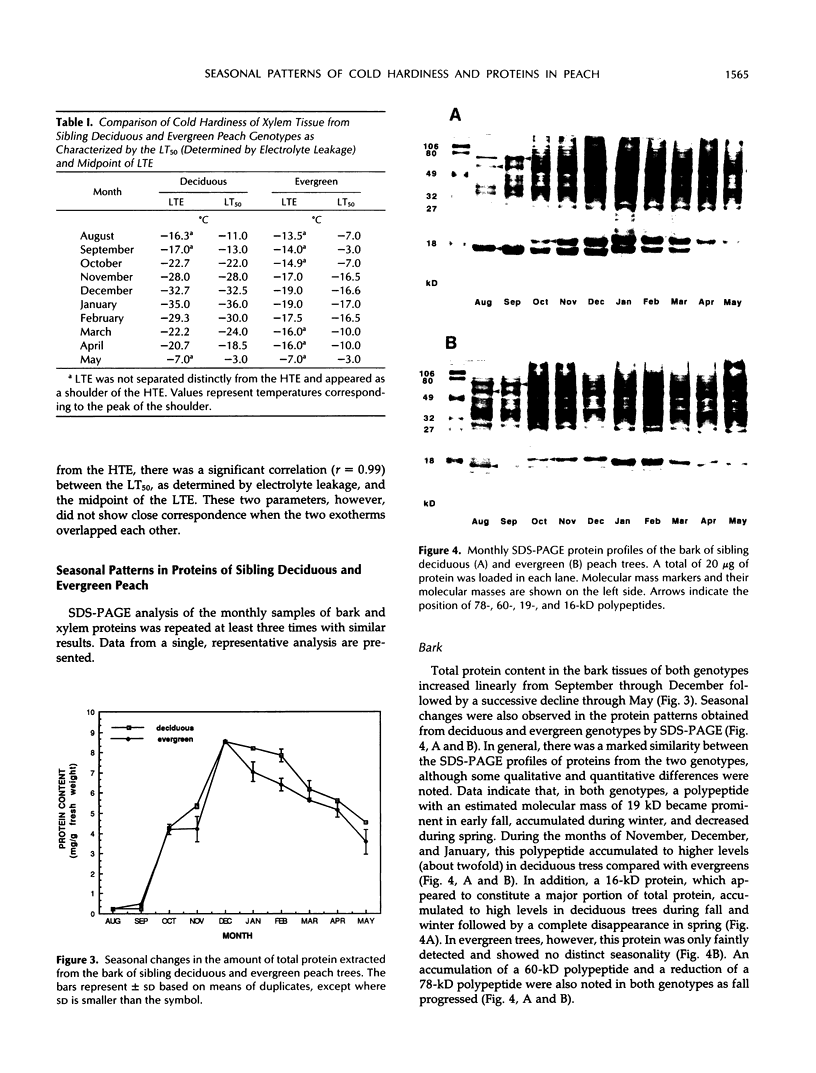
Seasonal patterns of proteins and of cold hardiness were characterized in bark and xylem tissues of genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch). In contrast with deciduous trees, which entered endodormancy and abscised leaves in the fall, evergreen trees retained their leaves and exhibited shoot elongation under favorable environmental conditions. A successive increase in the cold hardiness of bark and xylem was observed during the fall in both genotypes. This was followed by a subsequent decrease from midwinter to spring. Xylem tissue in both genotypes exhibited deep supercooling and a significant correlation (r = 0.99) between the midpoint of the low-temperature exotherm and the subzero temperature at which 50% injury occurred (assessed by electrolyte leakage) was noted. The maximum hardiness level attained in deciduous trees was more than twofold that of evergreens. Seasonal pattern of proteins from bark and xylem of the sibling genotypes was characterized by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Among other qualitative and quantitative changes, accumulation of a 19-kilodalton polypeptide in the bark of both genotypes was observed during fall followed by a decrease in spring. This polypeptide accumulated to higher levels in the deciduous peach compared with the evergreen. Additionally, a 16-kilodalton protein exhibited the same pattern in deciduous trees but not in the evergreen trees. Both the 19- and a 16-kilodalton bark proteins conform to the criteria of a bark storage protein. The relationship of seasonal changes in protein to cold hardiness and dormancy in these genetically related peach genotypes is discussed.
Full text
PDF

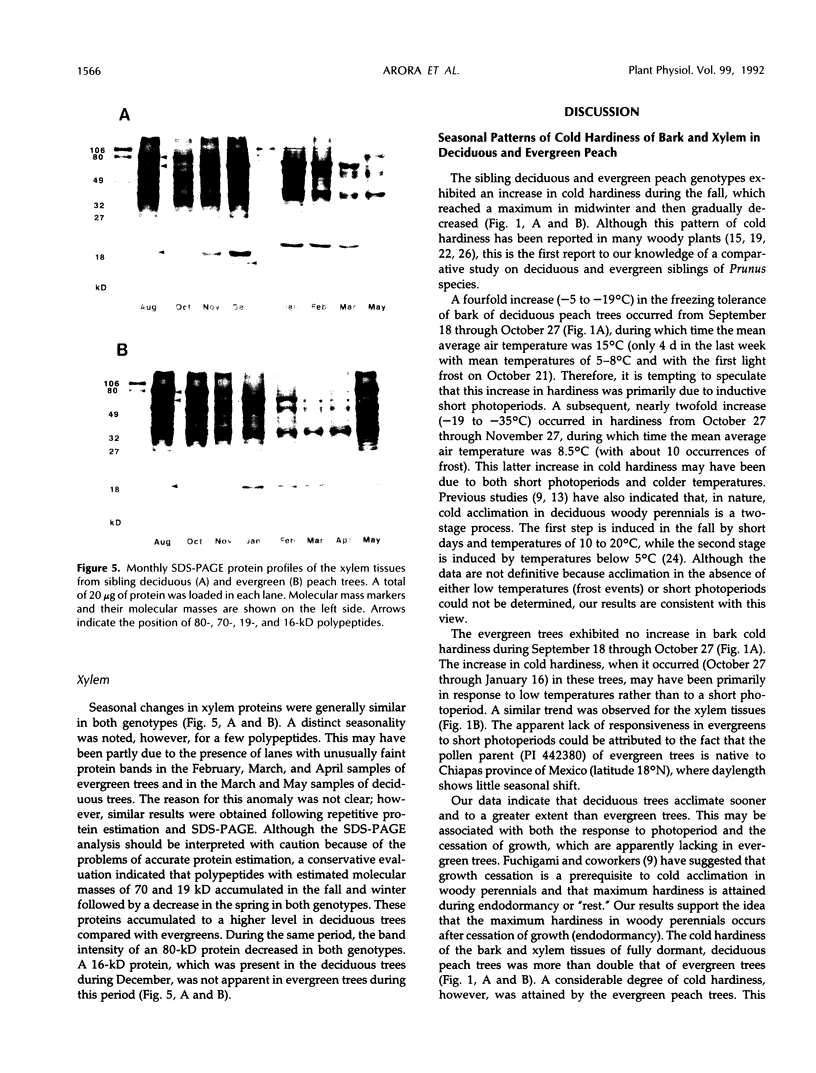


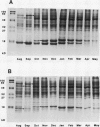
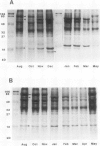
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bixby J. A., Brown G. N. Ribosomal Changes during Induction of Cold Hardiness in Black Locust Seedlings. Plant Physiol. 1975 Nov;56(5):617–621. doi: 10.1104/pp.56.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman G. D., Chen T. H., Ernst S. G., Fuchigami L. Photoperiod control of poplar bark storage protein accumulation. Plant Physiol. 1991 Jul;96(3):686–692. doi: 10.1104/pp.96.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchigami L. H., Weiser C. J., Evert D. R. Induction of Cold Acclimation in Cornus stolonifera Michx. Plant Physiol. 1971 Jan;47(1):98–103. doi: 10.1104/pp.47.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mac Irving R., Lanphear F. O. The long day leaf as a source of cold hardiness inhibitors. Plant Physiol. 1967 Oct;42(10):1384–1388. doi: 10.1104/pp.42.10.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D., Rheaume B., Pomeroy K., Lepage M. Phospholipid, protein, and nucleic acid increases in protoplasm and membrane structures associated with development of extreme freezing resistance in black locust tree cells. Cryobiology. 1968 Nov-Dec;5(3):202–225. doi: 10.1016/s0011-2240(68)80164-6. [DOI] [PubMed] [Google Scholar]