Abstract
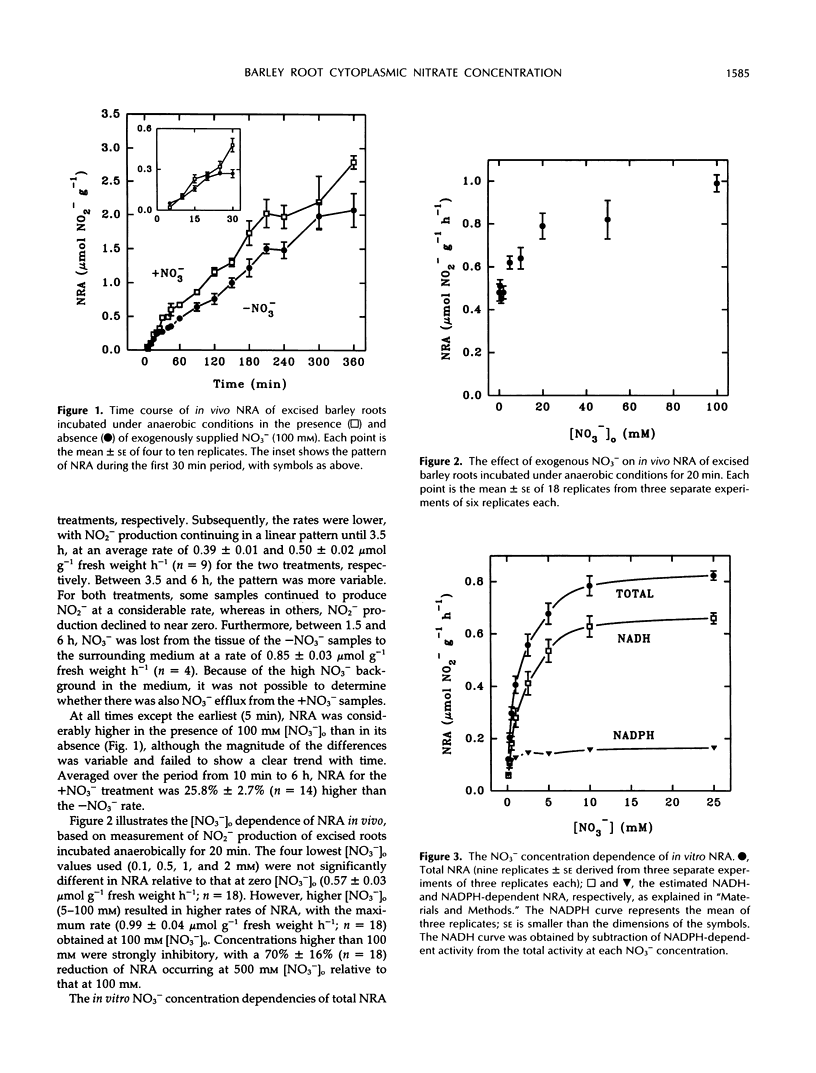
The cytoplasmic NO3− concentration ([NO3−]c) was estimated for roots of barley (Hordeum vulgare L. cv Klondike) using a technique based on measurement of in vivo nitrate reductase activity. At zero external NO3− concentration ([NO3−]o), [NO3−]c was estimated to be 0.66 mm for plants previously grown in 100 μm NO3−. It increased linearly with [NO3−]o between 2 and 20 mm, up to 3.9 mm at 20 mm [NO3−]o. The values obtained are much lower than previous estimates from compartmental analysis of barley roots. These observations support the suggestion (MY Siddiqi, ADM Glass, TJ Ruth [1991] J Exp Bot 42: 1455-1463) that the nitrate reductase-based technique and compartmental analysis determine [NO3−]c for two separate pools; an active, nitrate reductase-containing pool (possibly located in the epidermal cells) and a larger, slowly metabolized storage pool (possibly in the cortical cells), respectively. Given the values obtained for [NO3−]c and cell membrane potentials of −200 to −300 mV (ADM Glass, JE Schaff, LV Kochian [1992] Plant Physiol 99: 456-463), it is very unlikely that passive influx of NO3− is possible via the high-concentration, low-affinity transport system for NO3−. This conclusion is consistent with the suggestion by Glass et al. that this system is thermodynamically active and capable of transporting NO3− against its electrochemical potential gradient.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M. Reevaluation of anaerobic nitrite production as an index for the measurement of metabolic pool of nitrate. Plant Physiol. 1981 Aug;68(2):305–308. doi: 10.1104/pp.68.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti N., Hageman R. H. Comparison of in Vivo and in Vitro Assays of Nitrate Reductase in Wheat (Triticum aestivum L.) Seedlings. Plant Physiol. 1976 Oct;58(4):583–587. doi: 10.1104/pp.58.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey F. A., Warner R. L., Somers D. A., Kleinhofs A. Characteristics of a Nitrate Reductase in a Barley Mutant Deficient in NADH Nitrate Reductase. Plant Physiol. 1982 May;69(5):1200–1204. doi: 10.1104/pp.69.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane-Drummond C. E., Glass A. D. Nitrate Uptake into Barley (Hordeum vulgare) Plants : A New Approach Using ClO(3) as an Analog for NO(3). Plant Physiol. 1982 Jul;70(1):50–54. doi: 10.1104/pp.70.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Yoder O. C., Filner P. Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol. 1973 Mar;51(3):423–431. doi: 10.1104/pp.51.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D., Shaff J. E., Kochian L. V. Studies of the Uptake of Nitrate in Barley : IV. Electrophysiology. Plant Physiol. 1992 Jun;99(2):456–463. doi: 10.1104/pp.99.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D., Siddiqi M. Y., Ruth T. J., Rufty T. W. Studies of the Uptake of Nitrate in Barley : II. Energetics. Plant Physiol. 1990 Aug;93(4):1585–1589. doi: 10.1104/pp.93.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D. M., Oaks A. Stabilization of nitrate reductase in maize roots by chymostatin. Plant Physiol. 1990 Jul;93(3):846–850. doi: 10.1104/pp.93.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Higinbotham N. Transmembrane electropotential in barley roots as related to cell type, cell location, and cutting and aging effects. Plant Physiol. 1976 Feb;57(2):123–128. doi: 10.1104/pp.57.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Thomas J. F., Remmler J. L., Campbell W. H., Volk R. J. Intercellular localization of nitrate reductase in roots. Plant Physiol. 1986 Nov;82(3):675–680. doi: 10.1104/pp.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi M. Y., Glass A. D., Ruth T. J., Fernando M. Studies of the Regulation of Nitrate Influx by Barley Seedlings Using NO(3). Plant Physiol. 1989 Jul;90(3):806–813. doi: 10.1104/pp.90.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomino Y., Sakai H., Woodroffe A. J., Clarkson A. R. Studies on glomerular immune solubilization by complement in patients with IgA nephropathy. Acta Pathol Jpn. 1987 Nov;37(11):1763–1767. doi: 10.1111/j.1440-1827.1987.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Warner R. L., Huffaker R. C. Nitrate transport is independent of NADH and NAD(P)H nitrate reductases in barley seedlings. Plant Physiol. 1989;91:947–953. doi: 10.1104/pp.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]


