Abstract
Introduction
The peritoneum is the second most affected organ for the dissemination of colorectal cancer (CRC). Patients with colorectal peritoneal metastases (CPM) face a poor prognosis, despite the majority of patients being treated with palliative systemic therapy. The efficacy of palliative systemic therapy is limited due to the plasma-peritoneum barrier. The poor prognosis of unresectable CPM patients has resulted in the development of new treatment strategies where systemic therapy is combined with local, intraperitoneal chemotherapy. In the recently published phase I study, the maximum tolerated dose and thus the recommended phase II dose of intraperitoneal irinotecan was investigated and determined to be 75 mg. In the present study, the overall survival after treatment with 75 mg irinotecan with concomitant mFOLFOX4 and bevacizumab will be investigated.
Materials and methods
In this single-arm phase II study in two Dutch tertiary referral centres, 85 patients are enrolled. Eligibility criteria are an adequate performance status and organ function, histologically confirmed microsatellite stable and unresectable CPM, no previous palliative therapy for CRC, no systemic therapy<6 months for CRC prior to enrolment and no previous cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS and HIPEC). Patients will undergo a diagnostic laparoscopy as standard work-up for CPM and if the peritoneal disease is considered unresectable (eg, Peritoneal Cancer Index (PCI)>20, too extensive small bowel involvement), a peritoneal access port and a port-a-cath are placed for administration of intraperitoneal and intravenous chemotherapy, respectively. Patients may undergo up to 12 cycles of study treatment. Each cycle consists of intravenous mFOLFOX4 with bevacizumab and concomitant intraperitoneal irinotecan (75 mg), which is repeated every 2 weeks, with a maximum of 12 cycles. Modified FOLFOX-4 regimen consists of 85 mg/m2 oxaliplatin plus 200 mg/m2 LV and 5-FU 400 mg/m2 bolus on day 1 followed by 1600 mg/m2 5-FU as a 46 hours infusion. Study treatment ends after the 12th cycle, or earlier in case of disease progression or unacceptable toxicity. The primary outcome is overall survival and key secondary outcomes are progression-free survival, safety (measured by the amount of grade ≥3 adverse events (Common Terminology Criteria for Adverse Events V.5.0)), patient-reported outcomes and pharmacokinetics of irinotecan. It is hypothesised that the trial treatment will lead to a 4 month increase in overall survival; from a median of 12.2 to 16.2 months.
Ethics and dissemination
This study is approved by the Dutch Authority (CCMO, the Hague, the Netherlands), by a central medical ethics committee (MEC-U, Nieuwegein, the Netherlands) and by the institutional research boards of both research centres. Results will be submitted for publication in peer-reviewed medical journals and presented to patients and healthcare professionals.
Trial registration number
Keywords: chemotherapy, clinical trial, gastrointestinal tumours, colorectal surgery
STRENGTHS AND LIMITATIONS OF THIS STUDY.
First prospective phase II study assessing the survival, safety and feasibility of treatment of intraperitoneal irinotecan with concomitant FOLFOX and bevacizumab for patients with unresectable colorectal peritoneal metastases (CPM).
Assessment of multiple secondary outcomes such as patient-reported outcomes, costs and the pharmacokinetics of intraperitoneally administered irinotecan.
Translational research of the present study may provide fundamental insight in CPM.
The INTERACT-II study may be an important step towards a more effective, life-prolonging treatment modality for this specific patient group.
It is a non-randomised phase II study and therefore no comparison can be made to a control group.
Introduction
The peritoneum is the second most common metastatic site in colorectal cancer (CRC), affecting approximately 10% of patients.1 2 For a long time, the presence of colorectal peritoneal metastases (CPM) was considered to render the disease non-curable.3
The introduction of cytoreductive surgery and hyperthermic intraperitoneal (IP) chemotherapy (CRS and HIPEC) resulted in improved survival in selected patients with limited CPMs as compared with palliative systemic therapy.4 However, only a small portion of patients is eligible for CRS and HIPEC, as the majority of patients have too extensive CPM to benefit from CRS and HIPEC.5
The extent of peritoneal metastases is evaluated with the Peritoneal Cancer Index (PCI), which divides the abdomen in nine regions and the small bowel in four regions. Each region is given a score of 0–3 and the regions are summed up subsequently; a score of 0 reflects the absence of peritoneal metastases, while a maximum score of 39 indicates extensive disease in all regions.6 In general, CRS and HIPEC is not considered beneficial when the PCI exceeds 20 or when a macroscopic complete resection is not deemed feasible, for example in case of extensive small bowel involvement.7 The situation in which the patient has a PCI>20, or when complete resection is deemed unfeasible, is referred to as unresectable CPM.
Currently, patients with unresectable CPM receive palliative systemic therapy or best supportive care. The prognosis of these patients is dismal, with a median overall survival (OS) of 6–8 months with best supportive care and 10–14 months with palliative systemic therapy.5 The plasma-peritoneum barrier is suggested to reduce efficacy of systemic therapy in the treatment of CPM, as compared with patients with lung or liver metastases from a colorectal origin.8 The plasma-peritoneum barrier is a complex structure that regulates the IP homeostasis, thus hampering an effective transportation of the systemic therapy to the peritoneal metastases.9
By applying cytostatic therapies intraperitoneally, the traits of the plasma-peritoneum barrier can be used advantageously.9–13 Due to the limited absorption into the systemic circulation caused by the plasma-peritoneum barrier, higher IP drug concentrations and prolonged exposure of PM to those drugs can be achieved compared with systemic administration.13
The aforementioned CRS–HIPEC is based in part on these here described traits of the plasma-peritoneum barrier.10 In addition, different techniques, through which palliative chemotherapy can be applied, intraperitoneally exist. With pressurised IP aerosol chemotherapy (PIPAC), chemotherapy is administered as aerosol during repetitive laparoscopies, while the INTERACT I study investigated the IP administration of chemotherapy through an IP access port.14–16
In addition to various techniques for the IP application of chemotherapy, a variety of cytotoxic agents can be used.13 17 One of the chemotherapeutic groups that has been studied and that shows promise is the group of topoisomerase inhibitors.13
Irinotecan is a topoisomerase I inhibitor and was the chemotherapeutic agent that was studied in the INTERACT I study. Irinotecan is a prodrug and its main efficacy is attributed to its metabolite SN-38, which is 100–1000 fold more cytotoxic than irinotecan. The conversion to SN-38 takes place in both the liver and IP space.18–24 Several studies showed that the IP area under the curve of irinotecan and SN-38 was much higher after IP administration than after systemic administration. Additionally, the peritoneal clearance of intraperitoneally administered irinotecan was 10-fold lower than after systemic administration of irinotecan.21 25–28
IP chemotherapy, such as irinotecan, can either be applied as monotherapy, or in combination with systemic therapy. In both ovarian and gastric cancer, the addition of IP chemotherapy to systemic chemotherapy showed promising results.20 29–32 Moreover, in ovarian cancer, a beneficial effect was proven by a large randomised controlled trial.32 These findings, in combination with the promising results of the INTERACT I study, suggest that IP chemotherapy in addition to systemic therapy could be beneficial in patients with unresectable CPMs as well.33
The recent INTERACT study (NL63809.078.18) was a dose-escalation study and was performed to find the maximum tolerated dose (MTD) of IP irinotecan.16 In this study, 18 patients with unresectable CPMs were treated with first-line palliative systemic therapy with FOLFOX/bevacizumab and concomitant IP irinotecan at flat dose levels of 50 mg (n=4), 75 mg (n=9) and 100 mg (n=4). For the 50 mg and 75 mg dose cohorts, no dose-limiting toxicities were observed. After two dose-limiting toxicities at the 100 mg dose level, the MTD was thus established at 75 mg.
The INTERACT-II study is a multicentre, single-arm, phase II study, aimed to assess OS, progression-free survival (PFS), safety, patient-reported outcomes (PROs), costs and pharmacokinetics of 75 mg IP irinotecan with concomitant first-line systemic therapy (consisting of FOLFOX and bevacizumab) in patients with unresectable CPM.
Methods and analysis
This protocol summary follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Statement.34
Setting
This study is a single-arm, open-label, phase II study that is performed in two large Dutch tertiary referral centres for the treatment of CPM; The Catharina Cancer Institute in Eindhoven and the Erasmus MC Cancer Institute in Rotterdam. Further tertiary referral centres may join later.
Objectives
The primary objective is to explore OS after treatment with IP irinotecan (75 mg) to mFOLFOX4/bevacizumab in patients with unresectable CPMs, henceforth referred to as trial treatment.
Secondary objectives are as follows:
To assess PFS (which is calculated from the interval from the start of trial treatment until first evidence of IP and/or systemic disease progression and/or start of second-line systemic therapy, or last follow-up).
To assess the feasibility of trial treatment; to assess the toxicity profile (defined as the number of grade 3–5 adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE)) of trial treatment.
To assess PROs during trial treatment.
To assess costs of trial treatment.
To assess the nephrotoxicity, hepatotoxicity and haematological toxicity during trial treatment.
To assess tumour marker fluctuations during trial treatment.
To determine the number of patients completing trial treatment, required dose reductions and reasons for discontinuation.
To determine the number of patients with an objective radiological response during and after trial treatment.
To systematically collect, process and store blood, tumour tissue and ascites for future translational research.
To determine the systemic and IP pharmacokinetics of IP irinotecan.
Exploratory objectives are to determine if, and how many patients are able to undergo salvage procedures, such as CRS and HIPEC following successful treatment with IP irinotecan (75 mg) and concomitant palliative systemic therapy.
Eligibility criteria
Eligibility criteria are:
Histologically confirmed colorectal carcinoma.
Microsatellite stable (MSS) primary tumour.
Radiologically and clinically or pathologically confirmed unresectable CPMs (eg, PCI>20, extensive small bowel involvement, unresectable disease due to anatomical location).
WHO performance score of 0–1 with a life expectancy of>3 months.
Aged 18 years or older.
Adequate organ functions (haemoglobin of ≥5 mmol/L, neutrophil count of ≥1.5 x 109 /L, platelet count of ≥100 x 109 /L, serum creatinine of <1.5 x upper limit of normal (ULN), creatinine clearance of ≥30 mL/min, Bilirubin<2 x ULN and liver transaminases of <5 x ULN).
Absence of extensive systemic metastases that are deemed to be the dominant factor determining prognosis in terms of life expectancy and performance status (eg, no imminent threat of impaired organ functioning due to the presence of systemic metastases).
No prior cytoreductive surgery.
No prior palliative systemic therapy for CRC.
No (neo)adjuvant/adjuvant systemic therapy for CRC within 6 months prior to enrollment.
No homozygous UGT1A1*28 genotype.35
No dihydropyrimidine dehydrogenase (DPD) deficiency.
No contraindications for the planned chemotherapy (eg, active infection, serious concomitant disease and severe allergy), as determined by the medical oncologist.
Study treatment
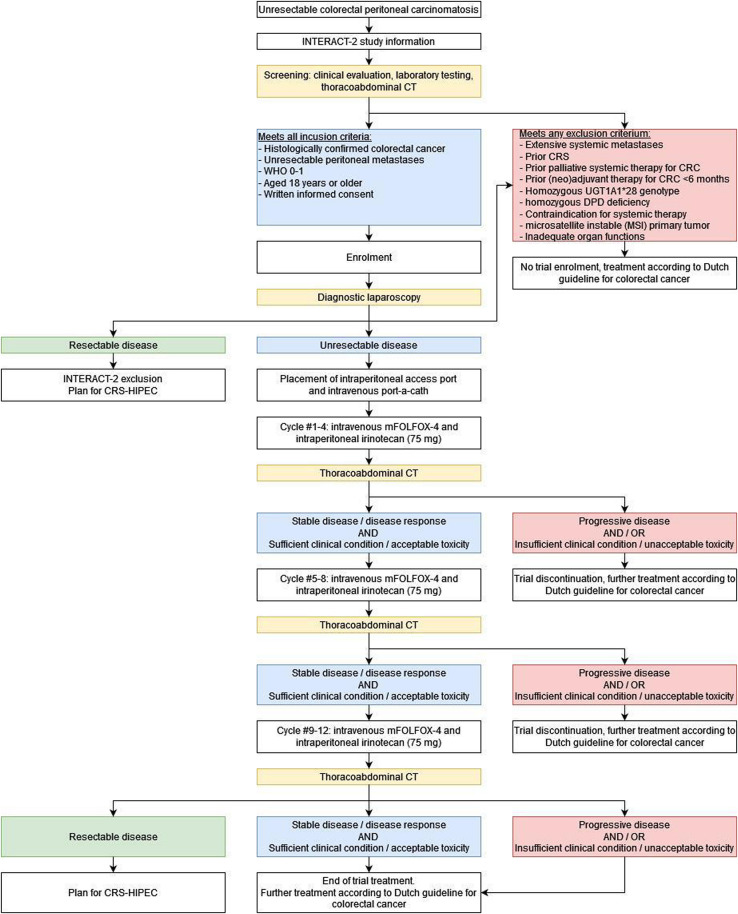
The study flowchart is presented in figure 1. The study schedule of enrolment, treatment and assessment is shown in online supplemental table 1.
Figure 1.
Study flowchart. CRC, colorectal cancer; CRS, cytoreductive surgery; DPD, dihydropyrimidine dehydrogenase; HIPEC, hyperthermic intraperitoneal chemotherapy; WHO 0–1, World Health Organization performance status.
bmjopen-2023-077667supp001.pdf (42.6KB, pdf)
Diagnostic laparoscopy and port placement
Patients who are candidates for CRS and HIPEC are discussed in a multidisciplinary oncology team meeting, after which they are scheduled for a diagnostic laparoscopy. Patients who are considered to have a high chance of unresectable CPM, based on radiological or clinical investigations, may be enrolled in the study. After enrolment, a diagnostic laparoscopy is performed to inspect the peritoneal cavity. The diagnostic laparoscopy is performed under general anaesthesia. If peritoneal disease is considered unresectable (eg, due to PCI>20, too extensive small bowel involvement or anatomical location), two ports are placed: one regular intravenous port-a-cath for the intravenous administration of chemotherapy according to local standard of care, and one peritoneal access port for the IP administration of chemotherapy. The peritoneal access port is placed on the fascia just above or just below the lower rib cage at the discretion of the surgeon. The catheter is tunnelled and inserted into the peritoneal cavity. The tip is positioned in the pelvis. In case of adhesions during the laparoscopy that hampers the positioning of the tip in the pelvis, a different place in the peritoneal cavity may be chosen to place the tip of the catheter. Ascites (or 0.9% NaCl lavage) is collected for translational research. Patients may be discharged the same day after having received instructions for hygiene and wound care.
Chemotherapy
In the absence of postoperative complications, the first cycle will start at least 1 week after placement of the ports to allow for sufficient wound healing. Each cycle consists of intravenous mFOLFOX4 with bevacizumab and concomitant IP irinotecan (75 mg). IP irinotecan (75 mg) will be dissolved in 1 L NaCl 0.9% and prewarmed to 37°C. Cycles are repeated every 2 weeks, with a maximum of 12 cycles. Modified FOLFOX-4 regimen consists of 85 mg/m2 oxaliplatin plus 200 mg/m2 LV and 5-FU 400 mg/m2 bolus on day 1 followed by 1600 mg/m2 5-FU as a 46 hours infusion.27 In case of symptomatic ascites, the ascites will be (partly) drained through the peritoneal access port prior to the start of the therapy cycle.
Response evaluation
Before each cycle, the patient is evaluated (based on clinical and biochemical parameters) by the treating medical oncologist. After every fourth cycle, a thoracoabdominal CT scan is performed for response evaluation. After each CT scan, the decision to continue trial treatment is based on disease response and clinical performance:
In case of physician-determined disease progression (either IP, systemic or both), trial treatment is discontinued. The patient will receive second-line palliative systemic treatment or best supportive care according to the Dutch national guideline for CRC.36
In case of physician-determined disease response or stable disease (both IP and systemic) but severe clinical deterioration or unacceptable toxicity to treatment, rendering the patient unsuited to continue with treatment, trial treatment is discontinued. The patient will receive further palliative systemic treatment or best supportive care according to the Dutch national guideline for CRC.36
In case of physician-determined disease response or stable disease (both IP and systemic) and sufficient clinical condition and acceptable toxicity to treatment, trial treatment is continued.
For all patients, study treatment ends after completing the 12th cycle of intravenous mFOLFOX4 with bevacizumab and concomitant IP irinotecan (75 mg), regardless of response on the thoracoabdominal CT performed after the 12th cycle. On patient’s request, the peritoneal access port is removed after the last cycle of trial treatment. After the evaluation after the 12th cycle, IP chemotherapy will be discontinued definitively and further treatment is scheduled with the medical oncologist and will be in according to local standard of care and may include of CRS–HIPEC.36
Sample size
Population-based studies have described an OS of approximately 12.2 months5 for patients with isolated unresectable CPMs treated with palliative systemic chemotherapy. Based on clinical experience, expert consensus and the preliminary results of the INTERACT study, we hypothesise that the study treatment will result in a median OS of at least 16.2 months. This entails an expected increase of 4 months in the study population in comparison to the general population of patients with unresectable CPM. To render this assumption plausible, with a power of 80% and a type I error rate of 0.05, a sample size of 85 is needed.
Given the previous experience with the trial treatment from the INTERACT study and the low expected additional toxicity of IP irinotecan, the investigators consider it reasonable and safe to expose 85 patients to trial treatment.
Replacement of individual patients
If a patient is withdrawn from the study prior to completing one cycle of IP irinotecan with concomitant systemic therapy, an additional patient is enrolled to replace the withdrawn patient.
Statistical analyses
All patients who complete at least one cycle of IP irinotecan (75 mg) with concomitant systemic therapy will be included in the analyses. Categorical variables will be presented as n (%) and compared with the χ2 test. Continuous variables will be presented as mean±SD or median (IQR), depending on distribution. Paired data will be compared with the paired t-test or Wilcoxon signed rank test, depending on distribution. Unpaired data will be compared with the unpaired t-test or Kruskal Wallis test, depending on distribution. A p value<0.05 will be considered statistically significant. Correction for multiple testing will be applied if necessary. Statistical analyses will be performed with SPSS (V.25.0, Armonk, NY, USA).
Analysis of primary study parameter(s)
The OS is calculated from (a) the interval from diagnosis of peritoneal metastases until death or last follow-up; (b) the interval from the first day of the first cycle until death or last follow-up). OS will be presented with the Kaplan Meier method, and subgroups (eg, stratification based on the presence of systemic metastases or peritoneal carcinomatosis index) will be compared with the log rank test.
Analysis of secondary study parameter(s)
PFS (calculated from the interval from the start of trial treatment until first evidence of IP and/or systemic disease progression or last follow-up) will be presented with the Kaplan Meier method, and subgroups (eg, stratification based on the presence of systemic metastases or PCI) will be compared with the log rank test.
Toxicity, defined as the number of patients who experience/the total number of Common Terminology Criteria for Adverse Events (CTCAE, V.5.0) grade 3–5 adverse events, measured up to 4 weeks after trial treatment. Given the non-randomised design of the study, these analyses will be exploratory and results will be presented as n (%). Differences in subgroups (eg, stratification based on the presence of systemic metastases or peritoneal carcinomatosis index) will be compared with the unpaired t-test or Kruskall Wallis test, depending on distribution.
PROs during trial treatment, assessed with the EQ-5D-5L, EORTC QLQ-C30 and EORTC QLQ-CR29 at baseline, 1 week after the first cycle, 1 week after the fourth cycle, 1 week after the eighth cycle and 1 week after the 12th cycle will be analysed according to the corresponding manuals.37–39 Given the novelty of the trial treatment, no a priori hypotheses are defined for PRO analyses. Therefore, PRO assessment will be explorative, providing the mean±SD of each PRO category at each time-point. Linear mixed modelling analyses will be performed to compare differential effects over time and scores at each time-point, with the use of maximum likelihood estimation and an unstructured covariance matrix with a two-level structure (ie, repeated time-points (lower level), patients (higher level)). To correct for multiple testing, a post-hoc Bonferroni correction will be performed per item, where the p-value will be divided by the number of timepoint-comparisons. In case of statistically significant differences, clinical relevance is determined by a Cohen’s D>0.500.
Healthcare costs and costs due to productivity losses during trial treatment will be assessed with the iMTA Medical Consumption Questionnaire and iMTA Productivity Cost Questionnaire at baseline, 1 week after the first cycle, 1 week after the fourth cycle, 1 week after the eighth cycle and 1 week after the twelfth cycle. An overview of the total costs of trial treatment ((1) per protocol healthcare costs; (2) additional healthcare costs; (3) costs due to productivity losses) is established according to the Dutch Manual for Cost Analysis in Healthcare.40 41
Tumour marker fluctuations during trial treatment will be assessed by carcino-embryonic antigen analysis before each subsequent cycle. Given the novelty of the trial treatment, no a priori hypotheses are defined. Linear mixed modelling analyses will be performed to compare differential effects over time and scores at each time-point, with the use of maximum likelihood estimation and an unstructured covariance matrix with a two-level structure (ie, repeated time-points (lower level) and patients (higher level)). To correct for multiple testing, a pragmatically chosen p<0.01 is considered statistically significant.
Feasibility of trial treatment is assessed through completion of 12 cycles of trial treatment, required dose reductions and reasons for discontinuation. These results are presented as n (%).
Radiological response (according to radiological PCI and RECIST39) during and after trial treatment will be assessed by thoracoabdominal CT at baseline, after the fourth cycle, after the eighth cycle and after the 12th cycle. These results are presented as n (%).
To further investigate the pharmacokinetics of IP irinotecan, peritoneal fluid and peripheral blood samples will be withdrawn at several time points during the first and fourth cycles. The maximum plasma concentration (Cmaxp), the time to maximum plasma concentration (Tmaxp), plasma area under the curve (AUCp), maximum IP concentration (Cmaxip), the time to maximum IP concentration (Tmaxip), IP area under the curve (AUCip) of irinotecan (plasma only) and SN-38 (plasma and peritoneal fluid) will be determined.
Recruitment
The study commenced in November 2022 and the first patients were enrolled in December 2022. It is expected to complete accrual within 2 years. To generate more awareness and to increase referrals of potential study candidates, a short Dutch summary of the study will be published in The Dutch Journal for Oncology (NTvO in Dutch). Further strategies to optimise accrual have not been defined a priori.
Data collection and data management
Outcomes are collected in all patients who completed at least one treatment cycle. All data are prospectively collected by a local investigator in each study centre using standardised electronic case report forms linked to an ISO 27001 certified central study database (De Research Manager, Deventer, the Netherlands). This ISO 27001 certified system optimises data quality by standardised data entry, coding, security and storage.
Data monitoring
Interim analyses are performed by principal investigators and trial coordinators 4 weeks after the first chemotherapy cycle of the 20th included patient and after the second chemotherapy cycle of the 43th patient, after half of the study procedures and systemic cycles have been performed and applied. These analyses will only focus on the safety aspect. The study may be prematurely terminated by the sponsor if there is evidence of an unacceptable risk for study patients. The sponsor will notify all concerned investigators, the medical ethics committee and regulatory authorities of the decision to terminate the study.
Serious adverse events (SAEs) and suspected unexpected serious adverse reaction (SUSARs)
The investigator will report all SAEs and SUSARs to the sponsor without undue delay after obtaining knowledge of the events. The sponsor will report the SAEs and SUSARs through the web portal ToetsingOnline to the accredited METC that approved the protocol, within 7 days of first knowledge for SAEs or SUSARs that result in death or are life threatening followed by a period of maximum of 8 days to complete the initial preliminary report. All other SAEs and SUSARS will be reported within a period of maximum 15 days after the sponsor has first knowledge of the serious adverse events.
Auditing
Auditing is performed by independent qualified monitors of the study centres. The study is considered a low-risk study according to the brochure ‘Kwaliteitsborging mensgebonden onderzoek 2.0’ by the Dutch Federation of University Medical Centres.
Patient and public involvement
None.
Ethics and dissemination
Research ethics approval
The present study is approved by a central ethics committee (MEC-U, Nieuwegein, Netherlands, number R22.052) and the institutional review boards of both study centres.
Protocol amendments
Important modifications to the study protocol need to be authorised by the central ethics committee. After authorisation, these modifications are communicated to the Dutch competent authority, the institutional review boards of both study centres, all investigators, study registries and patients (if required by the central ethics committee).
Informed consent
Patients are enrolled by their treating physician and provide written informed consent. Patients are able to consent to questionnaires and participation in translational side studies separately.
Confidentiality
Personal data of patients are collected and processed in strict adherence to the Dutch law.
Access to data
All authors have access to the final data set, without any contractual agreements that limit access.
Ancillary or poststudy care
The Catharina Hospital is insured to cover harms caused by study participation and extends its insurance to any participating hospital. After trial treatment is stopped, patients will be treated according to Dutch guidelines, as aforementioned in section response evaluation.
Dissemination policy
Study results will be submitted for publication in peer-reviewed medical journals and presented to patients, healthcare professionals and the public, during (inter)national meetings. Authorship eligibility guidelines are not defined a priori. The full study protocol and the Dutch informed consent form are made available on written request to the corresponding author. After study completion, the participant-level data set and statistical code will be made available on reasonable request.
Discussion
In this single-arm, open-label, phase II, patients with unresectable CPMs are treated with concomitant IP and systemic cytotoxic therapy. The primary objective of the study is to assess OS after treatment with IP irinotecan with concomitant mFOLFOX4 and bevacizumab. Secondary objectives are to assess PFS, safety, PROs, costs, feasibility and pharmacokinetic parameters of IP irinotecan with concomitant mFOLFOX4 and bevacizumab.
During this study, ascites and peritoneal biopsy samples will be collected and processed for translational research purposes. These samples will be used to establish organoids, in order to study drug response and resistance ex vivo in detail. This might aid in improved patient selection for both palliative and curative treatments, as well as enable a more personalised treatment approach.42
Multiple studies have studied the effect of another strategy to apply chemotherapy intraperitoneally: PIPAC.15 43 44 In contrast to PIPAC, the IP chemotherapy administered in this study is applied simultaneously with systemic chemotherapy without the need for complex (and expensive) devices or surgery. Furthermore, in comparison to PIPAC, INTERACT treatment has the potential benefit of exposing tumour cells to the cytotoxic agent much more frequent and for a much longer timespan.33
To the best of our knowledge, after the INTERACT study, this is only the second study in patients with peritoneal metastases of colorectal origin that combines standard of care systemic chemotherapy with intraperitoneally administered chemotherapy. As such, the present study will provide essential information about OS and PFS, as well as on safety, feasibility, costs and PROs of treatment with IP irinotecan, and will provide a framework for the conduction of further clinical research. The INTERACT-II study may be an important step towards a more effective, life-prolonging treatment modality for this specific patient group, with the possibility for curative treatment consisting of CRS–HIPEC in specific patients with excellent response to the treatment.
Supplementary Material
Footnotes
VCJvdV and NADG contributed equally.
Contributors: TBMvdH and NADG are the coordinating investigators. VCJvdV, RJL, TBMvdH, RJFB, IvH, G-JMC, SWN, IHJTdH and JWAB are the local investigators of the first study centre. NADG, SLWK, EvM, ARMBK, EVEM, CV and RHJM are the local investigators of the second study centre. SK is the study pharmacologist supervising the pharmacokinetic analyses. JN is the study radiologist performing the central radiological review. SLWK is responsible for translational research on blood. OK is responsible for translational research on ascites and peritoneal lavage. JWAB is the principal investigator. VCJvdV, NADG, RJL, RHJM and JWAB made substantial contributions to the conception and design of the study, drafted the protocol and drafted the manuscript. IEGvH, G-JMC, IHJTdH, SWN, OK, SK, CV, TBMvdH and EvM made substantial contributions to conception and design of the study and critically revised the protocol and the manuscript for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding: This study is supported by the Catharina Research Foundation (unrestricted grant, grant number CZE-2022.08).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Lurvink RJ, Bakkers C, Rijken A, et al. Increase in the incidence of synchronous and Metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. Eur J Surg Oncol 2021;47:1026–33. 10.1016/j.ejso.2020.11.135 [DOI] [PubMed] [Google Scholar]
- 2.van Gestel Y, de Hingh I, van Herk-Sukel MPP, et al. Patterns of Metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38:448–54. 10.1016/j.canep.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 3.van de Vlasakker VCJ, Lurvink RJ, Cashin PH, et al. The impact of PRODIGE 7 on the current worldwide practice of CRS-HIPEC for colorectal peritoneal metastases: A web-based survey and 2021 statement by peritoneal surface oncology group International (PSOGI). Eur J Surg Oncol 2021;47:2888–92. 10.1016/j.ejso.2021.05.023 [DOI] [PubMed] [Google Scholar]
- 4.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of Cytoreduction and Hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal Carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. 10.1200/JCO.2003.04.187 [DOI] [PubMed] [Google Scholar]
- 5.Bakkers C, Lurvink RJ, Rijken A, et al. Treatment strategies and prognosis of patients with synchronous or Metachronous colorectal peritoneal metastases: A population-based study. Ann Surg Oncol 2021;28:9073–83. 10.1245/s10434-021-10190-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacquet P, Sugarbaker P. Current Methodologies for clinical assessment of patients with peritoneal Carcinomatosis. J Experiment Clin Cancer Res 1996;15:49–58. 10.1007/978-1-4613-1247-5 [DOI] [Google Scholar]
- 7.Simkens GA, Rovers KP, Nienhuijs SW, et al. Patient selection for Cytoreductive surgery and HIPEC for the treatment of peritoneal metastases from colorectal cancer. Cancer Manag Res 2017;9:259–66. 10.2147/CMAR.S119569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res 1996;82:53–63. 10.1007/978-1-4613-1247-5_4 [DOI] [PubMed] [Google Scholar]
- 9.Flessner MF. The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol 2005;288:F433–42. 10.1152/ajprenal.00313.2004 [DOI] [PubMed] [Google Scholar]
- 10.Al-Quteimat OM, Al-Badaineh MA. Intraperitoneal chemotherapy: rationale, applications, and limitations. J Oncol Pharm Pract 2014;20:369–80. 10.1177/1078155213506244 [DOI] [PubMed] [Google Scholar]
- 11.Averbach AM, Sugarbaker PH. Methodologic considerations in treatment using intraperitoneal chemotherapy. Peritoneal Carcinomatosis: Principles of Management 1996:289–309. 10.1007/978-1-4613-1247-5 [DOI] [PubMed] [Google Scholar]
- 12.Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration. Cancer Treat Rep 1978;62:1–13. [PubMed] [Google Scholar]
- 13.Guchelaar NAD, Noordman BJ, Koolen SLW, et al. Intraperitoneal chemotherapy for Unresectable peritoneal surface malignancies. Drugs 2023;83:159–80. 10.1007/s40265-022-01828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovers KP, Wassenaar ECE, Lurvink RJ, et al. Pressurized intraperitoneal aerosol chemotherapy (Oxaliplatin) for Unresectable colorectal peritoneal metastases: a multicenter, single-arm, phase II trial (CRC-PIPAC). Ann Surg Oncol 2021;28:5311–26. 10.1245/s10434-020-09558-4 [DOI] [PubMed] [Google Scholar]
- 15.Lurvink RJ, Rovers KP, Nienhuijs SW, et al. Pressurized intraperitoneal aerosol chemotherapy with Oxaliplatin (PIPAC-OX) in patients with colorectal peritoneal metastases-a systematic review. J Gastrointest Oncol 2021;12:S242–58. 10.21037/jgo-20-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer NL, Brandt-Kerkhof ARM, Madsen EVE, et al. Concomitant intraperitoneal and systemic chemotherapy for extensive peritoneal metastases of colorectal origin: protocol of the Multicentre, open-label, phase I, dose-escalation INTERACT trial. BMJ Open 2019;9:e034508. 10.1136/bmjopen-2019-034508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol 2010;2:109–16. 10.4251/wjgo.v2.i2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathijssen RH, van Alphen RJ, Verweij J, et al. Clinical pharmacokinetics and metabolism of Irinotecan (CPT-11). Clin Cancer Res 2001;7:2182–94. [PubMed] [Google Scholar]
- 19.Ahn B-J, Choi MK, Park YS, et al. Population pharmacokinetics of CPT-11 (Irinotecan) in gastric cancer patients with peritoneal seeding after its Intraperitoneal administration. Eur J Clin Pharmacol 2010;66:1235–45. 10.1007/s00228-010-0885-3 [DOI] [PubMed] [Google Scholar]
- 20.Choi MK, Ahn B-J, Yim D-S, et al. Phase I study of intraperitoneal Irinotecan in patients with gastric adenocarcinoma with peritoneal seeding. Cancer Chemother Pharmacol 2011;67:5–11. 10.1007/s00280-010-1272-6 [DOI] [PubMed] [Google Scholar]
- 21.Maruyama M, Toukairin Y, Baba H, et al. Pharmacokinetic study of the intraperitoneal administration of CPT-11 for patients with peritoneal seedings of gastric and Colonic cancers. Gan to Kagaku Ryoho Cancer & Chemotherapy 2001;28:1505–7. [PubMed] [Google Scholar]
- 22.Matsui A, Okuda M, Tsujitsuka K, et al. Pharmacology of intraperitoneal CPT-11. Surg Oncol Clin N Am 2003;12:795–811, 10.1016/s1055-3207(03)00033-4 [DOI] [PubMed] [Google Scholar]
- 23.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and Leucovorin for metastatic colorectal cancer. N Engl J Med 2000;343:905–14. 10.1056/NEJM200009283431302 [DOI] [PubMed] [Google Scholar]
- 24.de Man FM, Goey AKL, van Schaik RHN, et al. Individualization of Irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and Pharmacogenetics. Clin Pharmacokinet 2018;57:1229–54. 10.1007/s40262-018-0644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guichard S, Chatelut E, Lochon I, et al. Comparison of the pharmacokinetics and efficacy of Irinotecan after administration by the intravenous versus intraperitoneal route in mice. Cancer Chemother Pharmacol 1998;42:165–70. 10.1007/s002800050801 [DOI] [PubMed] [Google Scholar]
- 26.Hribaschek A, Kuhn R, Pross M, et al. Intraperitoneal versus intravenous CPT-11 given intra-and Postoperatively for peritoneal Carcinomatosis in a rat model. Surg Today 2006;36:57–62. 10.1007/s00595-004-3096-7 [DOI] [PubMed] [Google Scholar]
- 27.Nagahama T, Maruyama M, Goseki N. Intraperitoneal administration of CPT-11 in rats--experimental study for pharmacokinetics. Gan to Kagaku Ryoho Cancer & Chemotherapy 2000;27:1866–9. [PubMed] [Google Scholar]
- 28.Turcotte S, Sideris L, Younan R, et al. Pharmacokinetics of intraperitoneal Irinotecan in a pig model. J Surg Oncol 2010;101:637–42. 10.1002/jso.21569 [DOI] [PubMed] [Google Scholar]
- 29.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950–5. 10.1056/NEJM199612263352603 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34–43. 10.1056/NEJMoa052985 [DOI] [PubMed] [Google Scholar]
- 31.Speyer JL. The rationale behind intraperitoneal chemotherapy in gastrointestinal malignancies. Semin Oncol 1985;12:23–8. [PubMed] [Google Scholar]
- 32.Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and Prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. Obstet Gynecolog Survey 2015;70:505–6. 10.1097/01.ogx.0000469913.56599.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Eerden RAG, de Boer NL, van Kooten JP, et al. Phase I study of intraperitoneal Irinotecan combined with palliative systemic chemotherapy in patients with colorectal peritoneal metastases. Br J Surg 2023;110:1502–10. 10.1093/bjs/znad228 [DOI] [PubMed] [Google Scholar]
- 34.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulshof EC, de With M, de Man FM, et al. Ugt1A1 genotype-guided dosing of Irinotecan: A prospective safety and cost analysis in poor Metaboliser patients. Eur J Cancer 2022;162:148–57. 10.1016/j.ejca.2021.12.009 [DOI] [PubMed] [Google Scholar]
- 36.Nederland VKG. Landelijke werkgroep Gastrointestinale tumoren. Landelijke richtlijn erfelijke darmkanker Versie.1. [Google Scholar]
- 37.Fayers P, Aaronson NK, Bjordal K, et al. EORTC QLQ–C30 scoring manual. European Organisation for Research and Treatment of Cancer, 1995. [Google Scholar]
- 38.Foundation ER . EQ-5D-5L user guide. EuroQol Research Foundation Rotterdam, The Netherlands, 2019. [Google Scholar]
- 39.Whistance RN, Conroy T, Chie W, et al. Clinical and Psychometric validation of the EORTC QLQ-Cr29 questionnaire Module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45:3017–26. 10.1016/j.ejca.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 40.Kanters TA, Bouwmans CAM, van der Linden N, et al. Update of the Dutch manual for costing studies in health care. PLoS One 2017;12:e0187477. 10.1371/journal.pone.0187477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan SS, Bouwmans CAM, Rutten FFH, et al. Update of the Dutch manual for costing in economic evaluations. Int J Technol Assess Health Care 2012;28:152–8. 10.1017/S0266462312000062 [DOI] [PubMed] [Google Scholar]
- 42.Lau HCH, Kranenburg O, Xiao H, et al. Organoid models of gastrointestinal cancers in basic and Translational research. Nat Rev Gastroenterol Hepatol 2020;17:203–22. 10.1038/s41575-019-0255-2 [DOI] [PubMed] [Google Scholar]
- 43.Sgarbura O, Eveno C, Alyami M, et al. Consensus statement for treatment protocols in Pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2022;7:1–7. 10.1515/pp-2022-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tempfer C, Giger-Pabst U, Hilal Z, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal Carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch Gynecol Obstet 2018;298:243–57. 10.1007/s00404-018-4784-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077667supp001.pdf (42.6KB, pdf)