Abstract
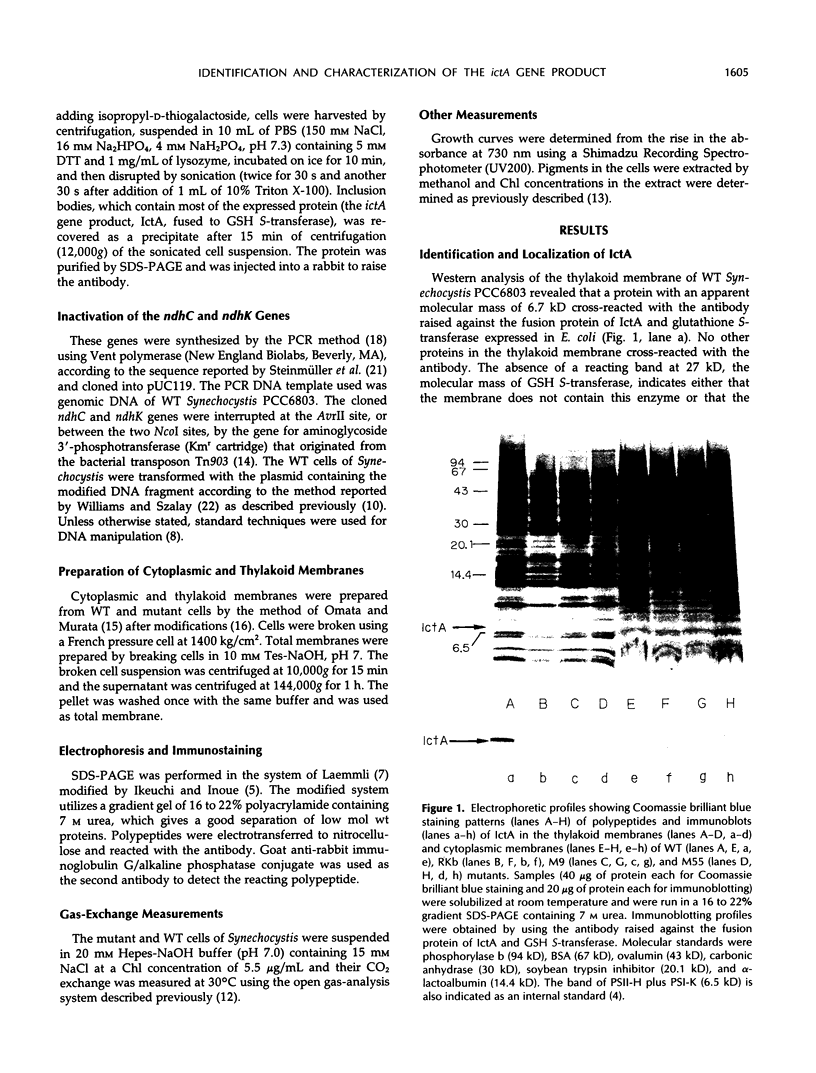
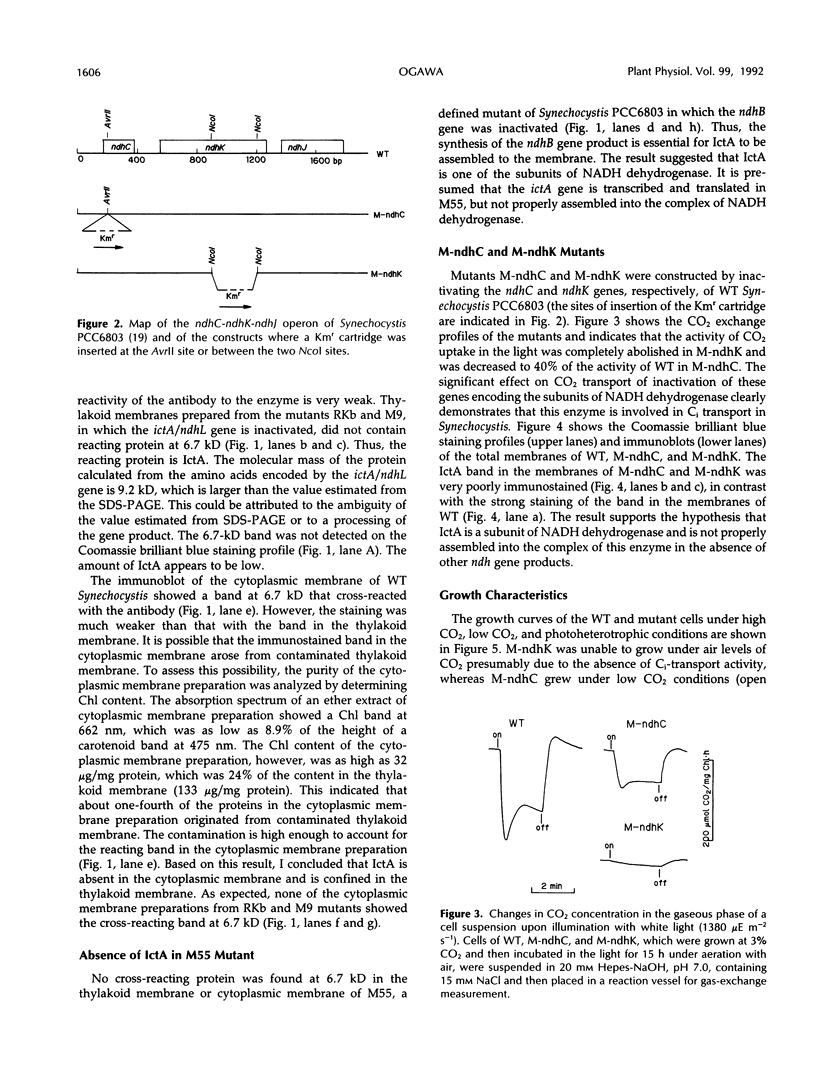
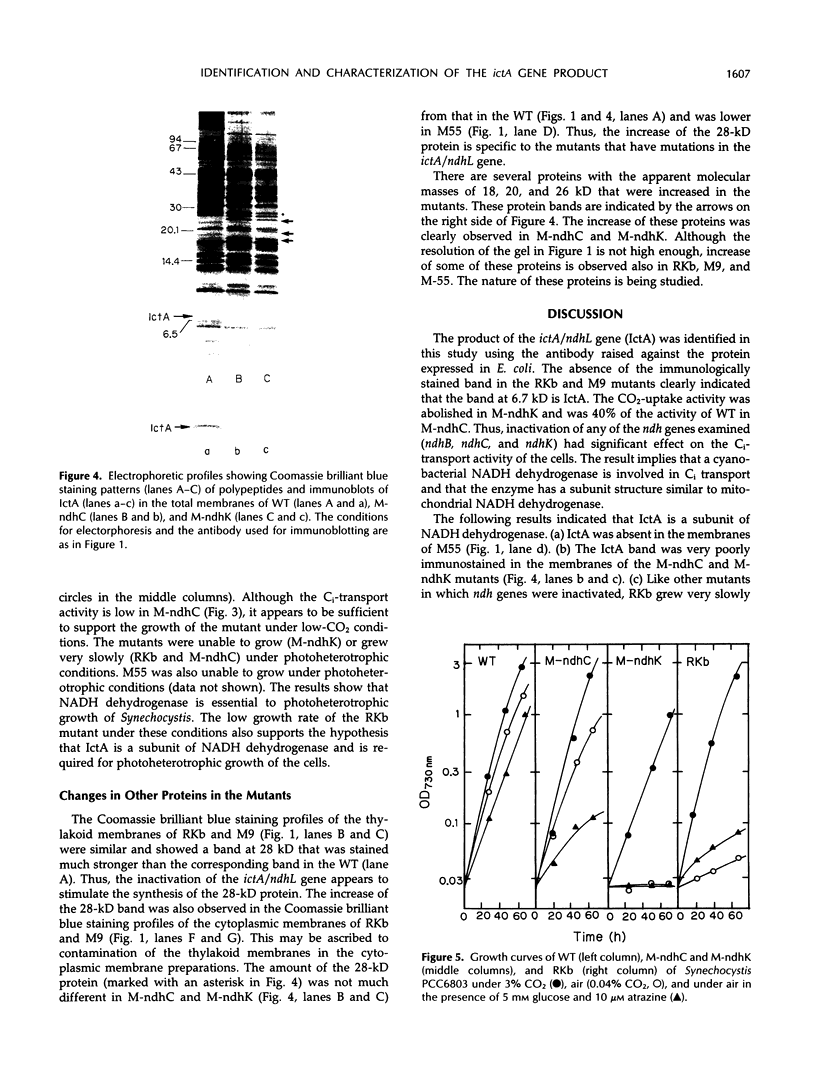
The ictA gene, renamed ndhL in this paper, essential to inorganic carbon transport of Synechocystis PCC6803, was expressed in Eschericia coli as a fusion protein with glutathione S-transferase. An antibody was raised against this fusion protein. Western analysis of the thylakoid membrane of wild-type (WT) Synechocystis revealed that a protein with an apparent molecular mass of 6.7 kilodaltons cross-reacted with this antibody. No immunoreactive protein was present in the thylakoid membranes of the Synechocystis mutants, RKb and M9, which have defects in the ictA/ndhL gene, or in the cytoplasmic membranes of the WT and mutant cells. Thus, the protein reacted with the antibody is the ictA gene product (IctA) and is localized in the thylakoid membrane of WT cells. IctA was absent in the thylakoid membranes of the M55 mutant, in which the ndhB gene is inactivated, and was poorly immunostained in the membranes of the mutants (M-ndhC and M-ndhK) constructed by inactivating the ndhC and ndhK genes of WT Synechocystis, respectively. The carbon dioxide uptake activity was nearly zero in M-ndhK and was about 40% of the activity of WT cells in M-ndhC. The RKb, M-ndhC, and M-ndhK mutants were unable to grow or grew very slowly under photoheterotrophic conditions. These results indicated that NADH dehydrogenase is essential to inorganic carbon transport and photoheterotrophic growth of Synechocystis and that IctA is one of the subunits of this enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. L., McIntosh L. Partial conservation of the 5' ndhE-psaC-ndhD 3' gene arrangement of chloroplasts in the cyanobacterium Synechocystis sp. PCC 6803: implications for NDH-D function in cyanobacteria and chloroplasts. Plant Mol Biol. 1991 Apr;16(4):487–499. doi: 10.1007/BF00023416. [DOI] [PubMed] [Google Scholar]
- Berger S., Ellersiek U., Steinmüller K. Cyanobacteria contain a mitochondrial complex I-homologous NADH-dehydrogenase. FEBS Lett. 1991 Jul 29;286(1-2):129–132. doi: 10.1016/0014-5793(91)80957-5. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Eggers B., Shen G. Z., Webber A., Yu J. J., Hirano A., Inoue Y., Vermaas W. Cloning of the psbK gene from Synechocystis sp. PCC 6803 and characterization of photosystem II in mutants lacking PSII-K. J Biol Chem. 1991 Jun 15;266(17):11111–11115. [PubMed] [Google Scholar]
- Kaplan A., Schwarz R., Lieman-Hurwitz J., Reinhold L. Physiological and molecular aspects of the inorganic carbon-concentrating mechanism in cyanobacteria. Plant Physiol. 1991 Nov;97(3):851–855. doi: 10.1104/pp.97.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ogawa T. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. Cloning and Inactivation of a Gene Essential to Inorganic Carbon Transport of Synechocystis PCC6803. Plant Physiol. 1991 May;96(1):280–284. doi: 10.1104/pp.96.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. Mutants of Synechocystis PCC6803 Defective in Inorganic Carbon Transport. Plant Physiol. 1990 Oct;94(2):760–765. doi: 10.1104/pp.94.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Omata T., Ogawa T. Biosynthesis of a 42-kD Polypeptide in the Cytoplasmic Membrane of the Cyanobacterium Anacystis nidulans Strain R2 during Adaptation to Low CO(2) Concentration. Plant Physiol. 1986 Feb;80(2):525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmüller K., Ley A. C., Steinmetz A. A., Sayre R. T., Bogorad L. Characterization of the ndhC-psbG-ORF157/159 operon of maize plastid DNA and of the cyanobacterium Synechocystis sp. PCC6803. Mol Gen Genet. 1989 Mar;216(1):60–69. doi: 10.1007/BF00332231. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]