Abstract
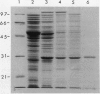
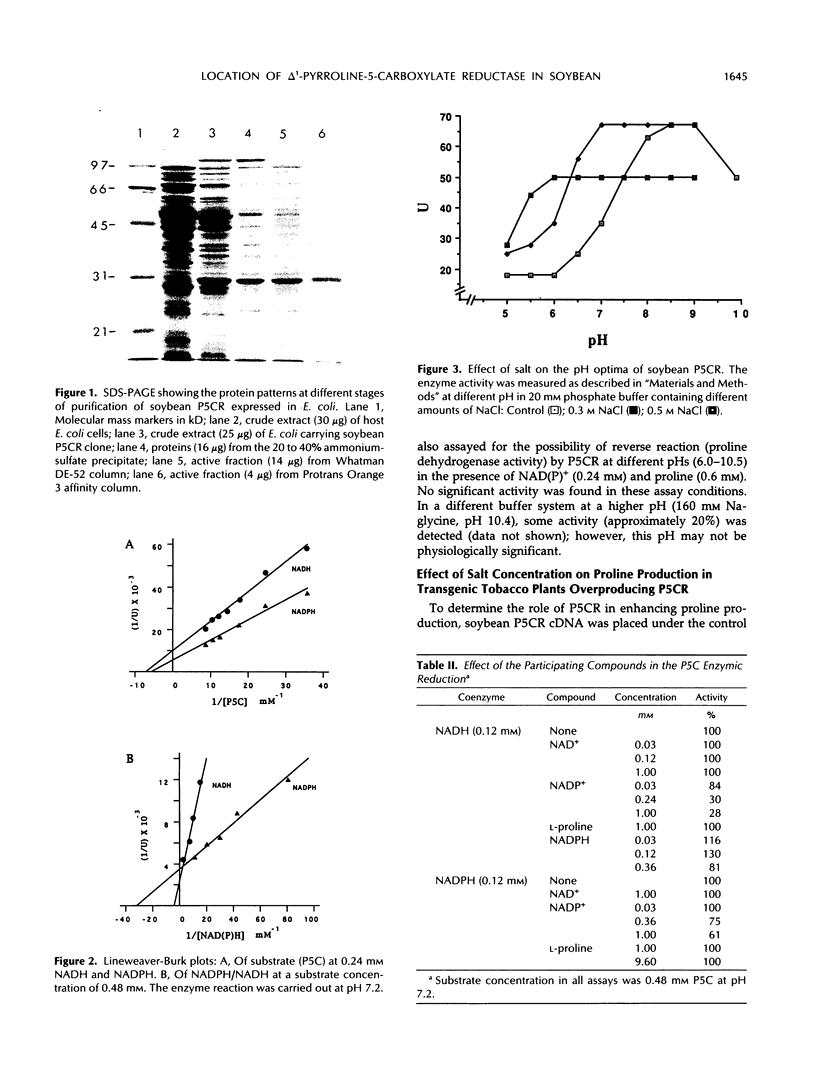
The expression of Δ1-pyrroline-5-carboxylate reductase (P5CR) gene was found to be higher in soybean root nodules than in leaves and roots, and its expression in roots appeared to be osmoregulated (AJ Delauney, DPS Verma [1990] Mol Gen Genet 221: 299-305). P5CR was purified to homogeneity as a monomeric protein of 29 kilodaltons by overexpression of a soybean P5CR cDNA clone in Escherichia coli. The pH optimum of the purified P5CR was altered by increasing the salt concentration, and maximum enzyme activity was attainable at a lower pH under high salt (0.2-1 molar NaCl). Kinetic studies of the purified enzyme suggested that nicotinamide adenine dinucleotide phosphate+ inhibited P5CR activity, whereas nicotinamide adenine dinucleotide+ did not. Subcellular fractionation and antibodies raised against purified soybean P5CR were used to investigate location of the enzyme in different parts of soybean as well as in leaves of transgenic tobacco plants synthesizing soybean P5CR. P5CR activity was present in cytoplasm of soybean roots and nodules as well as in leaves, but in leaves, about 15% of the activity was detected in the plastid fraction. The location of P5CR was further confirmed by western blot assay of the proteins from cytosol and plastid fractions of different parts of the plant. Expression of soybean nodule cytosolic P5CR in transgenic tobacco under the control of cauliflower mosaic virus 35S promoter led to the accumulation of this protein exclusively in the cytoplasm, suggesting that the chloroplastic activity may be due to the presence of a plastid form of the enzyme. The different locations of P5CR in root and leaf suggested that proline may be synthesized in different subcellular compartments in root and leaf. Proline concentration was not significantly increased in transgenic plants exhibiting high level P5CR activity, indicating that reduction of P5C is not a rate-limiting step in proline production.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Kelly-Chilson A. E., Siegel N. R. Pyrroline-5-carboxylate reductase in soybean nodules: isolation/partial primary structure/evidence for isozymes. Arch Biochem Biophys. 1991 Aug 1;288(2):350–357. doi: 10.1016/0003-9861(91)90206-x. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A. J., Tabaeizadeh Z., Verma D. P. A stable bifunctional antisense transcript inhibiting gene expression in transgenic plants. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4300–4304. doi: 10.1073/pnas.85.12.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Iwai Y., Hakuba A., Noguchi K., Nishimura S. A gigantic neurilemoma originating in the pterygopalatine fossa. Case report. Surg Neurol. 1988 Dec;30(6):452–456. doi: 10.1016/0090-3019(88)90030-4. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Schubert K. R., Carter M. B., Hagedorn C. H., Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R., Jäger H. J., Hintz M., Pahlich E. Purification to homogeneity of pyrroline-5-carboxylate reductase of barley. Plant Physiol. 1986 Jan;80(1):142–144. doi: 10.1104/pp.80.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberté G., Hellebust J. A. Pyrroline-5-Carboxylate Reductase in Chlorella autotrophica and Chlorella saccharophila in Relation to Osmoregulation. Plant Physiol. 1989 Nov;91(3):917–923. doi: 10.1104/pp.91.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa P. C., Rhodes D., Rhodes J. C., Bressan R. A., Csonka L. N. Elevated Accumulation of Proline in NaCl-Adapted Tobacco Cells Is Not Due to Altered Delta-Pyrroline-5-Carboxylate Reductase. Plant Physiol. 1991 May;96(1):245–250. doi: 10.1104/pp.96.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A., RADHAKRISHNAN A. N., BUCKLEY S. D. Enzymatic synthesis of L-pipecolic acid and L-proline. J Biol Chem. 1957 Dec;229(2):789–800. [PubMed] [Google Scholar]
- Miao G. H., Hirel B., Marsolier M. C., Ridge R. W., Verma D. P. Ammonia-regulated expression of a soybean gene encoding cytosolic glutamine synthetase in transgenic Lotus corniculatus. Plant Cell. 1991 Jan;3(1):11–22. doi: 10.1105/tpc.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang J. M. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Rayapati P. J., Stewart C. R., Hack E. Pyrroline-5-Carboxylate Reductase Is in Pea (Pisum sativum L.) Leaf Chloroplasts. Plant Physiol. 1989 Oct;91(2):581–586. doi: 10.1104/pp.91.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcher B. C., Galione M. J. Purification and comparison of elastins from different animal species. Anal Biochem. 1976 Aug;74(2):441–447. doi: 10.1016/0003-2697(76)90224-4. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]