Abstract
Objectives
There is limited qualitative research on patients’ experiences with long COVID-19, and how specific symptoms impact their daily lives. The study aimed to understand patients’ lived experiences of long COVID-19 and to develop a conceptual model representing the symptoms and their impact on overall quality of life.
Setting
Qualitative study consisting of a comprehensive literature review, and in-depth clinician and patient semistructured interviews.
Participants
Forty-one adult patients with long COVID-19, of whom 18 (44%) were recruited through Regeneron Pharmaceuticals’s clinical trials and 23 (56%) through recruitment agencies; 85.4% were female and 73.2% were White. Five independent clinicians treating patients with long COVID-19 were interviewed. Concept saturation was also assessed.
Primary and secondary outcomes
Interview transcripts were analysed thematically to identify concepts of interest spontaneously mentioned by patients, including symptoms and their impacts on daily life, to guide the development of the conceptual model.
Results
Findings from the literature review and clinician and patient interviews resulted in the development of a conceptual model comprising two overarching domains: symptoms (upper respiratory tract, lower respiratory tract, smell and taste, systemic, gastrointestinal, neurocognitive and other) and impacts (activities of daily living, instrumental activities of daily living, physical impacts, emotional, social/leisure activities and professional impacts). Saturation was achieved for the reported impacts. The symptoms reported were heterogenic; neurocognitive symptoms, such as numbness, ringing in ears, haziness, confusion, forgetfulness/memory problems, brain fog, concentration, difficulties finding the right word and challenges with fine motor skills, were particularly pertinent for several months.
Conclusion
The conceptual model, developed based on patient experience data of long COVID-19, highlighted numerous symptoms that impact patients’ physical and mental well-being, and suggests humanistic unmet needs. Prospective real-world studies are warranted to understand the pattern of long COVID-19 experienced in larger samples over longer periods of time.
Keywords: Patient Reported Outcome Measures, INFECTIOUS DISEASES, COVID-19
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study included a comprehensive review of published literature related to long COVID-19, alongside in-depth, qualitative interviews with patients recruited from both clinical trials and healthcare research firms, as well as interviews with independent clinicians, to understand the patient experience of long COVID-19.
While knowledge about acute COVID-19 symptoms and patient experience is relatively comprehensive, this study adds to the limited literature on the patient experience of long COVID-19 and its impacts on daily life, including neurocognitive, physical and emotional functioning.
A limitation of this study is that the participants were predominantly white and female. While the pathology of long COVID-19 is known to affect mostly women, it would be desirable to perform additional research in males and more diverse patient groups for better representation of the affected population.
Introduction
Patients infected with SARS-CoV-2, the virus that causes COVID-19, can experience long-term effects even if the virus is no longer detected with standard methods.1 These effects are often referred to as ‘long COVID’, ‘post-COVID-19 syndrome’ or ‘postacute sequelae of SARS-CoV-2’,2–4 and definitions may vary. According to the Centers for Disease Control and Prevention (CDC), patients with long COVID-19 continue to experience symptoms for ≥4 weeks after the initial infection with SARS-CoV-2.1 The National Institute for Health and Care Excellence (NICE) uses the term ‘post-COVID-19 syndrome’ and defines it as ‘signs and symptoms that develop during or after an infection consistent with COVID-19, which continue for more than 12 weeks and are not explained by an alternative diagnosis’.5 While the WHO defines the condition as ‘the illness that occurs in people who have a history of probable or confirmed SARS-CoV-2 infection; usually within 3 months from the onset of COVID-19, with symptoms and effects that last for at least 2 months.’ Similar to the NICE guidelines, the WHO specifies that ‘the symptoms and effects of post-COVID-19 condition cannot be explained by an alternative diagnosis’.6
The most commonly reported symptoms of long COVID-19 in current literature are fatigue, chest pain, muscle aches, persistent cough, fever, shortness of breath or difficulty breathing, loss of smell or taste, depression or anxiety, trouble speaking, memory, concentration and/or sleep problems.1 6–10 The CDC and NICE have indicated that long COVID-19 can affect anyone who has been infected, regardless of the severity of the initial infection.1 5 However, risk factors for long COVID-19 have been reported to include female sex, older age and a history of more than five symptoms during the infection.11 12 Specific features of long COVID-19 have been identified via immune profiling, and include elevated humoral responses against SARS-CoV-2, and decreased levels of cortisol.13 The reported prevalence of long COVID-19 ranges considerably, from 8% to 57% depending on the source and patient population evaluated.1 14 15 A recent study reported an elevated risk of both hospitalisation and death during 2 years of follow-up for patients who were hospitalised during acute COVID-19 infection.16 While the exact prevalence of long COVID-19 is unclear, it is evident that management of patients with the disease poses a potentially significant burden for healthcare systems globally.17 The health and economic consequences of long COVID-19 are predicted to be in line with acute disease, when calculating for a reduced quality-adjusted life expectancy, long COVID-19 has been estimated to cost around US$2.6 trillion in the USA.18 19
Although COVID-19 is a global public health issue, and there is an increasing body of clinical and epidemiological literature on the incidence and prevalence of symptoms, to date, there has been little empirical evidence directly from patients regarding the symptoms associated with long COVID-19 and how these symptoms impact their lives.7 20–23 Previously, we reported that the manifestation of symptoms in patients who were diagnosed with COVID-19 was heterogeneous and significantly affected all aspects of patients’ lives; however, the study focused on patients with acute disease.24 Due to its discrete characteristics, it is essential to understand and study long COVID-19 separately from acute COVID-19. We, therefore, sought to: (1) gain an in-depth understanding of the patient experience of long COVID-19 symptoms and the impact on their daily lives and (2) understand the patient experience within the framework of a conceptual model based on empirical patient-relevant evidence informed by literature and patient and clinician interviews.
Methods
Literature review
Search strategy
An electronic search was performed on 23 July 2021, using PubMed to identify qualitative papers published up to that date that explored the patient experience of long COVID-19. A combination of search terms related to the target population was used, including (online supplemental table S1): postacute sequelae of SARS-CoV-2 infection, long COVID-19 and patient experience (eg, symptoms, impacts). The search was limited to humans and English language publications. During the first stage of screening, articles were reviewed by title and abstract only. The second screening stage included a full-text review of articles that were retained after the first screening. It was decided a priori that the full text of up to 20 articles would be reviewed.
bmjopen-2023-076992supp001.pdf (7.6MB, pdf)
Qualitative in-depth interviews with patients and clinicians
Patients
A purposive sample of patients was initially identified through Regeneron Pharmaceuticals’s clinical trial programme (COV-2066 in hospitalised participants (NCT04426695) and COV-2067 in non-hospitalised participants (NCT04425629)).25 26 This patient sample was extended to the real-world through two independent healthcare research firms, Rare Patient Voice and PRC Corporation, which specialise in the recruitment of difficult-to-reach populations (online supplemental methods). Adults (aged ≥18 years) with a positive SARS-CoV-2 PCR test ≥180 days prior to enrolment into this study, experiencing long COVID-19 symptom(s) that could not be explained by an alternative diagnosis, and who were willing and able to participate in one 60 min audiorecorded telephone or online interview in English, were included in the study. Full inclusion and exclusion criteria are provided in online supplemental methods, along with details of patient information collected.
Clinicians
Clinicians were eligible to participate if they were regularly seeing/treating more than five patients a week with long COVID-19 in the USA and were willing to participate in a 60 min audiorecorded telephone interview in English. Clinicians who only treated patients who resided in an institutional setting (eg, nursing home) were excluded. There were no predefined sample quotas set for the five clinicians; however, the recruitment process sought a diverse representation of professions (eg, general practitioners, nurses), specialties and treatment settings (eg, hospital/non-hospital settings) where patients with long COVID-19 were regularly treated. A heterogeneous sample of five clinicians, who served as research consultants, was considered sufficient to obtain insights for input into the conceptual model.
Sampling
A purposive sampling approach was used and initially defined to target patients who were enrolled in Regeneron Pharmaceuticals’s clinical trials in the long COVID-19 substudy in the USA. Additional recruitment of patients from external recruitment agencies was matched to the trial patients according to the inclusion criteria. Due to the heterogeneity of both long COVID-19 symptoms and the affected population,27 sampling covered a target population experiencing a range of symptoms. This ensured a diverse sample of up to 50 patients, which was estimated as an adequate sample size to reach conceptual saturation in the concepts of interest (COIs).28 29 The sample size was anticipated to be adjusted downward based on recruitment feasibility and saturation analysis. In qualitative research, whether the sample size to obtain probable symptoms and impacts from a population is substantial or not is usually defined by the principle of data saturation (see Saturation Analysis section below).30 The final sample size was to be determined by saturation based on good research practice28 31 and requirements by the US Food and Drug Administration (FDA) for establishing content validity.32
Patient and clinician interviews
Semistructured patient interview guides were developed in line with best practices outlined in the FDA patient-focused drug development guidance.33 The patient interview guides provided the researcher with a general outline for the semistructured interview, but each interview was unique based on spontaneous patient responses to questions about symptoms and the impacts of long COVID-19 on daily activities and health-related quality of life (a copy of the patient interview guide is included in online supplemental material). Audiorecorded patient interviews were conducted via Microsoft Teams (use of camera optional by the patient) by four experienced qualitative researchers who received specific training for this study, and who had backgrounds in psychology and anthropology as well as ≥2 years’ experience in qualitative research. During the semistructured interview, patients were asked open-ended questions to provide spontaneous inputs regarding the symptoms of long COVID-19 (experienced after the first 4 weeks of acute COVID-19). For example, ‘Can you begin by telling me about the symptoms you have experienced with long COVID-19? and ‘How would you describe these symptoms in your own words?’ and the impacts the symptoms had on the patients’ daily lives. For example, ‘In what ways has your day-to-day life changed since you began to experience long COVID-19 symptoms?’ Specific questions for each reported symptoms were also asked, which included the symptom duration, as well as any changes over time such as improvement or worsening and symptom severity over time. Additional questions asked for further descriptions of symptoms and which symptoms were the most and least bothersome. Emotional impacts, effects on social interactions and disruption to day-to-day life and activities were also explored. Examples of these questions are, ‘How do your long COVID-19 symptoms affect your day-to-day life? and ‘Are there day-to-day activities that you engage in differently since experiencing long COVID-19 symptoms?’. Additional questions on activities were to assess the patients’ emotional well-being (eg, anxiety or depression) and their ability to engage in physical activities and their usual exercise routine, to care for other people and pets, to spend time with others, to do their usual hobbies and to do grocery shopping.
Clinician interviews were also conducted by experienced qualitative researchers via Microsoft Teams. During the interview process, clinicians provided insights into patients’ experiences of long COVID-19. All relevant findings extracted from the articles in the literature review and the clinician interviews were organised into a conceptual model to detail all potential COIs that could inform the patient experience of long COVID-19.
Patient and public involvement
Patients and/or the public were not involved in the development, design, reporting, dissemination plans of this qualitative study. Patients were interviewed as part of the study. The results will be made available via publication.
Thematic data analysis
All interviews were audiorecorded and transcribed verbatim. Transcripts from patient interviews were analysed thematically,34 using detailed line-by-line open and inductive coding35–37 within ATLAS.ti software (Scientific Software Development). Coding was tailored to the research objectives of the study, which were the identification of symptoms and impacts of long COVID-19, including those spontaneously mentioned by patients as well as further probing of COIs. Codes and quotations were compared with the rest of the data and inductively categorised into higher-order overarching categories referred to as concepts, subdomains and domains, reflecting their conceptual content underpinning. This categorisation was an iterative process performed by a research team that involved comparison and cross-referencing between different analytical categories.
Saturation analysis
Details of the saturation analysis are provided in online supplemental methods.
Conceptual model
A conceptual model identifies and characterises COIs related to a health condition.38 To develop a conceptual model of patient experiences with long COVID-19, data extracted from the literature review were inductively categorised into higher-order conceptual domains. Once coding was complete, a preliminary conceptual model was developed using the concepts extracted from PubMed; this was then revised with clinician input and further refined based on the results from patient interviews. Each concept was then considered and grouped into higher-order domains. If a concept was repeated across multiple sources, it was listed once. Ultimately, standard analytical techniques of conceptual model development were used to develop a visual representation of how the COIs relate to each other, grounded in a data-driven process based on patient-relevant empirical evidence.35–37 39
Analysis
Analysis details are provided in online supplemental methods.
Results
Literature review findings
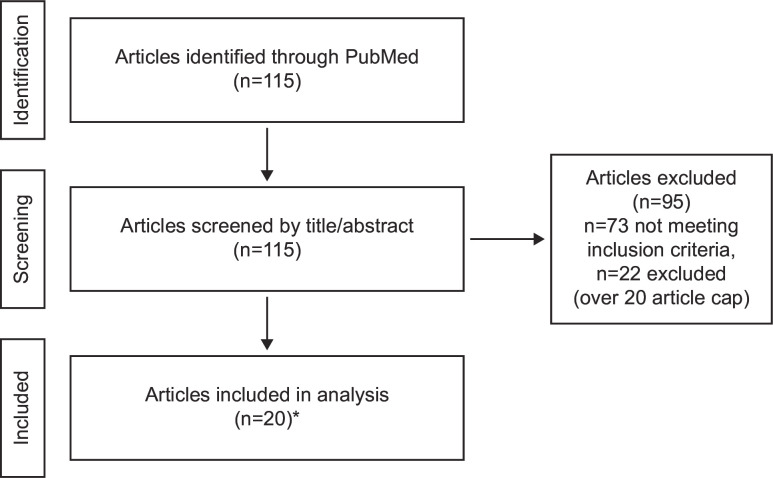
The PubMed search of qualitative studies identified 115 abstracts for screening: 95 articles were excluded after abstract review and 20 full-text articles related to symptom and impact concepts were included in the final literature analysis (figure 1). Articles presented symptoms at different conceptual levels (domain, subdomains and concepts). Impacts were also presented at a high level of abstraction, for example, worsened quality of life.
Figure 1.
Literature review process for concepts related to symptoms and impact concepts. *It was decided a priori that the full text of up to 20 articles would be reviewed.
Patient demographics and interviews
Interviews were conducted between 30 September 2021 and 12 May 2022. The study included 41 patients, of whom 18 (44%) were recruited through clinical trials and 23 (56%) through recruitment agencies (table 1). The mean age was 53.6 years, 85.4% were female and 73.2% were White. The mean time between the start of any COVID-19 symptoms and the interview was 12 months (range: 1–25 months). Twenty-two patients (54%) received two doses of either the Moderna (27%) or the Pfizer/BioNTech (27%) vaccine, and 49% had been hospitalised. Most patients reported good (34%) or fair (37%) general health. At the time of the interview, hypertension/high blood pressure, arthritis, asthma, diabetes and mood disorders were self-reported by 18 (44%), 16 (39%), 10 (24%), 9 (22%) and 7 (17%) patients, respectively.
Table 1.
Patient demographic and clinical characteristics
| Variable | All (N=41) | Clinical trials (n=18) | Recruited (n=23) |
| Age, mean (SD) | 53.6 (10.2) | 56.4 (11.0) | 51.3 (9.2) |
| Gender, n (%) | |||
| Female | 35 (85.4) | 14 (77.8) | 21 (91.3) |
| Male | 6 (14.6) | 4 (22.2) | 2 (8.7) |
| Race, n (%)* | |||
| White/Caucasian | 30 (73.2) | 12 (66.7) | 18 (78.3) |
| Black/African American | 9 (21.9) | 4 (22.2) | 5 (21.7) |
| American Indian/Alaskan | 1 (2.4) | 0 (0.0) | 1 (4.3) |
| Asian | 1 (2.4) | 0 (0.0) | 1 (4.3) |
| Other | 2 (4.9) | 1 (5.5) | 1 (4.4) |
| Prefer not to answer | 1 (2.4) | 1 (5.5) | 0 (0.0) |
| Self-reported health information at the time of the interview | |||
| General health ratings, n (%) | |||
| Excellent | 3 (7.3) | 2 (11.1) | 1 (4.3) |
| Very good | 3 (7.3) | 3 (16.7) | 0 (0.0) |
| Good | 14 (34.1) | 7 (38.9) | 7 (30.4) |
| Fair | 15 (36.6) | 5 (27.8) | 10 (43.5) |
| Poor | 6 (14.6) | 1 (5.5) | 5 (21.7) |
| COVID-19 vaccination status, n (%) | |||
| One dose of Pfizer/BioNTech | 2 (4.9) | 0 (0.0) | 2 (8.7) |
| Two doses of Pfizer/BioNTech | 11 (26.8) | 5 (27.8) | 6 (26.1) |
| Two doses of Moderna | 11 (26.8) | 8 (44.4) | 3 (13.0) |
| One dose of Johnson & Johnson | 1 (2.4) | 0 (0.0) | 1 (4.3) |
| Two doses of Pfizer/BioNTech AND a booster shot | 3 (7.3) | 0 (0.0) | 3 (13.0) |
| Two doses of Moderna AND a booster shot | 1 (2.4) | 0 (0.0) | 1 (4.3) |
| No COVID-19 vaccine received | 12 (29.3) | 5 (27.8) | 7 (30.4) |
| Number and duration of symptoms, mean (SD) | |||
| Time since symptoms began (months) | 12.2 (5.9) | 7.7 (2.1) | 15.6 (5.5) |
| Number of symptoms reported per patient | 7 (4.7) | 6 (4.5) | 8 (4.9) |
| Time between hospitalisation due to COVID-19 and interview (months) | |||
| Not hospitalised, n (%)† | 21 (51.2) | 8 (44.4) | 13 (56.5) |
| Mean (SD) | 10.4 (5.0) | 7.0 (6.7) | 13.8 (5.1) |
| Self-reported comorbidities,‡ n (%) | |||
| Hypertension/high blood pressure | 18 (43.9) | 5 (27.8) | 13 (56.5) |
| Arthritis | 16 (39.0) | 7 (38.9) | 9 (39.1) |
| Other§ | 19 (46.3) | 6 (33.3) | 13 (56.5) |
| Asthma | 10 (24.4) | 3 (16.7) | 7 (30.4) |
| Diabetes (type 1, type 2, gestational) | 9 (22.0) | 3 (16.7) | 6 (26.1) |
| Mood disorders (bipolar disorder, cyclothymia, etc) | 7 (17.1) | 2 (11.1) | 5 (21.7) |
| Cardiovascular disease (eg, heart failure, coronary artery) | 5 (12.2) | 0 (0.0) | 5 (21.7) |
| Chronic obstructive pulmonary disease | 5 (12.2) | 2 (11.1) | 3 (13.0) |
| Neurological conditions (eg, Parkinson’s disease) | 5 (12.2) | 1 (5.5) | 4 (17.4) |
| History of stroke | 4 (9.8) | 1 (5.5) | 3 (13.0) |
| Cancer | 2 (4.9) | 2 (11.1) | 0 (0.0) |
| None of the above | 7 (17.1) | 4 (22.2) | 3 (13.0) |
*Patients could select more than one choice to reflect individuals with mixed race.
†Missing data included in calculation of percentages.
‡Patients could select more than one choice.
§Other refers to a comorbidity that was described only once and includes but is not limited to, HIV/AIDS, multiple sclerosis, hypothyroidism, obesity, Hashimoto’s disease.
Clinician interviews
Participating clinicians included nurses and physicians who treated between 10 and 40 patients a week for COVID-19 (online supplemental table S2). All clinicians considered the CDC,1 WHO6 and NICE5 guidelines (of ≥4 weeks after initially experiencing COVID-19 symptoms that cannot be explained by another condition) as the most appropriate timepoint for describing symptoms of long COVID-19, reasoning that they expected symptoms such as a cough to typically resolve after the acute phase of COVID-19 within this time frame.
Clinicians described fatigue, shortness of breath, chest pain/discomfort, cough, and loss of smell and taste as the most common symptoms experienced by patients with long COVID-19. In addition, they emphasised that long COVID-19 may affect any system of the body and that there was a wide variety of other, less common symptoms, such as headache, dizziness and hair loss. One clinician highlighted that many patients received a combination of treatments in relation to their symptoms, and that it became challenging to distinguish between less common symptoms related to long COVID-19 and the side effects of these treatments.
Clinicians reported that cognitive impairment accompanied common and less common symptoms, highlighting several mental health components such as depression, anxiety and feeling vulnerable. Additional impacts on quality of life included loss of appetite, sleep interruptions, lack of physical activity, inability to work and inability to perform activities of daily living (ADLs). Clinicians noted that most patients presented with ongoing symptoms from their initial COVID-19 diagnosis; of these, some presented cyclically. They also reported that a small number of patients who presented with severe respiratory symptoms were hospitalised, but most received care in outpatient settings. During the interviews, clinicians suggested that most symptoms would eventually resolve, though they found it challenging to say exactly when this would happen.
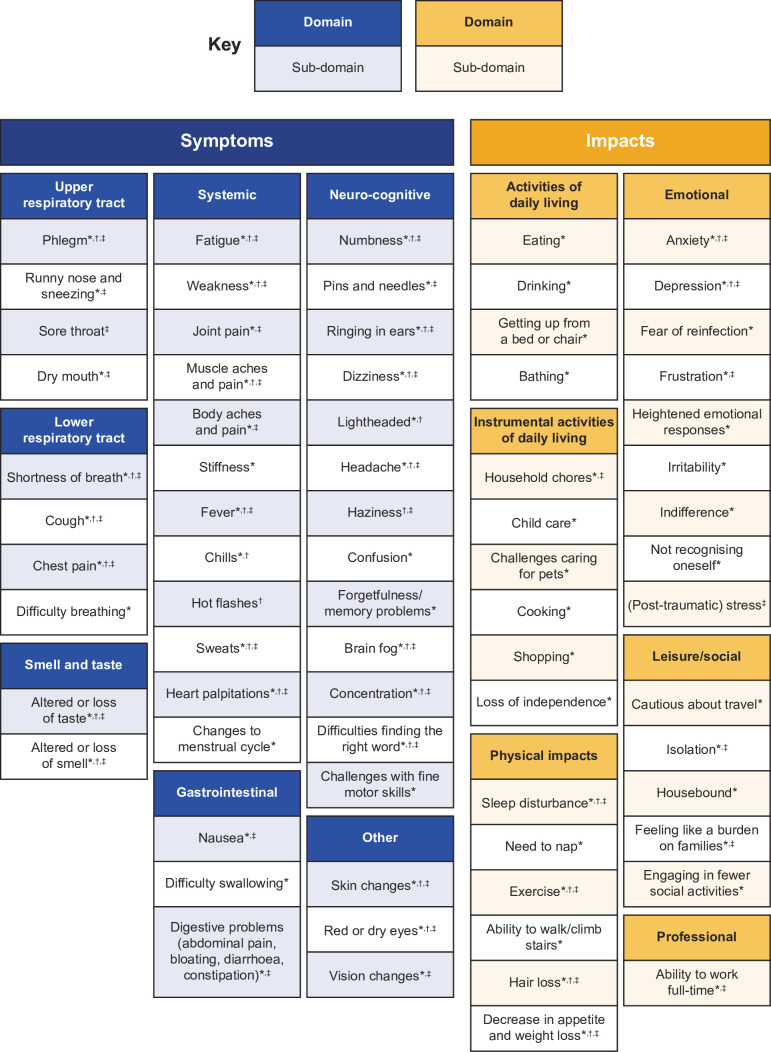
Conceptual model development and saturation
Data from the literature review and clinician/patient interviews yielded two overarching domains: symptoms and impacts. The refined conceptual model is presented in figure 2. Information was added in the conceptual model about the source of concepts included (literature review, clinician interviews and/or patient interviews). All patient-reported concepts included in the model were reported by at least two patients.
Figure 2.
Detailed conceptual model describing the patient experience of long COVID-19. *Supported by patient interviews. †Supported by clinician interviews. ‡Reported in literature.
Of the 41 symptoms presented, 93% were identified in the first 24 interviews (online supplemental table S3). In the remaining group of nine interviews, only two additional subdomains were identified: challenges with fine motor skills and stiffness. Stiffness added granularity to the body aches/pain subdomain previously identified and, with the highly heterogeneous nature of long COVID-19, we can expect that additional symptoms would emerge with additional interviews. This indicated that the current analysis was comprehensive regarding the key symptom domains. For subdomains in the impact domain, all unique subdomains (n=22) were identified in the first three groups of the saturation analysis (online supplemental table S4). No new impacts emerged in the final 16 interviews. This indicated that saturation was achieved for the reported impacts.
Symptoms
Symptoms experienced after the first 4 weeks of acute COVID-19, as reported by patients, were elicited during patient interviews. Symptoms were grouped into seven domains, such as upper respiratory tract, lower respiratory tract, smell and taste, systemic, gastrointestinal, neurocognitive and other. Due to the variation and complexity of symptoms experienced, the domains extended across specific areas of the body (ie, upper respiratory tract) to more general subdomains (ie, weakness or aches in the systemic domain, not exclusive or specific to one area of the body). A selection of quotations covering these symptoms is presented in table 2 to reflect the broad and often debilitating nature of symptoms of long COVID-19 experienced by patients.
Table 2.
Quotations from patients on the symptoms of long-COVID-19
| Concept/domain | Respondent demographics | Quotation |
| Symptom: upper respiratory tract | Female, 50–59 | ‘I would choke on it like a bread, cracker, peanut butter, anything—pudding even, pudding would get stuck. Anything that’s thick it didn’t go down easy. If it was liquidy [stet] I got it to go down. Oatmeal would get stuck. Just like it was stuck there, and I’d be choking on it.’ |
| Symptom: lower respiratory tract | Female, 40–49 | ‘So the coughing was… it felt the same as COVID. It was a dry cough. I couldn’t stop. It’d be like cough and I’d try to talk and here I’d start coughing again.’ |
| Symptom: loss of smell and taste | Female, 40–49 | ‘Now the coffee to me is very sour and I have to use 4–5 sugars for that sourness in my taste buds to go away. I never had a sweet tooth but the coffee itself it tastes burnt to me, I can’t have it black like I used to.’ |
| Symptom: systemic | Female, 50–59 | ‘Like, I literally could sleep all day and just lay there preferably sleeping. It just feels like I weigh 1000 pounds. I have no care for the things that need to get done. I don’t care to eat; I don’t care to work. I don’t care… It’s just a malaise, tiredness, and heaviness.’ |
| Symptom: gastrointestinal | Female, 30–39 | ‘I am having issues with digestion, appetite loss, I will have food that doesn’t get digested well and just goes right through. Or I will have… issues with constipation so that’s varying.’ |
| Symptom: neurocognitive | Female, 50–59 | ‘A fog comes over you and you can’t think what you’re trying to say. It’s just hard to explain. It’s a very weird feeling. You’re sitting there speaking and all of a sudden you can’t comprehend or concentrate to find the right words when you’re trying to speak.’ |
| Female, 50–59 | ‘Very much so, I am dizzy a lot. Just going up and down the stairs. I live on the second floor. I have to hang onto the railing to make sure I am not going to fall. I have to concentrate on walking down the stairs. I have to concentrate on anything that I do that requires movement from me.’ | |
| Female, 30–39 | ‘I’ve had a few severe situations with my memory. For instance, when I first started experiencing it, it’s horrible that you’re used to going and just driving, simple driving and knowing your way. For me being close to home and still forgetting how to get home, not retaining that, because I am kind of new to the area but still it shouldn’t have been an issue.’ | |
| Male, 50–59 | ‘Your recall is not as quick. Me trying to remember something I should remember… It comes back but it just isn’t as quick, you have to wait a little while. It’s not that you can’t remember it’s just that recall is slower.’ | |
| Female, 50–59 | ‘Grabbing things and making sure I have a hold of them properly, otherwise I might not really be holding it… So I’ll be smoking a cigarette and I’ll go to take a puff of the cigarette and be like where did the cigarette go and it’s fallen out of my hand and I didn’t even know it.’ |
The upper respiratory tract domain reflected issues related to the sinuses and throat, and included symptoms such as phlegm, runny nose and sneezing, difficulty swallowing, and dry mouth. Difficulty in swallowing was described by one patient as like choking. The lower respiratory tract domain reflected issues related to the airways and lungs, and the subdomains reflected symptoms such as shortness of breath, cough (including dry cough), difficulty breathing and chest pain. Loss of smell and taste was a commonly reported symptom in patients with long COVID-19. The severity of loss of senses varied widely, with some patients describing a total loss, while others reported that their senses were altered. The systemic domain included various symptoms, which affected the entire body, rather than a single organ or body part, such as fatigue, weakness, heart palpitations, body aches, joint pain, fever, stiffness and chills. Fatigue was specifically described by patients in terms of its severity, whether it improved or not, and how variable the fatigue was. The gastrointestinal symptoms reported were nausea, diarrhoea, vomiting and other stomach-related issues. Many patients experienced neurocognitive symptoms such as brain fog, dizziness, memory problems, difficulties finding the right word and challenges with fine motor skills. Brain fog reflected the challenges patients experienced to focus and stay on task. Neurocognitive symptoms were particularly pertinent for extended periods of times, with some patients reporting symptoms for several months.
Impact on daily life
The overarching domain ‘impacts’ is defined by the activities and health-related quality of life experiences that patients report have been affected because of having long COVID-19. ‘Impacts’ covered six subdomains related to ADLs, instrumental ADL (IADLs), physical impacts, emotional, social/leisure activities and professional impacts. A selection of quotations covering these domains is presented in table 3 to reflect the diverse and inconvenient negative impacts of long COVID-19 experienced by patients.
Table 3.
Quotations from patients on the impacts of long-COVID-19
| Concept/domain | Respondent demographics | Quotation |
| Impact: ADLs | Female, 50–59 | ‘With the lack of energy, the fatigue, the shortness of breath when I am active, simply daily hygiene tasks. It’s physically exhausting to take a shower, so I do wash with soap and water and wash the important parts. I feel gross taking a shower because I am just too exhausted to do it.’ |
| Impact: IADLs | Female, 40–49 | ‘I would say pretty much 90% of my meals have all been take-out since COVID. I’ve hardly cooked anything just because it’s been so difficult.’ |
| Impact: physical | Male, 30–39 | ‘I just couldn’t really go to the gym, I couldn’t do physical activity like that. I couldn’t lift my daughter too much but I would try. But it was a combination of the two that really affected me the most.’ |
| Impact: emotional | Female, 50–59 | ‘It’s hard to put a label on it because it’s almost like a loss of sense of self. That’s the best way I can put it into words. It’s overwhelming, like feeling of loss and depression where it’s not getting any better. Before, I had depression level come and go and now it’s just staying with me.’ |
| Impact: social and leisure activities | Female, 30–39 | ‘So things like I used to… be very active in my church. I used to go every week multiple times, different events. I used to go out and hang out with friends. And those are things that I can no longer do. Not because of risk of catching COVID or something like that. But just because I physically don’t have the ability.’ |
| Impact: professional | Female, 50–59 | ‘And like I said my job, I was going to attempt to work from home but my supervisor tells me oh everyone is back in the office now. I am not ready to get dressed in office clothes and drive to work and be on time. My extra income is just not… there. And it’s hard. I’ve tried to find jobs that I can when I feel okay, but nobody is trying to do that.’ |
ADLs, activities of daily living; IADLs, instrumental ADLs.
Quotations were categorised into ADLs if they referred to activities related to eating, drinking, getting up from a bed or chair, climbing stairs and routines of self-care (eg, bathing). IADLs included activities related to household chores, taking care of children or pets, shopping and meal preparation. The physical impacts domain included subdomains related to concepts associated with physical changes, such as changes to appetite and weight loss, hair loss and sleep disruption. It also included impacts on the ability to perform physical tasks such as walking and exercising. The emotional domain included subdomains associated with changes in the patient’s psyche and mood in general, including issues associated with various worries and anxieties of having long COVID-19, depression, irritability, frustration, heightened emotional responses and not recognising oneself any longer. Activities related to social life, hobbies and leisure activities as well as sports and exercise were categorised under social and leisure activities. The professional domain captured changes in the patient’s ability to work.
Discussion
There is limited information on the patient experience of living with long COVID-19, and to our knowledge, there is no current conceptual model that provides a visual representation of the symptoms and impacts of the disease. This qualitative study provides novel insight into the conceptualisation of the patient experience of long COVID-19. These rich patient qualitative insights are a useful resource to guide support and treatment requirements of those with long COVID-19.40
Following in-depth patient and physician interviews, we summarised the data captured in a simple and clinically grounded conceptual model comprising upper and lower respiratory symptoms, systemic symptoms, gastrointestinal symptoms, neurocognitive symptoms, altered or loss of smell and taste, and other symptoms. These symptoms were consistent with those reported by national and international health bodies such as the CDC,1 WHO6 and NICE,5 and by systematic reviews.27 41 In addition to headache, dizziness and lightheadedness, which were also identified in the acute COVID-19 conceptual model,24 the long COVID-19 conceptual model included various neurocognitive symptoms such as numbness, ringing in ears, haziness, confusion, forgetfulness/memory problems, brain fog, concentration, difficulties finding the right word and challenges with fine motor skills, and some of these symptoms were reported to be experienced for several months. Furthermore, while emotional concepts such as anxiety, depression, irritability and frustration were presented in the acute COVID-19 conceptual model,24 in the patients with long COVID-19 additional concepts included fear of reinfection, heightened emotional responses, indifference, not recognising oneself and post-traumatic stress. At the time our study was initiated, no instruments were available to assess the impact of long COVID-19. Subsequently several instruments are now available. The modified COVID-19 Yorkshire Rehabilitation Scale, Symptom Burden Questionnaire for Long Covid and postacute (long) COVID-19 quality of life instrument each assess symptom burden in long COVID-19, but are not comprehensive in all symptoms identified in our model; particularly neurocognitive symptoms.42–44 However, a handful of qualitative studies have demonstrated that patients with long COVID-19 report a very low state of mind and felt that their self-identity was affected, findings that are consistent with our own.21 45 Neurorehabilitation programmes for patients with long COVID-19 have proved helpful to improve working memory, verbal fluency and anxious-depressive symptomatology.46 Our study further highlights the importance of understanding the impact these neurocognitive symptoms, which are not often reported during acute disease, can have on patients’ emotional, social and professional lives.
In addition to symptoms, we also captured the impact of long COVID-19 and the changes in the daily lives of affected patients. The range of impacts due to long COVID-19 was consistent with those experienced during acute disease;24 however, this study highlighted the emphasis on long-term impacts. A reduction in the number and/or severity of symptoms may mitigate the negative impact of long COVID-19 on patient health-related quality of life. Studies in populations with acute COVID-19 have shown that improvements in symptoms are correlated with improvements in outcomes and patient quality of life. However, additional qualitative and quantitative research is required to address this current knowledge gap, and further explore how management of symptoms in long COVID-19 could improve patients’ lives.
Our study has two main limitations. First, the patients who were recruited from the external healthcare research firms closely matched the inclusion and exclusion criteria from the clinical trials. This approach may have resulted in a missed profile of patients with long COVID-19 who may be experiencing symptoms differently. Additional interviews with a broader exclusion and inclusion criteria could be considered as future research to determine whether they experience symptoms differently. Second, most of the patients in the present study were female and White. Nevertheless, this is consistent with other studies where the persistence of long COVID-19 symptoms for ≥12 weeks was higher in females than males.47 There have also been reports on differences in prevalence of long COVID-19 symptoms in different ethnicities. One study that used data from community-based samples (>600 000) reported that an Asian population had a lower risk of persistent symptoms compared with a white population.47 However, in other studies, black Afro-Caribbean ethnic groups and other minority ethnic groups (native American, Middle Eastern or Polynesian) had a higher risk of long COVID-19 compared with a white population.12 Additional studies are needed in different ethnic groups to fully understand the presentation and impact of long COVID-19 in different populations.
The present qualitative study provides unique and valuable insights on symptoms in patients with confirmed diagnoses of long COVID-19 and how it impacts their daily lives, including physical, emotional and psychological functioning. We found that patients typically experienced symptoms across of number of clinical domains during long COVID-19. Improving our understanding of the patient experience of the disease will help healthcare providers make informed decisions on optimised treatment options and the support needed for patients to improve their overall health-related quality of life, while also easing the burden on patient’s families and society.45 Although no obvious symptomatic profiles were found during the interviews with patients, the heterogeneity of the symptom profiles should be further explored in longitudinal studies to aid in understanding any patterns in onset of symptoms, progression and possible long-term implications.
Conclusion
Our qualitative research reveals that long COVID-19 impacts all aspects of patients’ daily life, particularly neurocognitive and mental health issues. To the best of our knowledge, this is the first study to report a conceptual model of long COVID-19 with neurocognitive and emotional concepts, based on empirical evidence from patient and clinician interviews. The model highlights, from a patient perspective, symptoms and impacts associated with long COVID-19, all of which showed significant negative effects on patient health-related quality of life.
Supplementary Material
Acknowledgments
We thank the study participants, their families and the clinicians involved in this trial. The authors also thank Prime, Knutsford, UK for manuscript formatting and copyediting suggestions.
Footnotes
Contributors: DR contributed to conceptualisation, funding acquisition, methodology, project administration, supervision, visualisation and writing (original draft, review and editing). SS-K contributed to conceptualisation, supervision and writing (review and editing). JYC contributed to interview conduct, data curation, formal analysis, project administration, visualisation and writing (review and editing). KP contributed to interview conduct, data curation, formal analysis, project administration, supervision, visualisation and writing (review and editing). YZ contributed to operations and writing (review and editing). MH contributed to conceptualisation, supervision and writing (review and editing). TDN contributed to operations and writing (review and editing). AJP contributed to supervision and writing (review and editing). EM contributed to supervision and writing (review and editing). GPG contributed to conceptualisation, supervision and writing (review and editing). DR as the guarantor of the submitted work.
Funding: This study was funded by Regeneron Pharmaceuticals, Inc. Certain aspects of this project have been funded in whole or in part with federal funds from the Department of Health and Human Services; Office of the Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under OT number: HHSO100201700020C.
Competing interests: DR is a Regeneron Pharmaceuticals, Inc. employee/stockholder and former Roche employee, and current stockholder. SS-K, YZ, MH, TDN and GPG are employees/stockholders at Regeneron Pharmaceuticals, Inc. JYC and KP are employees of Modus Outcomes, and consulted for Regeneron Pharmaceuticals, Inc. AJP reported receiving personal fees from Regeneron Pharmaceuticals, Inc. during the conduct of the study, personal fees from Imvaria, Boehringer Ingelheim, EBSCO/Dynamed and Roche outside the submitted work. EM reports payments to his institution received from SciClone Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Pfizer, Chemic Labs/KODA Therapeutics, Cidara and Leidos Biomedical Research/NCI; and reports Advisory board: Basilea and grants from NIH/NIAID, NIH/NIGMS and BARDA.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All study documents (the protocol, interview guides, demographic and health information form, screener form and informed consent form) were approved by the Western Institutional Review Board-Copernicus Group Independent Review Board (IRB) before study initiation (IRB tracking # 20214272). Electronic informed consent was obtained from all patients. Patients were paid a fee in line with fair market value to cover the time taken to participate in the study.
References
- 1.Centers for Disease Control and Prevention . Long COVID or post-COVID conditions. Available: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html [Accessed 24 Sep 2022].
- 2.Alghamdi F, Owen R, Ashton REM, et al. Post-acute COVID syndrome (long COVID): what should radiographers know and the potential impact for imaging services. Radiography (Lond) 2022;28 Suppl 1:S93–9. 10.1016/j.radi.2022.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bierle DM, Aakre CA, Grach SL, et al. Central sensitization phenotypes in post acute sequelae of SARS-Cov-2 infection (PASC): defining the post COVID syndrome. J Prim Care Community Health 2021;12:21501327211030826. 10.1177/21501327211030826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr 2021;15:869–75. 10.1016/j.dsx.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing the long-term effects of COVID-19. 2020. Available: https://www.nice.org.uk/guidance/ng188 [Accessed 24 Sep 2022]. [PubMed]
- 6.World Health Organization . Coronavirus disease (COVID-19): post COVID-19 condition. 2021. Available: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition [Accessed 26 Sep 2022].
- 7.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021;4:e210830. 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health 2021;6:e005427. 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazé ABC, Cerqueira-Silva T, Bomfim AP, et al. Prevalence and risk factors for long COVID after mild disease: a longitudinal study with a symptomatic control group. medRxiv 2022. 10.1101/2022.09.15.22279958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 2022;28:1706–14. 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. medRxiv 2022. 10.1101/2022.08.09.22278592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo SM, Liu TC, Motwani Y, et al. Factors associated with post-acute sequelae of SARS-Cov-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med 2022;37:1988–95. 10.1007/s11606-022-07523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowe B, Xie Y, Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat Med 2023;29:2347–57. 10.1038/s41591-023-02521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One 2021;16:e0254523. 10.1371/journal.pone.0254523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler DM. The costs of long COVID. JAMA Health Forum 2022;3:e221809. 10.1001/jamahealthforum.2022.1809 [DOI] [PubMed] [Google Scholar]
- 19.Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA 2020;324:1495–6. 10.1001/jama.2020.19759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuzuki S, Miyazato Y, Terada M, et al. Impact of long-COVID on health-related quality of life in Japanese COVID-19 patients. Health Qual Life Outcomes 2022;20:125. 10.1186/s12955-022-02033-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open 2022;12:e050979. 10.1136/bmjopen-2021-050979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . Caring for people with post-COVID conditions. Available: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/care-post-covid.html [Accessed 02 Oct 2022].
- 23.Wong AW, Shah AS, Johnston JC, et al. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J 2020;56:2003276. 10.1183/13993003.03276-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rofail D, McGale N, Podolanczuk AJ, et al. Patient experience of symptoms and impacts of COVID-19: a qualitative investigation with symptomatic outpatients. BMJ Open 2022;12:e055989. 10.1136/bmjopen-2021-055989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somersan-Karakaya S, Mylonakis E, Menon VP, et al. Casirivimab and Imdevimab for the treatment of hospitalized patients with COVID-19. J Infect Dis 2022;227:23–34. 10.1093/infdis/jiac320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021;385:e81. 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021;11:16144. 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyrick J. What is good qualitative research? A first step towards a comprehensive approach to judging rigour/quality. J Health Psychol 2006;11:799–808. 10.1177/1359105306066643 [DOI] [PubMed] [Google Scholar]
- 29.Morse JM. The significance of saturation. Qual Health Res 1995;5:147–9. 10.1177/104973239500500201 [DOI] [Google Scholar]
- 30.Rothman M, Burke L, Erickson P, et al. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR good research practices for evaluating and documenting content validity for the use of existing instruments and their modification PRO task force report. Value Health 2009;12:1075–83. 10.1111/j.1524-4733.2009.00603.x [DOI] [PubMed] [Google Scholar]
- 31.Leidy NK, Vernon M. Perspectives on patient-reported outcomes: content validity and qualitative research in a changing clinical trial environment. Pharmacoeconomics 2008;26:363–70. 10.2165/00019053-200826050-00002 [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration . Patient-reported outcome measures: use in medical product development to support labeling claims (guidance for industry). 2009. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims [Accessed 26 Sep 2022]. [DOI] [PMC free article] [PubMed]
- 33.US Food and Drug Administration . Patient-focused drug development: methods to identify what is important to patients. 2022. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients [Accessed 27 Sep 2022].
- 34.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 35.Bowling A. Research methods in health: investigating health and health services. 3rd ed. Maidenhead: Open University Press, 2009. [Google Scholar]
- 36.Bryman A, Burgess B. Analyzing qualitative data. New York: Routledge, 2002. [Google Scholar]
- 37.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2006;27:237–46. 10.1177/1098214005283748 [DOI] [Google Scholar]
- 38.Fabbrocini G, Cacciapuoti S, Monfrecola G. A qualitative investigation of the impact of Acne on health-related quality of life (HRQL): development of a conceptual model. Dermatol Ther (Heidelb) 2018;8:85–99. 10.1007/s13555-018-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klassen AF, Pusic AL, Scott A, et al. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health 2009;9:11. 10.1186/1472-6874-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration . CDER patient-focused drug development. 2023. Available: https://content.govdelivery.com/accounts/USFDA/bulletins/34aabd8 [Accessed 30 Mar 2023].
- 41.Han Q, Zheng B, Daines L, et al. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022;11:269. 10.3390/pathogens11020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes SE, Haroon S, Subramanian A, et al. Development and validation of the symptom burden questionnaire for long Covid (SBQ-LC): Rasch analysis. BMJ 2022;377:e070230. 10.1136/bmj-2022-070230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivan M, Preston N, Parkin A, et al. The modified COVID-19 yorkshire rehabilitation scale (C19-YRSm) patient-reported outcome measure for long Covid or post-COVID-19 syndrome. J Med Virol 2022;94:4253–64. 10.1002/jmv.27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jandhyala R. Design, validation and implementation of the post-acute (long) COVID-19 quality of life (PAC-19Qol) instrument. Health Qual Life Outcomes 2021;19:229. 10.1186/s12955-021-01862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samper-Pardo M, Oliván-Blázquez B, Magallón-Botaya R, et al. The emotional well-being of long COVID patients in relation to their symptoms, social support and stigmatization in social and health services: a qualitative study. BMC Psychiatry 2023;23:68. 10.1186/s12888-022-04497-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Molina A, García-Carmona S, Espiña-Bou M, et al. Neuropsychological rehabilitation for post-COVID-19 syndrome: results of a clinical programme and six-month follow up. Neurologia (Engl Ed) 2022. 10.1016/j.nrleng.2022.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun 2022;13:1957. 10.1038/s41467-022-29521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-076992supp001.pdf (7.6MB, pdf)
Data Availability Statement
No data are available.