Abstract
Background
Paraneoplastic pemphigus (PNP), also called paraneoplastic autoimmune multiorgan syndrome (PAMS), is a rare autoimmune disease with mucocutaneous and multi-organ involvement. PNP/PAMS is typically associated with lymphoproliferative or haematological malignancies, and less frequently with solid malignancies. The mortality rate of PNP/PAMS is elevated owing to the increased risk of severe infections and disease-associated complications, such as bronchiolitis obliterans.
Objectives
These guidelines summarise evidence-based and expert-based recommendations (S2k level) for the clinical characterization, diagnosis, and management of PNP/PAMS. They have been initiated by the Task Force Autoimmune Blistering Diseases of the European Academy of Dermatology and Venereology with the contribution of physicians from all relevant disciplines. The degree of consent among all task force members was included.
Results
Chronic severe mucositis and polymorphic skin lesions are clue clinical characteristics of PNP/PAMS. A complete assessment of the patient with suspected PNP/PAMS, requiring histopathological study and immunopathological investigations, including direct and indirect immunofluorescence, ELISA and, where available, immunoblotting/immunoprecipitation, is recommended to achieve a diagnosis of PNP/PAMS. Detection of anti-envoplakin antibodies and/or circulating antibodies binding to the rat bladder epithelium at indirect immunofluorescence is the most specific tool for the diagnosis of PNP/PAMS in a patient with compatible clinical and anamnestic features. Treatment of PNP/PAMS is highly challenging. Systemic steroids up to 1.5 mg/kg/day are recommended as first line option. Rituximab is also recommended in patients with PNP/PAMS secondary to lymphoproliferative conditions but might also be considered in cases of PNP/PAMS associated with solid tumours. A multidisciplinary approach involving pneumologists, ophthalmologists and onco-haematologists is recommended for optimal management of the patients.
Conclusions
These are the first European guidelines for the diagnosis and management of PNP/PAMS. Diagnostic criteria and therapeutic recommendations will require further validation by prospective studies.
Keywords: paraneoplastic pemphigus, paraneoplastic autoimmune multiorgan syndrome, haematological malignancies, Castleman disease, rituximab
INTRODUCTION
Paraneoplastic pemphigus (PNP), also called paraneoplastic autoimmune multiorgan syndrome (PAMS) is a potentially life-threatening autoimmune disease with mucocutaneous and multi-organ involvement typically associated with lymphoproliferative or haematological malignancies1–5. It is a very rare disease and there is little data allowing an estimation of its incidence and prevalence6. Based on published reports, one may nevertheless estimate that the incidence of PNP/PAMS is less than one new case per million inhabitants per year. Although there has been so far no consensus on and validation of diagnostic criteria for PNP/PAMS, most patients with PNP/PAMS show the following characteristics: (1) severe chronic stomatitis with multi-site mucosal involvement accompanied by variable cutaneous lesions; (2) association with an underlying neoplasm, which is either known at time of diagnosis of PNP/PAMS or is subsequently detected; (3) histopathologically, a variable combination of intraepithelial acantholysis, keratinocyte necrosis, vacuolar interface dermatitis and/or subepidermal blistering; (4) deposits of immunoreactants (IgG and/or C3) on the membrane of keratinocytes as well as along the epidermal and/or epithelial basement membrane zone (BMZ) by direct immunofluorescence (DIF) microscopy; (5) reactivity with rat bladder transitional epithelia by indirect immunofluorescence (IIF) studies; (6) binding to a variable set of autoantigens, including members of the plakin family, as detected by either immunoprecipitation, immunoblotting or ELISA1, 7–13.
Initially, a complex of 5 antigens with molecular weights of 250, 230, 210, 190 and 170 kDa was detected by immunoprecipitation from radiolabeled keratinocyte extracts in the sera of patients, as reported by Anhalt et al1. Subsequent studies demonstrated that PNP/PAMS sera typically react with members of the plakin family of proteins, most often with envoplakin10, 13–15, periplakin8, 10, 13, desmoplakin I and II10, 11, and, less frequently, with BP23010, plectin10, 16, and epiplakin17. Furthermore, binding to different cadherins, such as desmoglein 1 (Dsg1) and 3 (Dsg3)9, desmocollin 1 (Dsc1), 2 (Dsc2) and 3 (Dsc3)18 is also variably found. Up to 70% of PNP/PAMS sera show reactivity to α−2-macroglobulin-like protein 1 (A2ML1), initially described as the p170 kDa antigen12, 19 and most recently, transglutaminase 1 has been reported as target antigen20.
In 2001, Nguyen et al. proposed the acronym PAMS to emphasize its potential multi-organ involvement and its polymorphic mucocutaneous features3. According to these authors, the use of the term “PNP” might be misleading since patients with pemphigus vulgaris associated with underlying malignancy could be incorrectly diagnosed with “PNP” despite a very different immunologic profile and prognosis. They concluded that PNP would have been more properly regarded as a single pemphigus-like mucocutaneous phenotype of PAMS21, 22.
There is direct and indirect evidence indicating that both humoral and cell-mediated autoimmune responses are involved in the pathogenesis of PNP/PAMS. These autoreactive responses are mainly directed against components of adhesion complexes and of the BMZ of different stratified epithelia2, 3.
Lymphoproliferative and other haematological malignancies are the most frequently and characteristically associated neoplasms23–26. There are however racial and ethnic variations in the frequency of distinct neoplasms associated with PNP/PAMS. For example, Castleman disease, which has been observed in up to 56% of PNP/PAMS patients, is more frequent in Asian countries, such as Korea and China27–29. Castleman disease seems to be the most frequent tumor in children and adolescents with PNP/PAMS30. Solid tumors have been found in 14.8%−17% of PNP/PAMS patients23, 24. They can have epithelial or mesenchymal origin in about 9% and 6% of the cases, respectively24, 31–35. PNP/PAMS may rarely be triggered or exacerbated by either certain chemotherapy drugs (e.g. fludarabin, bendamustine and cyclophosphamide)36–39 or by radiotherapy40. Few cases of PNP/PAMS have been diagnosed in the absence of an underlying malignancy41–43. Accordingly, PNP/PAMS might rarely be a marker for occult malignancy, thus requiring an extended clinical follow-up44.
The mortality rate of PNP/PAMS is high. While in a first review by Anhalt et al45, 90% of 33 PNP/PAMS patients died within two years after diagnosis, a French multicenter retrospective study encompassing 53 PNP/PAMS patients showed a lower case‐fatality rate, with a one-year and 5-year overall survival rate of 49 % and 38%, respectively46. In the latter study, the main cause of death was severe infection due to the immunosuppressive treatment, followed by bronchiolitis obliterans-related respiratory failure and progression of the underlying malignancy46. Patients with erythema multiforme-like skin lesions and keratinocyte necrosis on histology, especially when associated with extensive skin and/or mucosal lesions at presentation were at higher risk for having a more severe and rapidly fatal outcome in the above-mentioned multicenter study46. A systematic review of 144 patients with PNP/PAMS associated with haematologic malignancies also found that patients with toxic epidermal necrolysis-like features and bronchiolitis obliterans have a poor prognosis47. Despite the strong association with malignancy, treatment of the underlying neoplasia rarely has a favorable impact on the clinical course of PNP/PAMS48, 49. However, in patients with an underlying resectable tumor, curative surgery may result in remission in up to half of patients29, 50, 51.
METHODS
Development of the guideline
The aim of this project was to standardize diagnostics and therapy of PNP/PAMS with support of the European Academy of Dermatology and Venereology (EADV).
A working group composed of 54 European and non-European experts was appointed by the EADV Task Force “Autoimmune Blistering Diseases” to develop a consensus-based (S2k) guideline following the directions of the Association of the Scientific Medical Societies in Germany (AWMF; https://www.awmf.org/en/clinical-practice-guidelines/awmf-guidance/cpg-development.html).
One member was onco-haematologist (F. D’Amore), one member was both dermatologist and oral medicine specialist (J. Setterfield) and all other members were dermatologists.
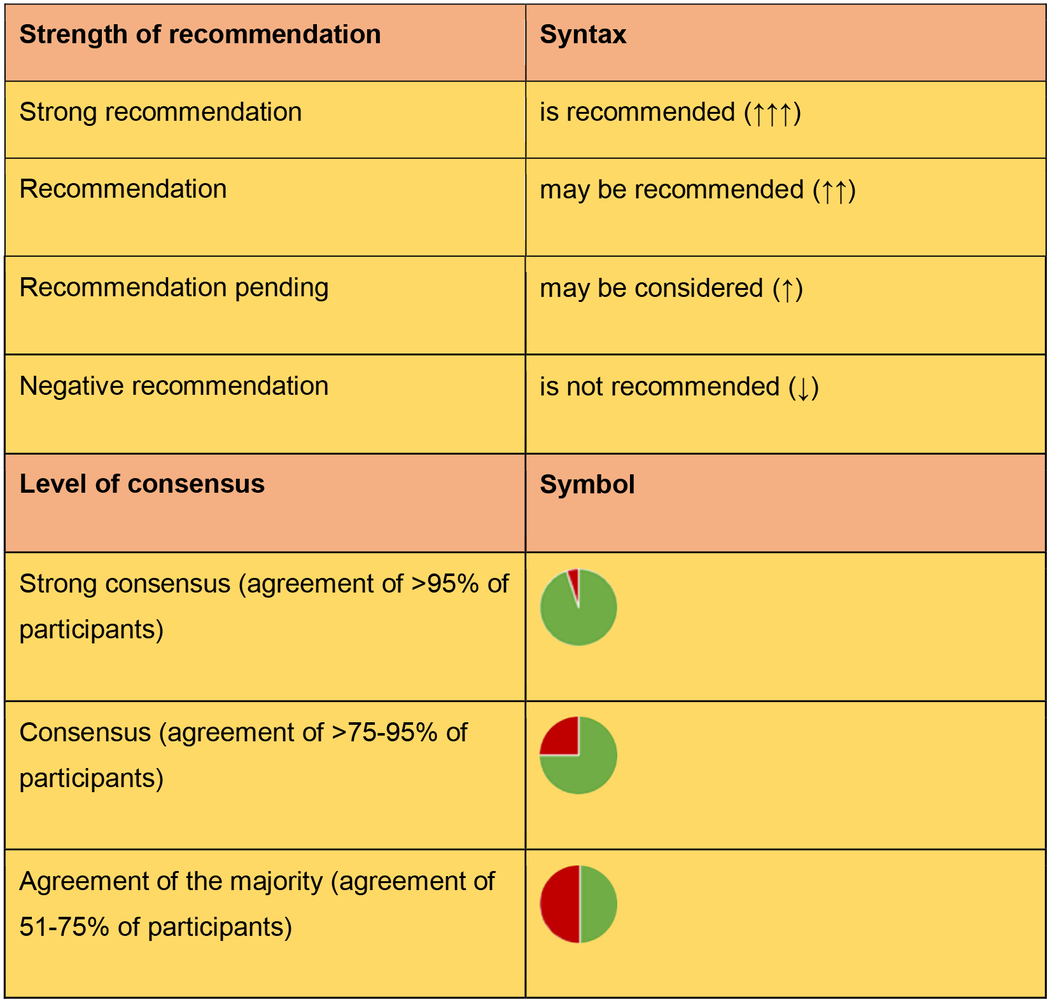
The writing group, i.e. R.B., E.A., R.M, G.G., A.V.M, L.B., J.M., wrote the first draft of the present guidelines. Recommendations were voted upon by the members of the working group with three possible options, i.e. „for”, „against”, „abstention”. Recommendations that reached a consensus of less than 50% were rephrased and voted again. Thereafter, the other members of the EADV Task Force “Autoimmune Blistering Diseases” reviewed the guideline draft and voted on each recommendation. Strength and agreement for each recommendation were expressed in a standardized form detailed in Table 1.
Table1.
Strength of recommendation and levels of consensus in these guidelines
|
DIAGNOSTIC APPROACH
The diagnosis of PNP/PAMS relies on a combination of clinical and immunopathological criteria with a number of steps and procedures summarized below. Additional specific diagnostic immunological tests are sometimes required.
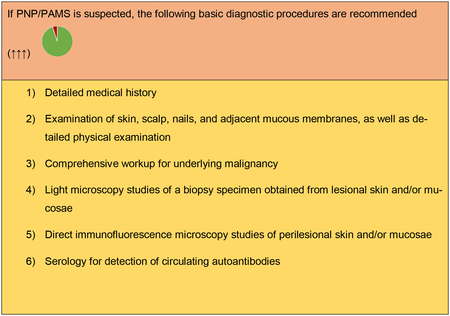
Medical history

Physical examination

Cutaneous and adnexal manifestations
The spectrum of mucocutaneous lesions in PNP/PAMS is broad and polymorphic. In a retrospective study on 104 patients, two-thirds had skin lesions in addition to mucosal lesions24. Cutaneous manifestations of PNP/PAMS have been classified into 5 major types: (i) pemphigus-like lesions; (ii) pemphigoid-like lesions; (iii) lichen planus-like lesions; (iv) erythema multiforme-like lesions; (v) graft versus host disease-like lesions3.
Pemphigus-like phenotype is characterized by flaccid blisters, erosions and erythematous lesions of variable severity and extent, which may affect the seborrheic areas and the trunk, or being widespread. Less frequently, a pemphigoid-like pattern is observed with either localized or widespread serous-haemorrhagic tense blisters with urticarial or eczematous lesions. In a substantial number of patients, a variably severe lichenoid reaction is observed. Lichen planus-like lesions comprise intensely itchy, violaceous, polygonal, flat-topped papules and plaques on the trunk, neck, and extremities. In another group of patients, erythema multiforme-like lesions predominate with erythematous targetoid lesions with sometimes a central vesicle or blister. The lesions often develop on the trunk and extremities. Interestingly, lichen planus-like lesions are more commonly seen in PNP/PAMS associated with Castleman disease and patients with bronchiolitis obliterans29. These lesions may be localized or become widespread, resulting in either a Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN)-like phenotype in the most severe cases. Finally, a graft-versus-host disease-like presentation consisting of lichenoid skin lesions and erosive mucositis has been observed. In a few PNP/PAMS patients, pustular or dyshidrosis-like lesions have been described52, 53. In presence of skin involvement, the scalp is often spared, while palmoplantar regions are frequently affected. Palmoplantar involvement with presence of discrete lichenoid lesions is a useful clue to differentiate PNP/PAMS from PV21. Nail involvement in patients with PNP/PAMS can lead to nail scarring and anonychia, resembling changes observed in lichen planus, TEN or epidermolysis bullosa54.
Oral manifestations
PNP/PAMS typically presents with severe erosive oral mucositis. Nearly all patients present with oral lesions. In contrast to most patients with PV, PNP/PAMS typically involves the entire oral cavity (panstomatitis). Lips tend to be haemorrhagic (more akin to severe EM or SJS) and often the vermillion is affected. Panstomatitis may take on very hyperplastic features with excess tissue and many folds4. The clinical aspect of oral lesions may range from erythema, lichenoid reticular and erosive lesions to diffuse painful haemorrhagic stomatitis involving the lips, tongue, cheeks and gingivae, or the entire oral cavity55. Oral involvement occurs usually early and is typically treatment-resistant56. Rarely, PNP/PAMS may present with a single oral lesion57. Odynophagia and dysphagia may be responsible for major malnutrition requiring nasoenteric tube or gastrostomy and nutritional support, contributing to an unfavourable prognosis. Lesions may extend to the nasal mucosa, pharynx, larynx or oesophagus57.
Ocular manifestations
Ocular involvement has been demonstrated in approximately 40% of cases from a large case series of 104 PNP/PAMS patients24. Conjunctival hyperaemia and erosions, which may ultimately lead to scarring and symblepharon formation may closely resemble those observed in mucous membrane pemphigoid58, 59. Pseudomembranous conjunctivitis, bilateral corneal ulcerations, forniceal shortening, and thickening of the palpebral margin may also be found60. Burning and pain, mucous discharge, and decreased visual acuity are the most frequent ocular symptoms. Ocular lesions may be present at the disease onset61. Early ophthalmological assessment is therefore recommended62. In one report, histopathologic and direct IF microscopy findings in ocular PNP/PAMS were similar to those found in PV62.

Anogenital mucosae
Involvement of the anogenital mucosae with presence of erosions and ulcers as well as lichenoid changes is often found24, 25. In the retrospective study of 104 PNP/PAMS patients, 28 of 79 patients (35%) had genital lesions24. In the latter study, there was a positive correlation between anti-Dsg3 reactivity and presence of genital lesions24. In a cohort of 32 children with Castleman disease-associated PNP/PAMS, genital lesions were present in 62% of the cases63.
Involvement of other organs
Bronchopulmonary system
The respiratory tract is frequently affected in PNP/PAMS with an involvement rate reported between 30% and 90% of cases22. Patients may develop progressive dyspnoea due to obstructive lung disease and bronchiolitis obliterans. The latter may ultimately lead to respiratory failure and severe hypoxia. Bronchiolitis obliterans is one of the leading causes of death in PNP/PAMS patients64. In the retrospective series of Ohzono et al. bronchiolitis obliterans was the cause of death in 40% of the 40 cases with fatal outcome24. Bronchiolitis obliterans manifests as an obstructive and/or restrictive lung disease. There is damage and shedding of the epithelium of the large airways and alveolar sacs, resulting in occlusion of terminal alveoli. Irreversible fibrosis and bronchiectasis are observed. IgG deposits are found on the bronchial epithelium in vivo and in autopsy specimens2, 65. Pulmonary involvement, and, specifically, bronchiolitis obliterans appear to be more frequently found in patients with an associated Castleman disease as well as in paediatric patients49, 66–69, and those presented with lichen planus-like lesions29. It has been reported that distinct autoantibody reactivities such as anti-epiplakin or anti-Dsg1 antibodies correlates with the presence of bronchiolitis obliterans17, 24. Nevertheless, the exact underlying pathomechanisms of bronchiolitis obliterans, which likely involve a cytotoxic T cell response, need to be further characterized70. It is of note that bronchiolitis obliterans may occur later on during the disease course; accordingly, in case of new symptoms suggesting pulmonary involvement, patients should be referred to the pneumologist.

Gastrointestinal tract
Besides the oral mucosa, PNP/PAMS may involve the upper and lower gastrointestinal tract even in absence of overt gastrointestinal symptoms71, 72. Miida et al. reported a case of PNP/PAMS with multifocal erosions in colonic mucosa and linear deposition of C3 along the colonic epithelial basement membrane71. Another study did not detect immunodeposits on gastrointestinal epithelia in the studied PNP/PAMS patients3.
Other organs
Other potentially affected organs include the thyroid gland, kidneys, and smooth muscle tissue although involvement of these organs is most likely due to associated diseases such as autoimmune thyroid disease and myasthenia gravis73. Myasthenia gravis with varying degrees of skeletal muscle weakness is also typically observed in PNP/PAMS, not only in those with thymoma but also with Castleman disease and other74. In the case of thymoma, clinical recovery from both PNP/PAMS and myasthenia gravis may be observed after radical thymectomy, resulting in a decline of the circulating antibodies against acetylcholine receptors and PNP/PAMS autoantibodies75.
Clinical, laboratory and instrumental assessment for the underlying neoplasm
In patients with suspected PNP/PAMS, the neoplasm can be present before the occurrence of PNP/PAMS symptoms. However, if the patient has not already received a diagnosis of neoplasm (or in the very rare case that a second neoplasm is suspected), an oncological screening based on the following recommendations is needed.

Histology
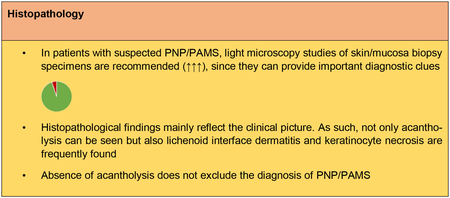
Light microscopic studies of a biopsy specimen of affected skin and/or mucosa are important and may provide useful diagnostic clues for PNP/PAMS. Biopsy specimens should ideally be obtained from an early lesion or from both lesional and perilesional skin/mucosa. The spectrum of histopathological features observed in PNP/PAMS is heterogeneous and broad, reflecting the high clinical polymorphism of the disorder1. Epidermal changes include suprabasal acantholysis, dyskeratotic keratinocytes, vacuolar change of the basal keratinocytes, keratinocyte necrosis and epidermal exocytosis of inflammatory cells of variable severity76. In some cases, keratinocyte necrosis is extensive and may cause full-thickness epidermal necrosis77, 78. A band-like lymphocytic lichenoid infiltrate at the BMZ is also observed with or without plasma cells. Subepithelial/subepidermal blister formation may also be found. Typically, multiple histologic patterns are observed in the same patient47, although acantholysis, keratinocyte necrosis and lichenoid dermatitis appear to be the most common changes47, 63, 79. The latter features are highly suggestive for PNP/PAMS, but have low diagnostic sensitivity77. Proper interpretation of histopathological findings should always consider patient’s clinical history. For example, presence of a lichenoid mucositis/dermatitis without acantholysis in a patient with concomitant neoplasm should raise the possibility of PNP/PAMS80.
Direct immunofluorescence microscopy
DIF studies of perilesional skin/mucosa from most PNP/PAMS patients show intercellular deposits of IgG and/or C3 in a so called “net-like” or “chicken wire” staining pattern within the epidermis77. Moreover, linear or granular deposits of IgG and/or C3 along the BMZ may also be found77. The deposits of immunoreactants may be found only focally and their staining intensity is variable1. The combination of intercellular and linear/granular deposits along the epidermal-epithelial BMZ of IgG and/or C3 (Fig. 2) was found in one study to be 97% specific for the diagnosis of PNP/PAMS81. However, this combined pattern is usually found in less than half of PNP/PAMS patients and has thus a relatively poor sensitivity (27–41%)22, 77, 81. This staining pattern is also rarely observed in distinct forms of pemphigus (such as in pemphigus erythematosus) and in pemphigus cases occurring in combination with either BP or cutaneous lupus erythematosus81.
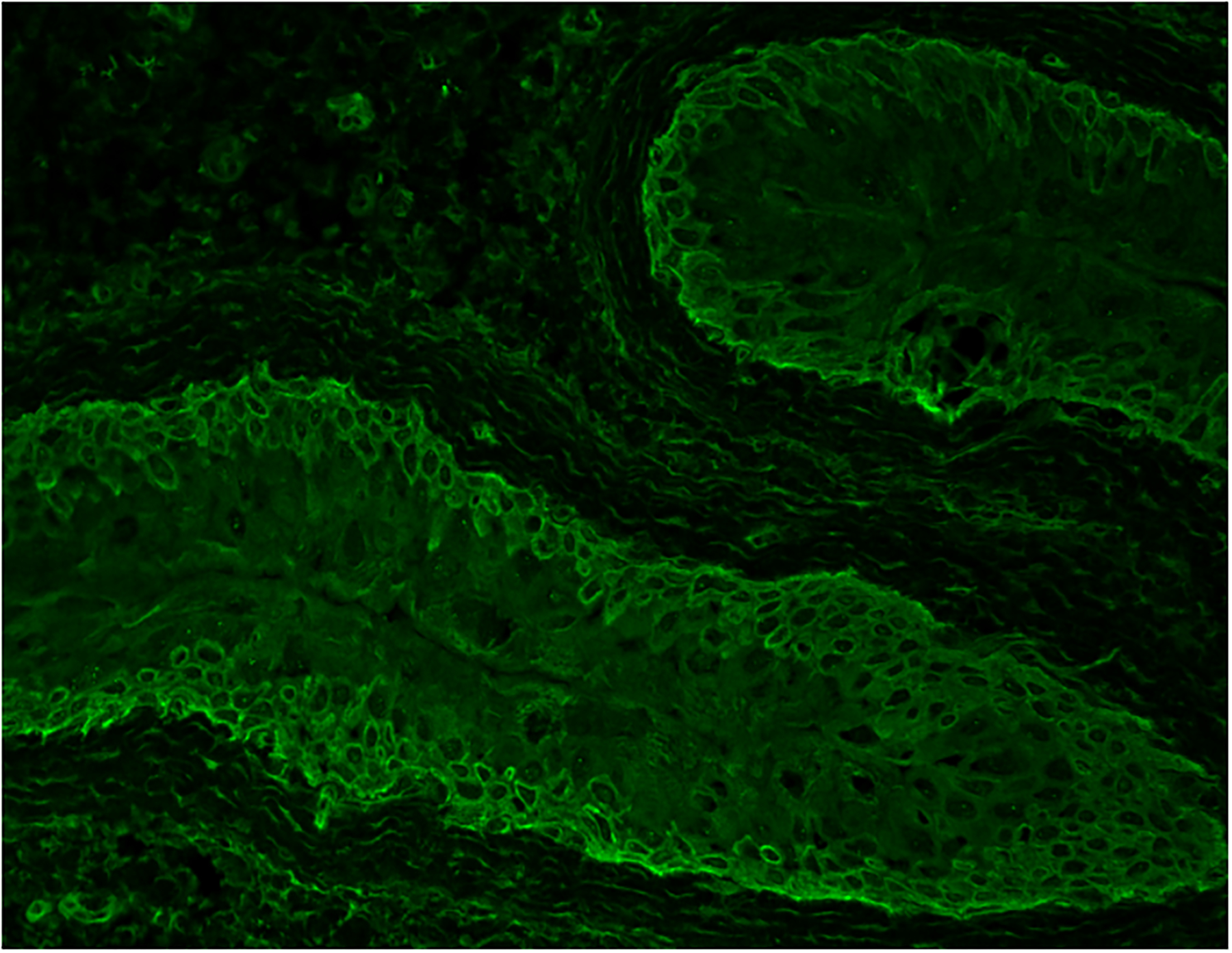
Figure 2:
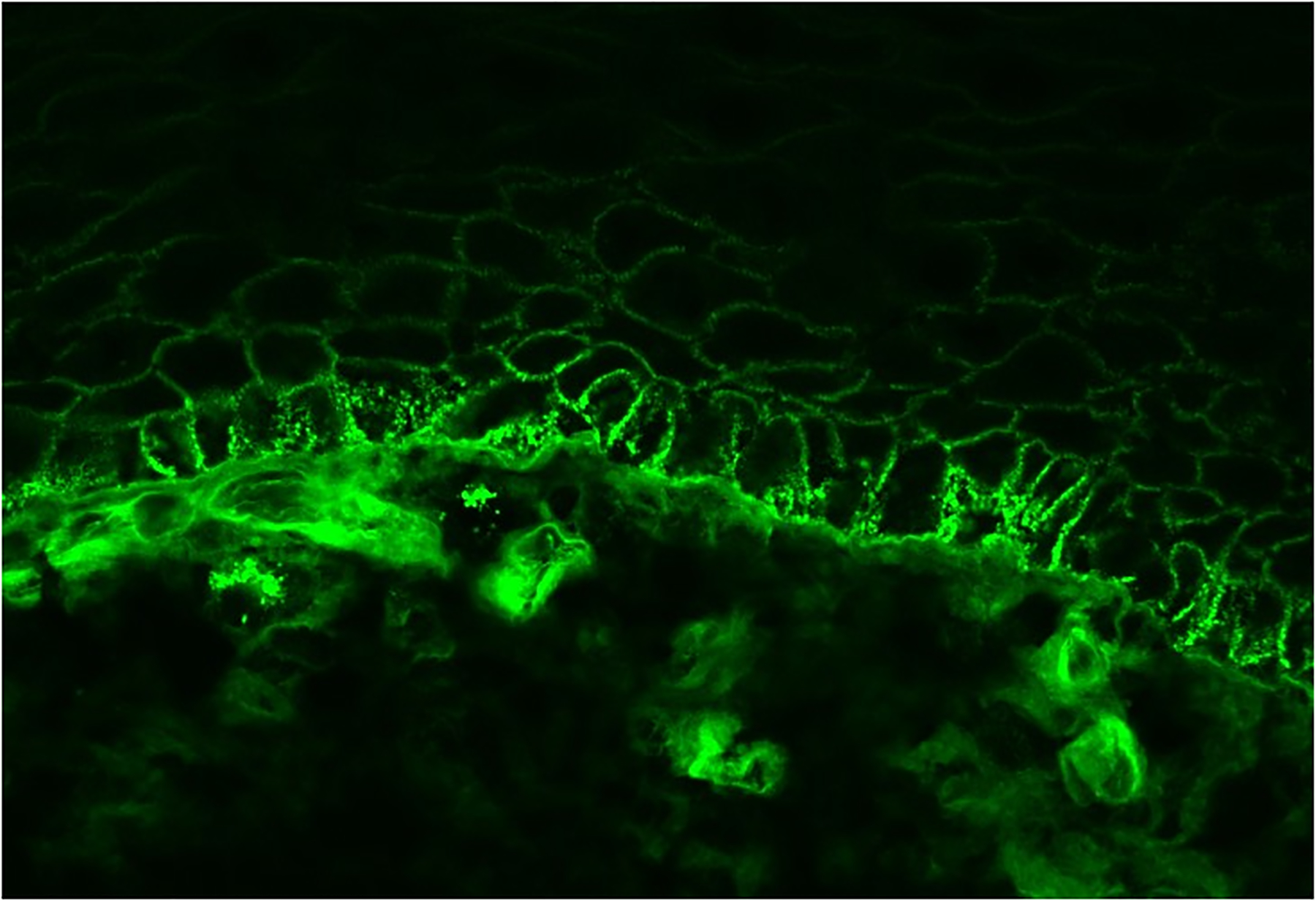
Direct immunofluorescence from a perilesional mucosal biopsy sample of a patient with paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome showing coexistence of intercellular IgG deposits and linear IgG deposits.
Finally, false negative DIF findings can occur and repeated biopsies are sometimes required to make the diagnosis2. Nevertheless, in some patients with clinicopathological and immunoserological findings typical for PNP/PAMS, DIF may remain negative80, 82, most likely because of either severe tissue damage or a predominant T-cell mediated immune response.
Serological examinations
PNP/PAMS patients characteristically exhibit autoantibodies directed against different antigens, including members of the plakin family of cytolinkers (such as envoplakin, periplakin, desmoplakin, plectin, BP230) and desmosomal cadherins (such as desmoglein 3, desmoglein 1 and desmocollins)7, 10, 13, 15, 16. The latter are components of desmosomes, and show a distinct tissue distribution profile. Furthermore, PNP/PAMS sera typically recognize the p170 antigen, identified as the protease inhibitor α2-macroglobulin-like 1 protein12.
Several techniques can be used to detect autoantibodies in PNP/PAMS, including IIF microscopy studies, ELISAs, immunoblotting (IB) and immunoprecipitation (IP) using epidermal extracts or recombinant proteins. While IIF and ELISA can be performed in most centres, IB and IP studies are usually available only in specialized laboratories. The latter are, however, very helpful to better characterize the reactivity profile of PNP/PAMS patients. In some cases, IB and IP studies are even indispensable to confirm the diagnosis of PNP/PAMS. Because of the lack of prospective studies in PNP/PAMS, in which the reactivity profile of PNP/PAMS sera has been systematically characterized by various complementary technical approaches, it is difficult to gain a good understanding of the frequency of the various autoantibody reactivities. The available studies are limited by recruitment and selection biases in specialized centres and laboratories using serologic tests of different performance.
A recent review indicates that PNP/PAMS sera show reactivities with envoplakin and periplakin antibodies in up to 88% of the patients. It has been found that the extremities of the N-terminus of envoplakin and C-terminus of its linker subdomain are major epitopes of PNP/PAMS83. Antibodies directed against epiplakin, plectin and BP230 were found less frequently, in 61%, 57% and approximately one third of cases, respectively. Binding to desmosomal cadherins is also frequent: anti-desmoglein 3 antibodies, anti-desmoglein 1 antibodies and anti-desmocollin antibodies are detectable in 70%, one third, and 62% of cases, respectively4.
Individual patients with purely lichenoid PNP/PAMS have been described with no detectable circulating autoantibodies by any diagnostic method80, 82. All these patients had received rituximab to treat an associated haematological neoplasm and this fact has certainly contributed if not caused the lack of serum autoantibodies.
Indirect immunofluorescence microscopy
In PNP/PAMS, IIF studies can be performed using different substrates. IIF on monkey esophagus may reveal intercellular deposition of IgG in most cases, with a sensitivity ranging from 68% to 100%4, 24, 81; however, findings obtained using monkey esophagus do not allow to reliably differentiate PNP/PAMS from other pemphigus variants. One important study underlined that PNP/PAMS sera typically stain uniformly throughout the epithelium of monkey esophagus, including both the cytoplasmic cell membrane of the basal epithelial cells as well as the epithelial BMZ, resulting in a combined staining pattern84. In analogy, using either normal intact or salt-split human skin as a substrate, different staining patterns can be observed, including intercellular, cytoplasmic and/or BMZ staining pattern. However, only the presence of a strong cytoplasmic staining in all epidermal layers may provide diagnostic clues for PNP/PAMS81. In one French study, 6 out of 22 (27%) PNP/PAMS patients show a combination of intercellular and dermal-epidermal junctional IgG deposition by indirect IIF, regardless of using normal or salt-split human skin as a substrate77. In contrast, in a Japanese study, only one (0.97%) out of 104 PNP/PAMS sera showed staining of both keratinocyte cell surface and epidermal BMZ by using normal human skin as substrate24. Ample evidence exists indicating that rat bladder epithelium (Fig. 3), a complex transitional epithelium, is the most useful, sensitive and specific IIF substrate for PNP/PAMS diagnosis. This substrate expresses high amounts of plakins, but not Dsg 1 and 32. PNP/PAMS sera most commonly and strongly stain both the urothelial cell surface and cytoplasm, although in some cases the staining may be faint and indistinct84. In one study, 86% of the 22 tested PNP/PAMS patients showed reactivity by IIF using rat bladder with an almost 100% specificity77, while in a Dutch study 74% of 19 PNP/PAMS sera were positive for rat bladder IIF85. In a Chinese study, the sensitivity of IIF on rat bladder varied based on the underlying tumour; in fact, it was 92.3% in PNP/PAMS patients with Castleman disease, while it was only 60% for PNP/PAMS patients with thymoma83.
Figure 3:
Positive indirect immunofluorescence on rat bladder substrate - intercellular and cytoplasmic staining of the transitional epithelium.
ELISA
ELISA is very useful to detect distinct characteristic reactivities, such as those for envoplakin and periplakin. An ELISA for the detection of anti-envoplakin antibodies is commercially available. In one study, this envoplakin-ELISA, which uses the N-terminal portion of envoplakin, detected antibodies in 25 out of 31 (81%) PNP/PAMS sera with a specificity of almost 99 %13. Due to the high sequence homology between the N-terminal regions of both envoplakin and periplakin, this envoplakin-ELISA also recognizes anti-periplakin antibodies cross-reacting with envoplakin13. In another study with 19 PNP/PAMS sera, the envoplakin-ELISA was positive in 63% of cases, whereas 89% of the sera immunoblotted envoplakin85. In the latter study, envoplakin-ELISA values decreased during immunosuppressive therapy85. By ELISA, reactivity with Dsg3 and Dsg1 is detectable in between 78.8% and 100% and in between 13.3% to and 26% of PNP/PAMS sera, respectively24, 86. In contrast to PV, PNP/PAMS sera predominantly recognize the COOH-terminal EC4 and EC5 domains of Dsg3, while IgG1 is the predominant subclass86, 87. Although experimental evidence indicating that anti-Dsg3 antibodies contribute to PNP/PAMS pathogenesis exists9, the presence of anti-Dsg antibodies does not seem to correlate neither to the clinical phenotype nor to disease activity86, 88. In a minority of PNP/PAMS sera, reactivity with BP180 and BP230 are also detectable by ELISA, especially in patients showing staining of the epidermal BMZ by direct IF studies85, 89. In one report, detection of IgG anti- BP180-NC16A antibody correlated with the presence of BP-like blistering89. Nonetheless, ELISAs for BP180 and BP230 are not specific for PNP/PAMS diagnosis85.
Approximately 60% of PNP/PAMS patients demonstrate antibodies against proteins of the Dsc family, including Dsc1, Dsc2 and Dsc34, 18, 84. By ELISAs for Dsc1-3 using recombinant proteins of human Dsc1-3 produced in mammalian cells binding to Dsc 3, Dsc 2 and Dsc 1 was found in 60.8%, 41.2% and 18.6% of the 102 tested samples, respectively24. A recent study described an ELISA to specifically detect reactivity with the alpha-2-macroglobulin-like-1 (A2ML1) by producing A2ML1 in fusion with enhanced green fluorescent protein in eukaryotic cells. This novel assay identified anti-A2ML1 autoantibodies in 61 % of 36 PNP/PAMS sera tested, with a specificity of 88.9 % and a sensitivity of 95 %90. These ELISAs for the specific detection of anti-Dsc and anti-A2ML1 are not yet commercially available.
Immunoblotting (IB) and immunoprecipitation (IP)
IP using radioactively labelled keratinocyte extracts is the technique which was originally used to identify the characteristic complex of PNP/PAMS antigens. Currently, IP has been almost invariably abandoned in favour of non-radioactive IP or non-radioactive IP/IB combined techniques. The latter may also be performed by using recombinant proteins produced by different approaches to increase sensitivity or facilitate the detection of specific reactivities7, 12, 85, 90, 91. IP studies still constitute the most sensitive diagnostic techniques for PNP/PAMS diagnosis, partly because of the detection of anti-A2ML1 antibodies, which are detectable only in non-reducing conditions12, 85. In one study comprising 19 PNP/PAMS sera the reported sensitivities were 95% for radioactive immunoprecipitation and 100% for non-radioactive immunoprecipitation85. The immunoprecipitated proteins found in different combinations predominantly include desmoplakin I and II, envoplakin, periplakin and/or A2ML18, 10, 12, 14. In several cases, IP reactivity may be limited to one protein band, such as alpha-2-macroglobulin-like protein12. IB studies are preferably performed using epidermal extracts, cultured keratinocytes or recombinant proteins produced using different expression systems92.
Both IB and IP studies have a number of advantages as diagnostic tool for PNP/PAMS: i) they have a high diagnostic performance when compared to rat bladder IIF and envoplakin-ELISA85; ii) they are particularly useful to detect autoantibodies against PNP/PAMS antigens for which specific ELISAs are not easily available, such as for periplakin, desmoplakins, desmocollins and A2ML1; iii) depending on the used substrate, IB and IP techniques allow to detect multiple reactivities, being IP the most sensitive tool for the diagnosis of PNP/PAMS;24 iv) finally, IB can also detect reactivities of other autoantibody isotypes, such as IgA93. These tests, however, have their limitations: little availability, technically demanding and time-consuming, and lack of standardization resulting in variable performance.

Diagnostic criteria for PNP/PAMS
Since the initial description of PNP/PAMS, several different diagnostic criteria for the classification of PNP/PAMS have been proposed1, 4, 30, 94, 95. However, so far, there are not generally accepted and validated diagnostic criteria for this disorder. Its diagnosis should thus rather rely on a combination of criteria, including presence of compatible or typical clinical features and histopathology findings, positive direct IF studies with a compatible staining pattern as well as the detection of circulating autoantibodies with distinct specificities. Although anti-plakin antibodies (mainly against desmoplakins) can be found sporadically in patients without PNP/PAMS96–98, detection of autoantibodies against rat urothelium, envoplakin, periplakin and alpha-2-macroglobulin-like proteins represent the most specific immunoserological findings for PNP/PAMS. Consequently, envoplakin ELISA, IIF studies using rat bladder epithelium and, where available, IB/IP studies are the gold standard for the diagnosis of PNP/PAMS.
Presence of an underlying neoplasm, most frequently a lymphoproliferative or haematological malignancy is an important diagnostic criterion. Nevertheless, in a subset of patients, the underlying malignancy has not been yet diagnosed at the time of PNP/PAMS development. In anecdotal patients with clinical and immune-pathological features typical for PNP/PAMS, no associated malignancy could be detected despite throughout search (see above).
Diagnostic criteria and diagnostic algorithm for PNP/PAMS diagnosis are reported below and in Fig 4.
Figure 4:
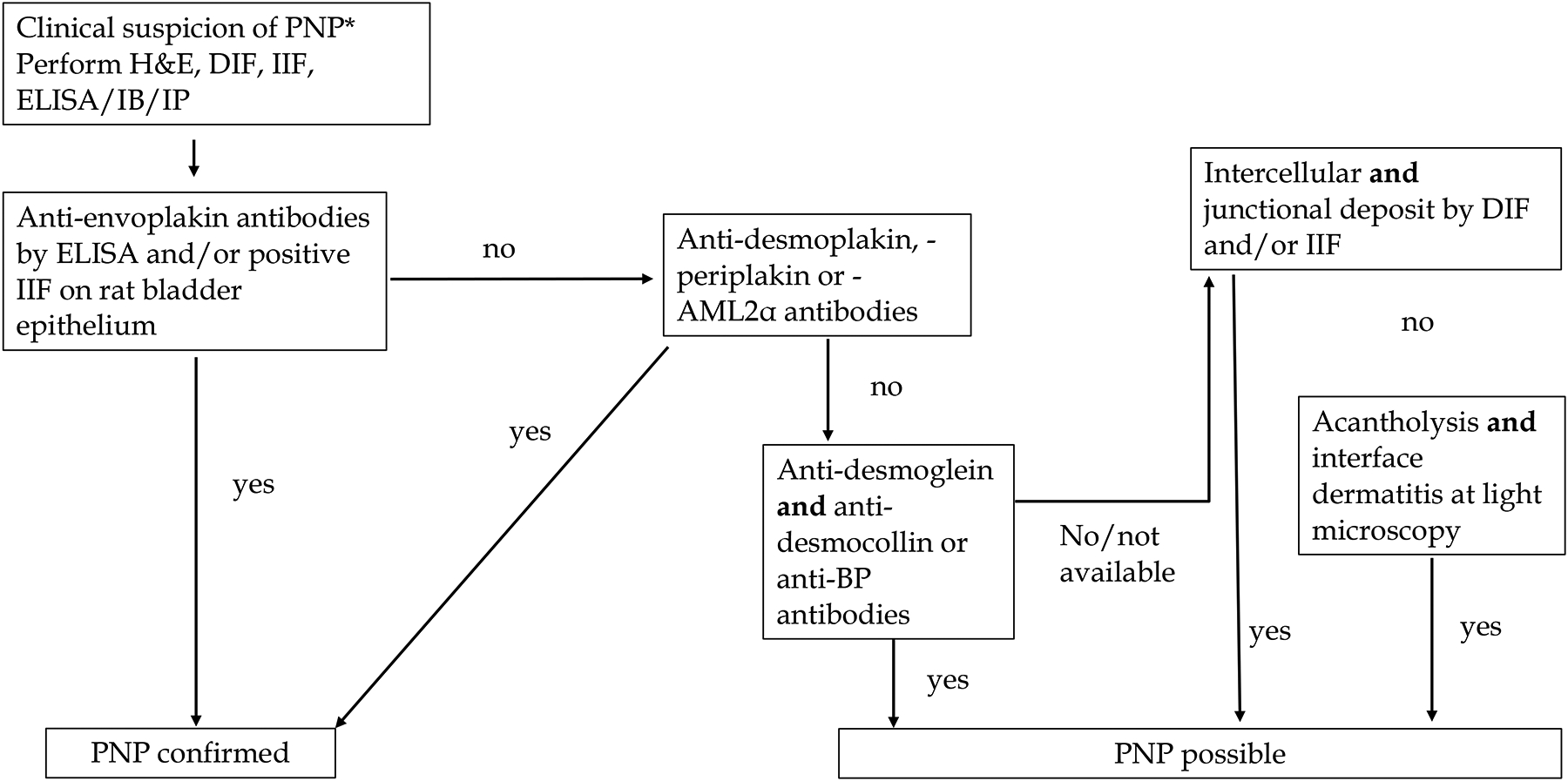
Proposed diagnostic algorithm for paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome. Abbreviations: PNP/PAMS = paraneoplastic pemphigus; paraneoplastic autoimmune multiorgan syndrome; ELISA = enzyme linked immunosorbent assay; IB = immunoblotting; DIF = direct immunofluorescence; IIF = indirect immunofluorescence; AML2α = a2-macroglobulin-like 1 protein.

DIFFERENTIAL DIAGNOSES
PNP/PAMS needs to be differentiated from all dermatoses associated with either acute or chronic lesions of mucosal sites, particularly stomatitis, in combination with polymorphic skin lesions of variable severity. The most important conditions include PV and mucous membrane pemphigoid, severe drug reactions and erythema multiforme majus and are discussed below.
Autoimmune bullous diseases
Pemphigus vulgaris
Differentiating PNP/PAMS from PV in the setting of underlying malignancy may represent a significant challenge. Indeed, malignancy-associated PV and PNP/PAMS may present similarly, and a comprehensive clinical and immunopathological assessment is necessary to differentiate these two conditions99. Severe refractory oral mucositis may be shared by both entities, particularly if PV is associated with high titers of anti-Dsg3 antibodies. Histologically, the presence of interface dermatitis and lichenoid infiltrates are suggestive and typical for PNP/PAMS and are not observed in PV. DIF may show linear immune deposits along the epithelial BMZ, which are absent in PV. IIF findings using rat bladder are highly specific for PNP/PAMS, and typically negative in PV. While both PNP/PAMS and PV may show anti-Dsg1 and anti-Dsg3 on ELISA, PNP/PAMS is characterized by the presence of additional autoantibodies directed against different plakins and other components of the desmosomes99. Isolated presence of anti-desmoplakin antibodies is not specific for PNP/PAMS and is rarely detected also in PV and in a subset of patients with erythema multiforme majus100,101.
Mucous membrane pemphigoid
Mucous membrane pemphigoid (MMP) is a subepithelial autoimmune bullous disease that primarily affect the mucous membranes. The predominant involvement of mucosal sites makes the differential diagnosis with PNP/PAMS sometimes difficult. Eye and mouth involvement are indeed very frequent in both conditions. Furthermore, it has been shown that the subset of MMP associated with anti-laminin 332 antibodies is at increased risk for neoplasia, usually solid tumours102–107. One case of PNP/PAMS in which reactivity with laminin‐332 was detected has been described108. However, MMP histopathology usually shows sub-epithelial detachment but not acantholysis. Moreover, DIF from perilesional skin/mucosa demonstrates linear IgG and/or IgA and/or C3 deposition along the epidermal/epithelial BMZ, but not intercellular deposits. Likewise, IIF performed on various substrates, including salt-split human skin, demonstrates IgG/IgA deposits along either the epidermal or dermal side of the BMZ; finally, MMP shows no reactivity at IIF using rat bladder as a substrate105.
Other diseases
Lichen planus and lichenoid eruptions associated with immune checkpoint inhibitor therapy
PNP/PAMS may present with predominant lichen planus-like lesions35, 109–111. Mucous membrane lesions of PNP/PAMS may closely mimic “true” oral lichen planus both clinically and histopathologically with both erosive and lichenoid reticular lesions112–116. Lichenoid drug reactions alone or more rarely in combination with skin blistering, are also observed in patients with various malignancies following treatment with immune checkpoint inhibitors, such as anti-PD-1117. Another entity that may be clinically confused with PNP/PAMS is Good Syndrome118. These patients have thymoma and combined B-cell and T-cell immunodeficiency of adult onset and may present with lichenoid oral and cutaneous lesions. Both DIF and IIF are negative in this syndrome.
Erythema multiforme majus, Stevens-Johnson syndrome and toxic epidermal necrolysis
Since the first seminal report on PNP/PAMS, it has been recognized that PNP/PAMS may characteristically present lesions very similar to those observed in erythema multiforme majus, Stevens-Johnson syndrome or even toxic epidermal necrolysis1, 2, 119, 120. Erosions may cover the entire surface of the body in the toxic epidermal necrolysis-like presentation of PNP/PAMS, including in children78.
Recently a patient with a PNP/PAMS-like eruption associated with envoplakin and periplakin antibodies, but negative IF studies and absence of malignancy has been reported as anti-plakin dermatosis121. This probably represents a variant of relapsing erythema multiforme with anti-plakin antibodies. However, these disorders are usually transient, compared to the chronic course of PNP/PAMS.
Graft-versus-host disease
PNP/PAMS may present with clinical and pathological features similar of those of acute cutaneous graft-versus-host disease (GVHD). However, GVHD is differentiated from PNP/PAMS on the ground of the clinical history and timing after allogeneic hematopoietic stem cell transplantation122. Cutaneous T-cell response in patients with graft-versus-host disease-like PNP/PAMS demonstrate a selective epidermal accumulation of activated CD8+ T cells together with an increased local production of interferon-γ and tumour necrosis factor-α123.
THERAPY
Treatment of PNP/PAMS remains challenging. The treatment of the underlying malignancy is always recommended, as management of the underlying neoplasm can result in PNP/PAMS improvement44, 124–127. Early detection and radical resection of tumors such as Castleman’s disease or thymoma have occasionally been shown to have a beneficial effect and lead to PNP/PAMS resolution48, 50 with long-term survival128.
There is no evidence supporting the use of any specific therapy due to the rarity of the condition129. However, systemic corticosteroids (prednisolone) 0.5mg/kg to 1.5 mg/kg/day in combination with steroid-sparing agents have been widely used. Although the responses to these regimens may be inconsistent and may result in an increased risk for severe or life-threatening complications, including infections, diabetes, osteoporosis, Cushing syndrome, etc, systemic corticosteroids still remain the first line of treatment for patients with PNP/PAMS. Systemic steroids usually have a beneficial effect on cutaneous lesions, while PNP/PAMS-associated mucositis and bronchiolitis obliterans may be less responsive. Azathioprine, mycophenolate mofetil, cyclosporine, cyclophosphamide and methotrexate have been used as steroid-sparing agents, even in variable combination44, 125–127. High doses of intravenous immunoglobulins (IVIG) have also been employed44. In PNP/PAMS associated with B-cell malignancies, the anti-CD20 drug rituximab has been successfully used in some patients44, 130, 131. In PNP/PAMS associated with chronic lymphocytic leukemia, the anti-CD52 drug alemtuzumab at low doses (i.e. 10 mg three times a week for 12 weeks) has also been employed132, 133. GJ Anhalt suggested the combination of prednisone, rituximab and the anti-CD25 monoclonal antibodies (like daclizumab or basiliximab) to down-regulate both the B and T-cell autoimmune response134. Plasmapheresis has also been tried44, 127. There is sporadic evidence for the use of thalidomide in the treatment of PNP/PAMS135.
The management of mucositis and bronchiolitis obliterans in PNP/PAMS is challenging. In fact, patients with bronchiolitis obliterans have poor survival rates47. Systemic corticosteroids are often not effective. Combination regimens with prednisolone, various immunosuppressants and targeted therapy, such as tocilizumab (anti-IL6), alemtuzumab, rituximab or ibrutinib might be tried since they showed some effectiveness in patients with PNP/PAMS,44 although in severe cases leading to respiratory failure lung transplantation could be the only available therapeutic option136.
As most of PNP/PAMS treatment options can result in immunosuppression they should always be consented in multidisciplinary teams including haematologists or oncologists who care for the underlying malignancies.

CONCLUDING REMARKS
This manuscript represents the first European guideline dedicated to PNP/PAMS. Which acronym is best suited to describe this complex disorder has been already a matter of debate in the literature. Here, both acronyms, PNP and PAMS, have been maintained throughout the guideline. Accordingly, besides its historical relevance, the term PNP highlights the importance of anti-keratinocyte antibodies and acantholysis in the pathogenesis of the disease, while the term PAMS better describes the extracutaneous involvement of the disease, which is highly prevalent and often compromises patients’ survival.
The diagnosis of PNP/PAMS remains challenging, owing to the rarity of the disease, the large spectrum of differential diagnoses, and the fact that the detection of circulating autoantibodies may require highly specialized tools, such as IB/IP, which are not broadly available. This guideline suggests novel diagnostic criteria and a diagnostic algorithm which could help clinicians to achieve a diagnosis of PNP/PAMS in various clinical scenarios. Of note, these criteria have been proposed by consensus agreement among experts, and thereby will require validation by large multicentric prospective investigations in the near future.
With regard to therapeutics, in PNP/PAMS associated with lymphoproliferative conditions, B-cell depleting therapies such as rituximab represent the preferred strategy by targeting both neoplastic and autoaggressive lymphocytes. In the case of PNP/PAMS associated with solid tumours, the use of either rituximab or other immunosuppressants should be based on a balance between the life-threatening course of the disease, especially in the short-term, and the risk of tumour progression favoured by a deep immunosuppressive state. In any case, a multidisciplinary approach to PNP/PAMS is of vital importance to correctly manage the patients.
Remarkably, despite a prompt recognition and management, the short-term prognosis of PNP/PAMS remains poor, owing to an elevated incidence of severe infections and disease-associated complications, such as bronchiolitis obliterans. In this regard, future prospective investigations should be intended to identify either patient- or disease-specific characteristics potentially predictive of a worse outcome, the early recognition of whom may lead to improve patients’ management and survival.
Figure 1:
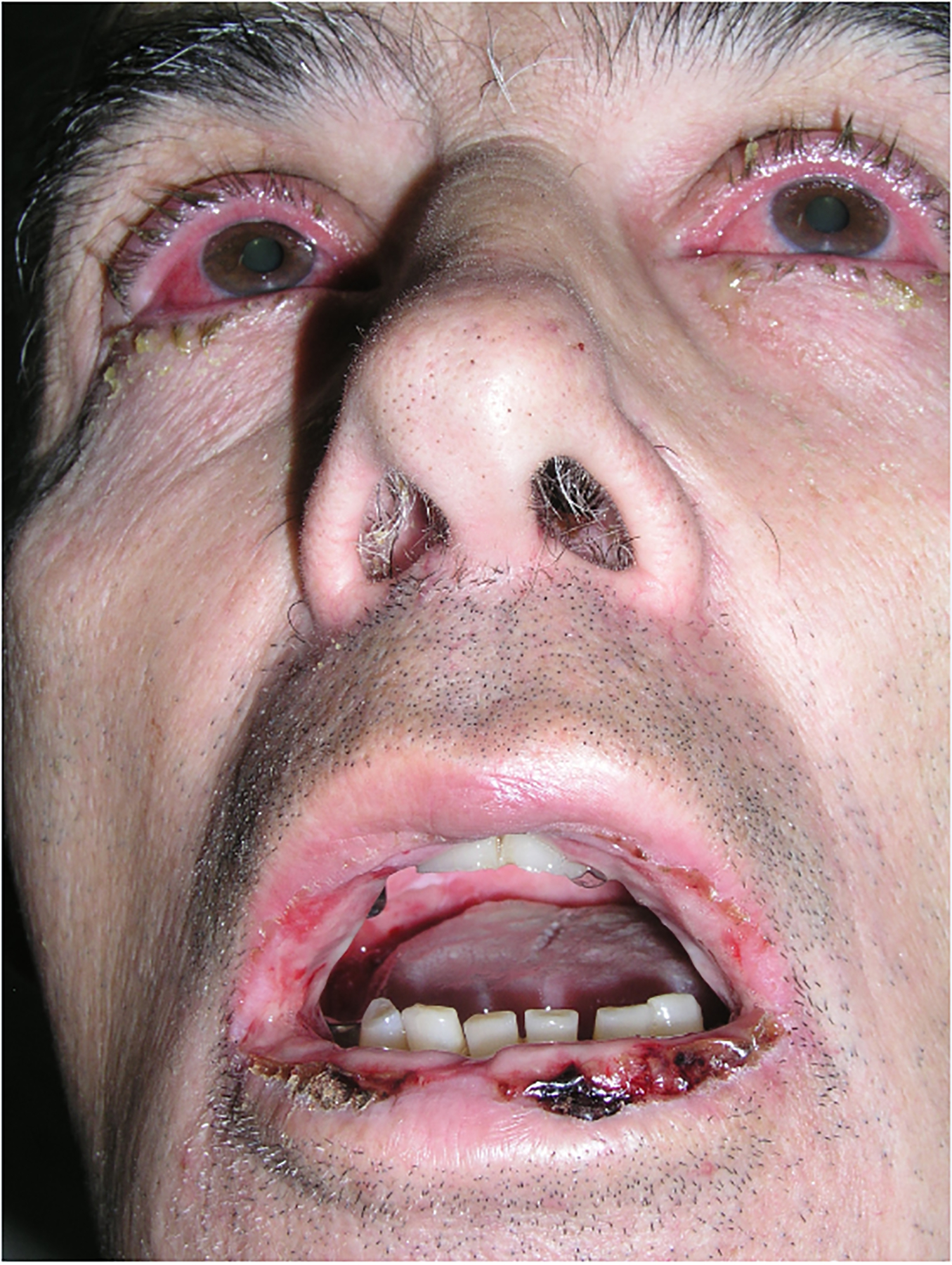
Severe mucositis – panstomatitis and bilateral conjunctivitis in a patient with paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome associated with B-cell chronic lymphatic leukemia.
Acknowledgements:
The patients in this manuscript have given written informed consent to publication of their case details.
Conflicts of Interest:
Emiliano Antiga, Rikke Bech, Barbara Bockle, Marzia Caproni, Nisha Suyen Chandran, Alberto Corrà, Francesco D’Amore, Maryam Daneshpazhooh, Dario Didona, Marian Dmochowski, Kossara Drenovska, Jan Ehrchen, Claudio Feliciani, Giovanni Genovese, Richard Groves, Sanjeev Handa, Takashi Hashimoto, Silke C. Hofmann, Dimitrios Ioannidis, Hana Jedlickova,Cezary Kowalewski, Khalaf Kridin, Roberto Maglie, Branka Marinovic, Angelo V. Marzano, Josè M.Mascarò Jr, Emanual Maverakis, Joost M. Meijer, Aikaterini Patsatsi, Catherine Prost, Jane Setterfield, Dusan Skiljevic, Eli Sprecher, Soner Uzun, Snejina Vassileva, Artem Vorobyev, Gang Wang, Mingyue Wang, Katarzyna Wozniak and Savas Yayli declared no conflict of interest.Luca Borradori had consulting fees from Chugai and Argenx; Frédéric Caux had grants with payment to his institution from Argenx, Regeneron and Roche; Dipankar De had a grant as an investigator from Argenx, Matthias Goebeler had a grant as an investigator with payment to his institution from Argenx, Claudia Gunther had a grant for attending a meeting by Boehringer; Barbara Horvath had grants with payment to her institutions from Janssen-Cilag, AbbVie, Novartis Pharma, Celgene/AMGEN, Akari therapeutics, Argenx, consulting fees with payment to her institution from Janssen-Cilag, AbbVie, Novartis Pharma, UCB Pharma, Leo Pharma, Akari therapeutics, Philips, Roche, Regeneron, Sanofi, Argenx, honoraria for lectures with payment to her institutions from Janssen-Cilag, AbbVie, Novartis Pharma, UCB Pharma, Leo Pharma, Solenne B.V., Celgene, Akari therapeutics, Philips, Roche, Regeneron, Sanofi, Argenx; Pascal Joly had consulting fees from Lilly, Novartis, Principia Biopharma, Roche Laboratories, Sanofi-aventis, Astra Zeneca, Argenx; Yen Loo Lim is in the Scientific programme committee and Organising committee of the World Congress of Dermatology 2023; Carlo Pincelli is consultant of Pincell and inventor in the patent:” Remedies for pemphigus containing anti Fas ligand antibodies” WO2010/066914; Enno Schmidt had grants with payment to his insititution from UCB, Incyte, Biotest, Argenx, Dompe, Euroimmun, Fresenius Medical Care, Bayer, Pharmaxis, Alpine Immune, CSL, had consulting fees from Leo, Chugai, Almirall, Janssen, had patents with Euroimmun and Dompe, had Participation on a Data Safety Monitoring Board or Advisory Board with Argenx and AstraZeneca; Kaisa Tasanen had support to attend EADV Congress from Novartis, participated to advisory board for Abbvie, Leo Pharma, Novartis, Sanofi Genzyme, Janssen-Cilag, Bristol-Myers Squibb and UCB Pharma and is Board member of ESDR and EDF; Nina Van Beek has a pending patent together with Euroimmun and received material for a joint project by Euroimmun; Igor Vujic had consulting fees from Abbvie, Novartis, Lilly, Amgen, Janssen; Giovanna Zambruno participated to advisory board with payment to her institution from Argenx.
Data availability statement:
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1.Anhalt GJ, Kim SC, Stanley JR, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990;323:1729–35. [DOI] [PubMed] [Google Scholar]
- 2.Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol 1997;12:77–96; discussion 97. [PubMed] [Google Scholar]
- 3.Nguyen VT, Ndoye A, Bassler KD, et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol 2001;137:193–206. [PubMed] [Google Scholar]
- 4.Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet 2019;394:882–94. [DOI] [PubMed] [Google Scholar]
- 5.Maglie R, Genovese G, Solimani F, et al. Immune-Mediated Dermatoses in Patients with Haematological Malignancies: A Comprehensive Review. Am J Clin Dermatol 2020;21:833–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kridin K, Schmidt E. Epidemiology of Pemphigus. JID Innov 2021;1:100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto T, Amagai M, Watanabe K, et al. Characterization of paraneoplastic pemphigus autoantigens by immunoblot analysis. J Invest Dermatol 1995;104:829–34. [DOI] [PubMed] [Google Scholar]
- 8.Borradori L, Trueb RM, Jaunin F, Limat A, Favre B, Saurat JH. Autoantibodies from a patient with paraneoplastic pemphigus bind periplakin, a novel member of the plakin family. J Invest Dermatol 1998;111:338–40. [DOI] [PubMed] [Google Scholar]
- 9.Amagai M, Nishikawa T, Nousari HC, Anhalt GJ, Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest 1998;102:775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahoney MG, Aho S, Uitto J, Stanley JR. The members of the plakin family of proteins recognized by paraneoplastic pemphigus antibodies include periplakin. J Invest Dermatol 1998;111:308–13. [DOI] [PubMed] [Google Scholar]
- 11.Oursler JR, Labib RS, Ariss-Abdo L, Burke T, O’Keefe EJ, Anhalt GJ. Human autoantibodies against desmoplakins in paraneoplastic pemphigus. J Clin Invest 1992;89:1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schepens I, Jaunin F, Begre N, et al. The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS One 2010;5:e12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst C, Schlumberger W, Stocker W, et al. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin Chim Acta 2009;410:13–8. [DOI] [PubMed] [Google Scholar]
- 14.Kiyokawa C, Ruhrberg C, Nie Z, et al. Envoplakin and periplakin are components of the paraneoplastic pemphigus antigen complex. J Invest Dermatol 1998;111:1236–8. [DOI] [PubMed] [Google Scholar]
- 15.Kim SC, Kwon YD, Lee IJ, Chang SN, Lee TG. cDNA cloning of the 210-kDa paraneoplastic pemphigus antigen reveals that envoplakin is a component of the antigen complex. J Invest Dermatol 1997;109:365–9. [DOI] [PubMed] [Google Scholar]
- 16.Proby C, Fujii Y, Owaribe K, Nishikawa T, Amagai M. Human autoantibodies against HD1/plectin in paraneoplastic pemphigus. J Invest Dermatol 1999;112:153–6. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchisaka A, Numata S, Teye K, et al. Epiplakin Is a Paraneoplastic Pemphigus Autoantigen and Related to Bronchiolitis Obliterans in Japanese Patients. J Invest Dermatol 2016;136:399–408. [DOI] [PubMed] [Google Scholar]
- 18.Ishii N, Teye K, Fukuda S, et al. Anti-desmocollin autoantibodies in nonclassical pemphigus. Br J Dermatol 2015;173:59–68. [DOI] [PubMed] [Google Scholar]
- 19.Numata S, Teye K, Tsuruta D, et al. Anti-alpha-2-macroglobulin-like-1 autoantibodies are detected frequently and may be pathogenic in paraneoplastic pemphigus. J Invest Dermatol 2013;133:1785–93. [DOI] [PubMed] [Google Scholar]
- 20.Landegren N, Ishii N, Aranda-Guillen M, et al. A gene-centric approach to biomarker discovery identifies transglutaminase 1 as an epidermal autoantigen. Proc Natl Acad Sci U S A 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amber KT. Paraneoplastic autoimmune multi-organ syndrome is a distinct entity from traditional pemphigus subtypes. Nat Rev Dis Primers 2018;4:18012. [DOI] [PubMed] [Google Scholar]
- 22.Amber KT, Valdebran M, Grando SA. Paraneoplastic autoimmune multiorgan syndrome (PAMS): Beyond the single phenotype of paraneoplastic pemphigus. Autoimmun Rev 2018;17:1002–10. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol 2004;40:553–62. [DOI] [PubMed] [Google Scholar]
- 24.Ohzono A, Sogame R, Li X, et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br J Dermatol 2015;173:1447–52. [DOI] [PubMed] [Google Scholar]
- 25.Marzano AV, Grammatica A, Cozzani E, Terracina M, Berti E. Paraneoplastic pemphigus. A report of two cases associated with chronic B-cell lymphocytic leukaemia. Br J Dermatol 2001;145:127–31. [DOI] [PubMed] [Google Scholar]
- 26.Lim JM, Lee SE, Seo J, Kim DY, Hashimoto T, Kim SC. Paraneoplastic Pemphigus Associated with a Malignant Thymoma: A Case of Persistent and Refractory Oral Ulcerations Following Thymectomy. Ann Dermatol 2017;29:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi Y, Nam KH, Lee JB, et al. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol 2012;39:973–81. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Bu DF, Li T, et al. Autoantibody production from a thymoma and a follicular dendritic cell sarcoma associated with paraneoplastic pemphigus. Br J Dermatol 2005;153:558–64. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Li F, Wang X, et al. Features and Risk Factors for Paraneoplastic Autoimmune Multiorgan Syndrome in 145 Chinese Patients. Acta Derm Venereol 2020;100:adv00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimouni D, Anhalt GJ, Lazarova Z, et al. Paraneoplastic pemphigus in children and adolescents. Br J Dermatol 2002;147:725–32. [DOI] [PubMed] [Google Scholar]
- 31.Mignogna MD, Fortuna G, Leuci S, Adamo D, Ruoppo E. Metastatic prostate cancer presenting as paraneoplastic pemphigus: a favourable clinical response to combined androgen blockade and conventional immunosuppressive therapy. Br J Dermatol 2009;160:468–70. [DOI] [PubMed] [Google Scholar]
- 32.Lepekhova A, Olisova O, Teplyuk N, Zolotenkov D, Allenova A. A rare association of paraneoplastic pemphigus with gastric signet cell ring carcinoma. Australas J Dermatol 2019;60:e168–e69. [DOI] [PubMed] [Google Scholar]
- 33.Cho JH, Kim NJ, Ko SM, et al. A case report of paraneoplastic pemphigus associated with esophageal squamous cell carcinoma. Cancer Res Treat 2013;45:70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su Z, Liu G, Liu J, et al. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma: report of a case and review of literature. Int J Clin Exp Pathol 2015;8:11983–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Marzano AV, Vezzoli P, Mariotti F, Boneschi V, Caputo R, Berti E. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma and Castleman disease. Br J Dermatol 2005;153:214–5. [DOI] [PubMed] [Google Scholar]
- 36.Anhalt GJ. Paraneoplastic pemphigus: the role of tumours and drugs. Br J Dermatol 2001;144:1102–4. [DOI] [PubMed] [Google Scholar]
- 37.Bazarbachi A, Bachelez H, Dehen L, Delmer A, Zittoun R, Dubertret L. Lethal paraneoplastic pemphigus following treatment of chronic lymphocytic leukaemia with fludarabine. Ann Oncol 1995;6:730–1. [DOI] [PubMed] [Google Scholar]
- 38.Higo T, Miyagaki T, Nakamura F, et al. Paraneoplastic pemphigus occurring after bendamustine and rituximab therapy for relapsed follicular lymphoma. Ann Hematol 2015;94:683–5. [DOI] [PubMed] [Google Scholar]
- 39.Preisz K, Horvath A, Sardy M, et al. Exacerbation of paraneoplastic pemphigus by cyclophosphamide treatment: detection of novel autoantigens and bronchial autoantibodies. Br J Dermatol 2004;150:1018–24. [DOI] [PubMed] [Google Scholar]
- 40.Lee MS, Kossard S, Ho KK, Barnetson RS, Ravich RB. Paraneoplastic pemphigus triggered by radiotherapy. Australas J Dermatol 1995;36:206–10. [DOI] [PubMed] [Google Scholar]
- 41.Ostezan LB, Fabre VC, Caughman SW, Swerlick RA, Korman NJ, Callen JP. Paraneoplastic pemphigus in the absence of a known neoplasm. J Am Acad Dermatol 1995;33:312–5. [DOI] [PubMed] [Google Scholar]
- 42.Verrini A, Cannata G, Cozzani E, Terracini M, Parodi A, Rebora A. A patient with immunological features of paraneoplastic pemphigus in the absence of a detectable malignancy. Acta Derm Venereol 2002;82:382–4. [DOI] [PubMed] [Google Scholar]
- 43.Park GT, Lee JH, Yun SJ, Lee SC, Lee JB. Paraneoplastic pemphigus without an underlying neoplasm. Br J Dermatol 2007;156:563–6. [DOI] [PubMed] [Google Scholar]
- 44.Kim JH, Kim SC. Paraneoplastic Pemphigus: Paraneoplastic Autoimmune Disease of the Skin and Mucosa. Front Immunol 2019;10:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anhalt GJ. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc 2004;9:29–33. [DOI] [PubMed] [Google Scholar]
- 46.Leger S, Picard D, Ingen-Housz-Oro S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol 2012;148:1165–72. [DOI] [PubMed] [Google Scholar]
- 47.Ouedraogo E, Gottlieb J, de Masson A, et al. Risk factors for death and survival in paraneoplastic pemphigus associated with hematologic malignancies in adults. J Am Acad Dermatol 2019;80:1544–49. [DOI] [PubMed] [Google Scholar]
- 48.Fang Y, Zhao L, Yan F, Cui X, Xia Y, Duren A. A critical role of surgery in the treatment for paraneoplastic pemphigus caused by localized Castleman’s disease. Med Oncol 2010;27:907–11. [DOI] [PubMed] [Google Scholar]
- 49.Chorzelski T, Hashimoto T, Maciejewska B, Amagai M, Anhalt GJ, Jablonska S. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol 1999;41:393–400. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Qiao QL, Chen XX, et al. Improved outcomes after complete resection of underlying tumors for patients with paraneoplastic pemphigus: a single-center experience of 22 cases. J Cancer Res Clin Oncol 2011;137:229–34. [DOI] [PubMed] [Google Scholar]
- 51.Jing L, Shan Z, Yongchu H, Xixue C, Xuejun Z. Successful treatment of a paraneoplastic pemphigus in a teenager using plasmapheresis, corticosteroids and tumour resection. Clin Exp Dermatol 2011;36:752–4. [DOI] [PubMed] [Google Scholar]
- 52.Rivollier C, Vaillant L, Machet MC, et al. [Paraneoplastic pemphigus: a pustular form during chronic lymphoid leukemia]. Ann Dermatol Venereol 2001;128:644–8. [PubMed] [Google Scholar]
- 53.van der Waal RI, Pas HH, Anhalt GJ, Schulten EA, Jonkman MF, Nieboer C. Paraneoplastic pemphigus as the presenting symptom of a lymphoma of the tongue. Oral Oncol 1998;34:567–70. [DOI] [PubMed] [Google Scholar]
- 54.Simmons BJ, Adams JA, Cho-Vega JH, Tosti A. Paraneoplastic pemphigus with cicatricial nail involvement. Cutis 2020;106:E4–E6. [DOI] [PubMed] [Google Scholar]
- 55.Rashid H, Lamberts A, Diercks GFH, et al. Oral Lesions in Autoimmune Bullous Diseases: An Overview of Clinical Characteristics and Diagnostic Algorithm. Am J Clin Dermatol 2019;20:847–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Decaux J, Ferreira I, Van Eeckhout P, Dachelet C, Magremanne M. Buccal paraneoplastic pemphigus multi-resistant: Case report and review of diagnostic and therapeutic strategies. J Stomatol Oral Maxillofac Surg 2018;119:506–09. [DOI] [PubMed] [Google Scholar]
- 57.Gissi DB, Bernardi A, D’Andrea M, Montebugnoli L. Paraneoplastic pemphigus presenting with a single oral lesion. BMJ Case Rep 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Broussard KC, Leung TG, Moradi A, Thorne JE, Fine JD. Autoimmune bullous diseases with skin and eye involvement: Cicatricial pemphigoid, pemphigus vulgaris, and pemphigus paraneoplastica. Clin Dermatol 2016;34:205–13. [DOI] [PubMed] [Google Scholar]
- 59.Tam PM, Cheng LL, Young AL, Lam PT. Paraneoplastic pemphigus: an uncommon cause of chronic cicatrising conjunctivitis. BMJ Case Rep 2009;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beele H, Claerhout I, Kestelyn P, Dierckxens L, Naeyaert JM, De Laey JJ. Bilateral corneal melting in a patient with paraneoplastic pemphigus. Dermatology 2001;202:147–50. [DOI] [PubMed] [Google Scholar]
- 61.Piscopo R, Romano M, Maria AD, Vinciguerra R, Vinciguerra P. Ocular Onset of Paraneoplastic Pemphigus Presenting as Hyperemic Conjunctivitis and Massive Bilateral Eyelid Ulceration: A Case Report and Literature Review. Ocul Immunol Inflamm 2018;26:265–68. [DOI] [PubMed] [Google Scholar]
- 62.Meyers SJ, Varley GA, Meisler DM, Camisa C, Wander AH. Conjunctival involvement in paraneoplastic pemphigus. Am J Ophthalmol 1992;114:621–4. [DOI] [PubMed] [Google Scholar]
- 63.Han SP, Fu LS, Chen LJ. Masked pemphigus among pediatric patients with Castleman’s disease. Int J Rheum Dis 2019;22:121–31. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi M, Shimatsu Y, Kazama T, Kimura K, Otsuka T, Hashimoto T. Paraneoplastic pemphigus associated with bronchiolitis obliterans. Chest 2000;117:603–7. [DOI] [PubMed] [Google Scholar]
- 65.Nousari HC, Deterding R, Wojtczack H, et al. The mechanism of respiratory failure in paraneoplastic pemphigus. N Engl J Med 1999;340:1406–10. [DOI] [PubMed] [Google Scholar]
- 66.Maldonado F, Pittelkow MR, Ryu JH. Constrictive bronchiolitis associated with paraneoplastic autoimmune multi-organ syndrome. Respirology 2009;14:129–33. [DOI] [PubMed] [Google Scholar]
- 67.Sano Y, Kikuchi Y, Morita S, et al. Paraneoplastic pemphigus and fatal bronchiolitis obliterans associated with Castleman disease: Report of an autopsy case. Pathol Int 2021;71:170–72. [DOI] [PubMed] [Google Scholar]
- 68.Chorzelski TP, Hashimoto T, Amagai M, et al. Paraneoplastic pemphigus with cutaneous and serological features of pemphigus foliaceus. Br J Dermatol 1999;141:357–9. [DOI] [PubMed] [Google Scholar]
- 69.Kanaoka M, Matsukura S, Ishikawa H, et al. Paraneoplastic pemphigus associated with fatal bronchiolitis obliterans and appearance of anti-BP180 antibodies in the late stage of the disease. J Dermatol 2014;41:628–30. [DOI] [PubMed] [Google Scholar]
- 70.Hata T, Nishimoto S, Nagao K, et al. Ectopic expression of epidermal antigens renders the lung a target organ in paraneoplastic pemphigus. J Immunol 2013;191:83–90. [DOI] [PubMed] [Google Scholar]
- 71.Miida H, Kazama T, Inomata N, et al. Severe gastrointestinal involvement in paraneoplastic pemphigus. Eur J Dermatol 2006;16:420–2. [PubMed] [Google Scholar]
- 72.Odani K, Itoh A, Yanagita S, et al. Paraneoplastic Pemphigus Involving the Respiratory and Gastrointestinal Mucosae. Case Rep Pathol 2020;2020:7350759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frew JW, Murrell DF. Paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome): clinical presentations and pathogenesis. Dermatol Clin 2011;29:419–25, viii. [DOI] [PubMed] [Google Scholar]
- 74.Wang R, Li J, Wang M, et al. Prevalence of myasthenia gravis and associated autoantibodies in paraneoplastic pemphigus and their correlations with symptoms and prognosis. Br J Dermatol 2015;172:968–75. [DOI] [PubMed] [Google Scholar]
- 75.Winkler DT, Strnad P, Meier ML, et al. Myasthenia gravis, paraneoplastic pemphigus and thymoma, a rare triade. J Neurol 2007;254:1601–3. [DOI] [PubMed] [Google Scholar]
- 76.Horn TD, Anhalt GJ. Histologic features of paraneoplastic pemphigus. Arch Dermatol 1992;128:1091–5. [PubMed] [Google Scholar]
- 77.Joly P, Richard C, Gilbert D, et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol 2000;43:619–26. [DOI] [PubMed] [Google Scholar]
- 78.Rybojad M, Leblanc T, Flageul B, et al. Paraneoplastic pemphigus in a child with a T-cell lymphoblastic lymphoma. Br J Dermatol 1993;128:418–22. [DOI] [PubMed] [Google Scholar]
- 79.Fournet M, Roblot P, Levillain P, Guillet G, Machet L, Misery L. [Paraneoplastic pemphigus: Retrospective study of a case series]. Ann Dermatol Venereol 2018;145:564–71. [DOI] [PubMed] [Google Scholar]
- 80.Cummins DL, Mimouni D, Tzu J, Owens N, Anhalt GJ, Meyerle JH. Lichenoid paraneoplastic pemphigus in the absence of detectable antibodies. J Am Acad Dermatol 2007;56:153–9. [DOI] [PubMed] [Google Scholar]
- 81.Poot AM, Siland J, Jonkman MF, Pas HH, Diercks GF. Direct and indirect immunofluorescence staining patterns in the diagnosis of paraneoplastic pemphigus. Br J Dermatol 2016;174:912–5. [DOI] [PubMed] [Google Scholar]
- 82.Kwatra SG, Boozalis E, Pasieka H, Anhalt GJ. Decreased recognition of paraneoplastic pemphigus in patients previously treated with anti-CD 20 monoclonal antibodies. Br J Dermatol 2019;180:1238–39. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Chen T, Zhao J, et al. Extremities of the N-terminus of envoplakin and C-terminus of its linker subdomain are major epitopes of paraneoplastic pemphigus. J Dermatol Sci 2016;84:24–29. [DOI] [PubMed] [Google Scholar]
- 84.Helou J, Allbritton J, Anhalt GJ. Accuracy of indirect immunofluorescence testing in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol 1995;32:441–7. [DOI] [PubMed] [Google Scholar]
- 85.Poot AM, Diercks GF, Kramer D, et al. Laboratory diagnosis of paraneoplastic pemphigus. Br J Dermatol 2013;169:1016–24. [DOI] [PubMed] [Google Scholar]
- 86.Brandt O, Rafei D, Podstawa E, et al. Differential IgG recognition of desmoglein 3 by paraneoplastic pemphigus and pemphigus vulgaris sera. J Invest Dermatol 2012;132:1738–41. [DOI] [PubMed] [Google Scholar]
- 87.Futei Y, Amagai M, Hashimoto T, Nishikawa T. Conformational epitope mapping and IgG subclass distribution of desmoglein 3 in paraneoplastic pemphigus. J Am Acad Dermatol 2003;49:1023–8. [DOI] [PubMed] [Google Scholar]
- 88.Ohyama M, Amagai M, Hashimoto T, Nousari HC, Anhalt GJ, Nishikawa T. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol 2001;44:593–8. [DOI] [PubMed] [Google Scholar]
- 89.Tsuchisaka A, Kawano H, Yasukochi A, et al. Immunological and statistical studies of anti-BP180 antibodies in paraneoplastic pemphigus. J Invest Dermatol 2014;134:2283–87. [DOI] [PubMed] [Google Scholar]
- 90.Bazzini C, Begre N, Favre B, et al. Detection of autoantibodies against alpha-2-macroglobulin-like 1 in paraneoplastic pemphigus sera utilizing novel green fluorescent protein-based immunoassays. J Dermatol Sci 2020;98:173–78. [DOI] [PubMed] [Google Scholar]
- 91.Hashimoto T, Amagai M, Ning W, et al. Novel non-radioisotope immunoprecipitation studies indicate involvement of pemphigus vulgaris antigen in paraneoplastic pemphigus. J Dermatol Sci 1998;17:132–9. [DOI] [PubMed] [Google Scholar]
- 92.Witte M, Zillikens D, Schmidt E. Diagnosis of Autoimmune Blistering Diseases. Front Med (Lausanne) 2018;5:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges 2018;16:1077–91. [DOI] [PubMed] [Google Scholar]
- 94.Camisa C, Helm TN. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch Dermatol 1993;129:883–6. [PubMed] [Google Scholar]
- 95.Billet SE, Grando SA, Pittelkow MR. Paraneoplastic autoimmune multiorgan syndrome: review of the literature and support for a cytotoxic role in pathogenesis. Autoimmunity 2006;39:617–30. [DOI] [PubMed] [Google Scholar]
- 96.Karlhofer FM, Hashimoto T, Slupetzky K, et al. 230-kDa and 190-kDa proteins in addition to desmoglein 1 as immunological targets in a subset of pemphigus foliaceus with a combined cell-surface and basement membrane zone immune staining pattern. Exp Dermatol 2003;12:646–54. [DOI] [PubMed] [Google Scholar]
- 97.Cozzani E, Dal Bello MG, Mastrogiacomo A, Drosera M, Parodi A. Antidesmoplakin antibodies in pemphigus vulgaris. Br J Dermatol 2006;154:624–8. [DOI] [PubMed] [Google Scholar]
- 98.Cozzani E, Di Zenzo G, Calabresi V, et al. Anti-desmoplakin antibodies in erythema multiforme and Stevens-Johnson syndrome sera: pathogenic or epiphenomenon? Eur J Dermatol 2011;21:32–6. [DOI] [PubMed] [Google Scholar]
- 99.Streifel AM, Wessman LL, Schultz BJ, Miller D, Pearson DR. Refractory mucositis associated with underlying follicular dendritic cell sarcoma of the thymus: Paraneoplastic pemphigus versus malignancy-exacerbated pemphigus vulgaris. JAAD Case Rep 2019;5:933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joly P, Horvath B, Patsatsi A, et al. Updated S2K guidelines on the management of pemphigus vulgaris and foliaceus initiated by the european academy of dermatology and venereology (EADV). J Eur Acad Dermatol Venereol 2020;34:1900–13. [DOI] [PubMed] [Google Scholar]
- 101.Foedinger D, Sterniczky B, Elbe A, Anhalt G, Wolff K, Rappersberger K. Autoantibodies against desmoplakin I and II define a subset of patients with erythema multiforme major. J Invest Dermatol 1996;106:1012–6. [DOI] [PubMed] [Google Scholar]
- 102.Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet 2001;357:1850–1. [DOI] [PubMed] [Google Scholar]
- 103.Goletz S, Probst C, Komorowski L, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol 2019;180:149–56. [DOI] [PubMed] [Google Scholar]
- 104.Rashid H, Meijer JM, Bolling MC, Diercks GFH, Pas HH, Horvath B. Insights into clinical and diagnostic findings as well as treatment responses in patients with mucous membrane pemphigoid: A retrospective cohort study. J Am Acad Dermatol 2021. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt E, Rashid H, Marzano AV, et al. European Guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part II. J Eur Acad Dermatol Venereol 2021;35:1926–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du G, Patzelt S, van Beek N, Schmidt E. Mucous membrane pemphigoid. Autoimmun Rev 2022;21:103036. [DOI] [PubMed] [Google Scholar]
- 107.Chiorean R, Danescu S, Virtic O, et al. Molecular diagnosis of anti-laminin 332 (epiligrin) mucous membrane pemphigoid. Orphanet J Rare Dis 2018;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamada H, Nobeyama Y, Matsuo K, et al. A case of paraneoplastic pemphigus associated with triple malignancies in combination with antilaminin-332 mucous membrane pemphigoid. Br J Dermatol 2012;166:230–1. [DOI] [PubMed] [Google Scholar]
- 109.Okahashi K, Oiso N, Ishii N, et al. Paraneoplastic pemphigus presenting lichen planus-like lesions. J Dermatol 2019;46:e140–e42. [DOI] [PubMed] [Google Scholar]
- 110.Coelho S, Reis JP, Tellechea O, Figueiredo A, Black M. Paraneoplastic pemphigus with clinical features of lichen planus associated with low-grade B cell lymphoma. Int J Dermatol 2005;44:366–71. [DOI] [PubMed] [Google Scholar]
- 111.Bowen GM, Peters NT, Fivenson DP, et al. Lichenoid dermatitis in paraneoplastic pemphigus: a pathogenic trigger of epitope spreading? Arch Dermatol 2000;136:652–6. [DOI] [PubMed] [Google Scholar]
- 112.Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol 2009;34:e754–6. [DOI] [PubMed] [Google Scholar]
- 113.Yokokura H, Demitsu T, Kakurai M, et al. Paraneoplastic pemphigus mimicking erosive mucosal lichen planus associated with primary hepatocellular carcinoma. J Dermatol 2006;33:842–5. [DOI] [PubMed] [Google Scholar]
- 114.Passeron T, Bahadoran P, Lacour JP, et al. Paraneoplastic pemphigus presenting as erosive lichen planus. Br J Dermatol 1999;140:552–3. [DOI] [PubMed] [Google Scholar]
- 115.Wang M, Wang X, Chen T, et al. Erosive oral lichen planus as a sign of paraneoplastic pemphigus. J Dermatol 2016;43:983. [DOI] [PubMed] [Google Scholar]
- 116.Kelly S, Schifter M, Fulcher DA, Lin MW. Paraneoplastic pemphigus: two cases of intra-abdominal malignancy presenting solely as treatment refractory oral ulceration. J Dermatol 2015;42:300–4. [DOI] [PubMed] [Google Scholar]
- 117.Chou S, Zhao C, Hwang SJE, Fernandez-Penas P. PD-1 inhibitor-associated lichenoid inflammation with incidental suprabasilar acantholysis or vesiculation-Report of 4 cases. J Cutan Pathol 2017;44:851–56. [DOI] [PubMed] [Google Scholar]
- 118.Malphettes M, Gerard L, Galicier L, et al. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis 2015;61:e13–9. [DOI] [PubMed] [Google Scholar]
- 119.Hayanga AJ, Lee TM, Pannucci CJ, et al. Paraneoplastic pemphigus in a burn intensive care unit: case report and review of the literature. J Burn Care Res 2010;31:826–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kokubu H, Nishikawa J, Kato T, et al. Paraneoplastic Pemphigus Mimicking Toxic Epidermal Necrolysis Associated with Follicular Lymphoma: Possible Pathological Role of CD8 T Cells. Acta Derm Venereol 2020;100:adv00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oiso N, Yanagihara S, Tateishi C, et al. Case of Antiplakin Dermatosis. JAMA Dermatol 2021;157:602–03. [DOI] [PubMed] [Google Scholar]
- 122.Mahler V, Antoni C, Anhalt GJ, et al. Graft-versus-host-like mucocutaneous eruptions with serological features of paraneoplastic pemphigus and systemic lupus erythematosus in a patient with non-Hodgkin’s lymphoma. Dermatology 1998;197:78–83. [DOI] [PubMed] [Google Scholar]
- 123.Reich K, Brinck U, Letschert M, et al. Graft-versus-host disease-like immunophenotype and apoptotic keratinocyte death in paraneoplastic pemphigus. Br J Dermatol 1999;141:739–46. [DOI] [PubMed] [Google Scholar]
- 124.Maruta CW, Miyamoto D, Aoki V, Carvalho RGR, Cunha BM, Santi CG. Paraneoplastic pemphigus: a clinical, laboratorial, and therapeutic overview. An Bras Dermatol 2019;94:388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Didona D, Fania L, Didona B, Eming R, Hertl M, Di Zenzo G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grando SA. New approaches to the treatment of pemphigus. J Investig Dermatol Symp Proc 2004;9:84–91. [DOI] [PubMed] [Google Scholar]
- 127.Paolino G, Didona D, Magliulo G, et al. Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Riera-Monroig J, Iranzo P, Ishii N, Hashimoto T, Mascaro JM Jr. Persistence of antienvoplakin and antiperiplakin antibodies in a patient with paraneoplastic pemphigus 20 years after remission. Br J Dermatol 2020;182:797–98. [DOI] [PubMed] [Google Scholar]
- 129.Joly P, Sin C. [Pemphigus: a review]. Ann Dermatol Venereol 2011;138:182–200. [DOI] [PubMed] [Google Scholar]
- 130.Borradori L, Lombardi T, Samson J, Girardet C, Saurat JH, Hugli A. Anti-CD20 monoclonal antibody (rituximab) for refractory erosive stomatitis secondary to CD20(+) follicular lymphoma-associated paraneoplastic pemphigus. Arch Dermatol 2001;137:269–72. [PubMed] [Google Scholar]
- 131.Barnadas M, Roe E, Brunet S, et al. Therapy of paraneoplastic pemphigus with Rituximab: a case report and review of literature. J Eur Acad Dermatol Venereol 2006;20:69–74. [DOI] [PubMed] [Google Scholar]
- 132.Frew JW, Murrell DF. Current management strategies in paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome). Dermatol Clin 2011;29:607–12. [DOI] [PubMed] [Google Scholar]
- 133.Bech R, Baumgartner-Nielsen J, Peterslund NA, Steiniche T, Deleuran M, d’Amore F. Alemtuzumab is effective against severe chronic lymphocytic leukaemia-associated paraneoplastic pemphigus. Br J Dermatol 2013;169:469–72. [DOI] [PubMed] [Google Scholar]
- 134.Anhalt G Paraneoplastic Pemphigus: diagnosis. Clinical associations and management. International Pemphigus Pemphigoid Foundation Fifteenth Annual Patient/Doctor Meeting; Boston (MA)May 18–20, 2012. [Google Scholar]
- 135.Wang J, Zhang Y, Pan M. Thalidomide as a potential adjuvant treatment for paraneoplastic pemphigus: A single-center experience. Dermatol Ther 2020;33:e14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen W, Zhao L, Guo L, et al. Clinical and pathological features of bronchiolitis obliterans requiring lung transplantation in paraneoplastic pemphigus associated with Castleman disease. Clin Respir J 2022;16:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


