Abstract
OBJECTIVES:
Clear cell carcinoma is a high-risk subtype of endometrial cancer. Some patients have a mixture of clear cell carcinoma with other histologic types (endometrioid or serous) or cannot be neatly assigned to one of these types. Protocol GOG-8032 within GOG-210 was designed to determine whether these tumors differ from pure clear cell carcinoma in stage at diagnosis, initial pattern of spread, or patient survival.
METHODS:
The term “mixed” was applied to tumors with multiple identifiable components, and “indeterminate” was applied to tumors with features intermediate between different histologic types. Three hundred eleven women with pure, mixed, or indeterminate clear cell carcinoma were identified in a larger cohort of patients undergoing hysterectomy for endometrial cancer in GOG-210. Histologic slides were centrally reviewed by expert pathologists. Baseline and follow-up data were analyzed.
RESULTS:
One hundred thirty-six patients had pure clear cell carcinoma and 175 had a mixed or indeterminate clear cell pattern. Baseline clinicopathologic characteristics were similar except for a small difference in age at presentation. Univariate survival analysis confirmed the significance of typical endometrial cancer prognostic factors. Patients in the mixed categories had disease-free and overall survival similar to pure clear cell carcinoma, but the indeterminate clear cell/endometrioid group had longer survival.
CONCLUSION:
In clear cell endometrial cancer, the presence of a definite admixed endometrioid or serous component did not correlate with a significant difference in prognosis. Patients whose tumors had indeterminate clear cell features had better prognosis. Some of these tumors may be endometrioid tumors mimicking clear cell carcinoma.
Keywords: Endometrial carcinoma, clear cell carcinoma, malignant mixed tumors, pathology, gynecologic oncology, survival analysis, clinical trials
INTRODUCTION
Endometrial carcinoma is the most common malignancy of the female genital tract, with an estimated 66,950 new cases and 12,550 deaths in the United States in 2022 [1]. The World Health Organization Classification of Tumours (5th edition) recognizes five major histologic types of endometrial epithelial malignancies [2], of which endometrioid adenocarcinoma is the most common. The histologic diagnosis or cell type of a tumor correlates with biologic behavior and can guide the selection of diagnostic/staging procedures, biomarker testing, and adjuvant treatment. In addition, because patients with tumors of different cell types may have different expected survival, the precise histologic diagnosis may be important for counseling.
Clear cell carcinoma (CCC) is a malignant tumor of the endometrium characterized by the presence of epithelial cells with clear or eosinophilic cytoplasm, hobnail cells, and typical histologic patterns described as solid, papillary, or tubulocystic growth, with these three patterns often intermingled in a single tumor [3]. Although no specific molecular finding is entirely sensitive or specific for CCC, the tumors often have somatic mutations in TP53, PPP2R1A, PIK3CA, PIK3R1, KRAS, and ARID1A [4–7]. Among all histologic types of endometrial cancer, between 1% and 6% of cases are CCC [3]. This “high risk” subtype [8,9] is intrinsically high grade, and patients have a prognosis similar to those with other type 2 endometrial malignancies [10] such as serous carcinoma. The prognosis of patients with CCC is worse than FIGO grade 1–2 endometrioid adenocarcinoma and similar to FIGO grade 3 endometrioid adenocarcinoma [11,12].
A persistent difficulty in gynecologic pathology is how to appropriately classify and predict the behavior of tumors that do not clearly fall into a specific diagnostic category. One such group of tumors is “mixed”, which is currently defined by the World Health Organization (5th edition) [2] as endometrial carcinomas that contain two or more identifiable types of tumor, of which at least one is serous or clear cell. The mixed category is important to identify because recent evidence suggests that any component of a high-risk histology confers an adverse outcome [13–15]. The World Health Organization definition of mixed carcinoma requires that the two components be spatially distinct and identifiable. Another group of tumors that present a challenge for pathologists is those that have a combination of features that do not fit neatly into any of the cell types. We refer to these tumors, which are encountered with some frequency, as “indeterminate”.
The present study reports on the behavior of endometrial cancers with a clear cell component, with an emphasis on mixed and indeterminate histologic types. The study draws upon Gynecologic Oncology Group (GOG) protocol GOG-210, under which a large cohort of women with various histologic types of endometrial carcinoma underwent complete epidemiologic evaluation and homogenous initial treatment including hysterectomy, salpingo-oophorectomy, and pelvic and para-aortic lymphadenectomy. A sub-protocol (GOG-8032) was established to use highly specialized pathology review to provide more detailed histopathologic annotation of tumor type. One aim of the protocol was to examine the significance of mixed and indeterminate tumor types. A previous manuscript described tumors with a serous component, comparing pure serous tumors to mixed serous/endometrioid tumors or those with histology indeterminate between serous and endometrioid [13]. We now report the clinical and pathologic characteristics of women enrolled in GOG-210 with pure and non-pure clear cell tumors to document differences in presentation and prognosis. Specifically, we asked whether a tumor with mixed endometrioid and clear cell carcinoma has the same pattern of spread and prognosis as a pure clear cell carcinoma. Additionally, we sought to determine the behavior of tumors that cannot be neatly classified as either clear cell or endometrioid carcinoma.
METHODS
Inclusion and Exclusion Criteria and Treatment:
GOG-210, “A Molecular Staging Study of Endometrial Carcinoma,” enrolled patients from September 22, 2003, to December 1, 2011. The protocol was approved by the Institutional Review Board at each participating institution. On September 24, 2007, the protocol was amended by restricting eligibility to patients with high-risk cell types and underrepresented minorities, in order to enhance accrual of these sub-populations. Entry into the study required a biopsy or curettage proving the diagnosis of endometrial carcinoma. Participants were asked to complete an epidemiologic questionnaire and underwent initial treatment with total hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymphadenectomy, with collection of serum, urine, fresh-frozen and formalin-fixed neoplastic and non-neoplastic tissues for other investigations. Adjuvant therapy and any treatment for metastatic or recurrent disease were not specified by the protocol. Survival data were frozen as of January 15, 2019.
Central Pathology Review:
For each patient, the local pathologist reviewed the material from the hysterectomy and provided slides for central review depicting pertinent pathologic characteristics including histologic type and tumor stage. Immunostains or slides depicting lymphovascular space invasion or depth of invasion were not required but were reviewed if provided. Slides were initially reviewed by rotating pairs of GOG Pathology Committee members attending semiannual meetings, including but not limited to the authors. After comparing the central review data on these cases with that from the submitting institution, it was determined that the reproducibility in assessment was sufficiently great that central review was not necessary for endometrioid, adenosquamous, and mucinous carcinomas of grades 1 and 2, stages IA-IC (FIGO 1988). All cases of other histologic types were submitted for a third review carried out at double-headed microscopes by rotating pairs of pathologists drawn from a group of six (“G6”) with special expertise in gynecologic pathology, following mutually agreed-upon criteria for diagnoses and interpretations of findings. The expert pathologists were not masked to the local diagnoses and were able to review the original reports (identifiers were redacted). When G6 determinations were not available, the results of central (GOG) pathology review or local institutional pathology review were substituted to minimize missing values.
Cell Type Definitions:
Clear cell carcinoma was defined per Crum and Lee’s Diagnostic Gynecologic and Obstetric Pathology [16], with the following additional stipulations agreed upon by the G6 pathologists: Architectural, cytoplasmic, and nuclear features together were used for diagnosis; clear cytoplasm by itself was considered neither necessary nor sufficient for diagnosis. Most CCC were expected to have papillary patterns present at least focally. Papillae were expected to be round and non-hierarchically branched, lined by only one or two layers of cells. Stromal hyalinization was expected to sometimes be prominent. Tumor cells were typically expected to be cuboidal, not tall columnar. Flattened and low columnar cells were permitted. Exfoliation (budding) of tumor cells was not expected. Cytoplasm was allowed to be clear or eosinophilic (oxyphilic). Nuclei in papillary tumors were expected to be round and uniform in size and could have prominent nucleoli. Occasional scattered larger nuclei were allowed. Tubulocystic CCC were expected to have flattened, hobnail, or cuboidal cells, with either small nuclei or nuclei resembling those of papillary CCC. The mitotic rate was expected to be low. Mitotic rates exceeding 8–10 mitotic figures per 10 high-power fields were considered an exceptional feature. No grade was given to these CCC.
On the basis of published literature, prior experience, and review of protocol cases, the G6 determined that, although some cases of CCC were composed purely of that individual cell type, others fell into a mixed or indeterminate group. Therefore, the following five categories were used: pure CC, mixed clear cell and endometrioid carcinoma (CC-EM-M), mixed clear cell and serous carcinoma (CC-SER-M), indeterminate clear cell vs. endometrioid carcinoma (CC-EM-I), and indeterminate clear cell vs. serous carcinoma (CC-SER-I). The “mixed” categories were used for tumors that displayed two or more well-defined patterns of neoplasm with any percentage of admixture. The “indeterminate” categories were used for tumors that displayed a single pattern (not a mixture) that had a combination of features that did not fit neatly into any of the cell types. Tumor classification was performed primarily on histologic grounds, supported by any immunohistochemical data provided in the original pathology report. Immunostained slides were not generally available.
Statistical Considerations:
SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. All statistical tests were two-sided. Based upon the protocol, 37 statistical tests were planned, and a Holm-Bonferroni stepdown correction was used to adjust P-values for multiple hypothesis testing with a family-wise error rate of 0.05 [17]. All observations with missing values (including not reported/not assessed) were excluded from statistical analyses.
The association of the five clear cell histologic subtypes (CC, CC-EM-M, CC-SER-M, CC-EM-I, and CC-SER-I) with each of the baseline patient or clinicopathologic characteristics was evaluated either by Monte Carlo permutation-based exact chi-square tests for discrete-type characteristics or by Monte Carlo permutation-based exact Kruskal-Wallis tests for interval-type characteristics.
Log-rank tests were used to compare progression-free survival (PFS) and overall survival (OS) among patients with the five tumor subtypes. PFS was defined as the time from study entry to disease recurrence or progression, death, or last contact, whichever occurred first. PFS was censored in patients who were alive and had not experienced disease progression or recurrence at last contact. OS was defined as the time from study entry to death due to any cause or last contact. The relationship of PFS and OS with baseline characteristics (whenever feasible) was also examined by log-rank tests. A Cox proportional hazards model was used to estimate corresponding hazard ratios [18]. Given the small numbers, patients with race other than black or white were not included in the survival analysis.
RESULTS
There were 6,124 patients enrolled in GOG-210. After removing patients with ineligible cell types, non-endometrial primary tumor site, history of pretreatment, or other factors, 5,866 were eligible for further analysis. After application of standardized terminology and criteria, 3,657 patients (62.3%) had pure endometrioid tumors of any grade, and 934 (15.9%) had a serous component without evidence of a clear cell component. These patients had either pure serous carcinoma, mixed serous and endometrioid carcinoma, or indeterminate serous versus endometrioid carcinoma. A group of six highly specialized pathologists conducted central pathology review of 3,566 (60.8%) tumors, including 308 out of 311 (99%) tumors with a clear cell component.
Among the 3,715 (63.3%) participants enrolled during the initial, unrestricted period of enrollment, 3.5% had CCC or one of its variants. Among the 2,151 (36.7%) enrolled after the protocol was modified to prioritize the inclusion of minorities and patients with rare tumor types, 8.5% had CCC or one of its variants (Table 1). In the entire cohort, 311 patients out of 5,866 (5.3%) had tumors that fell into one of five CCC categories: pure clear cell carcinoma (CC, 136 patients), mixed clear cell and endometrioid carcinoma (CC-EM-M, 70 patients), mixed clear cell and serous carcinoma (CC-SER-M, 52 patients), indeterminate clear cell versus endometrioid carcinoma (CC-EM-I, 32 patients), and indeterminate clear cell versus serous carcinoma (CC-SER-I, 21 patients). No tumors had mixtures or features of all three cell types.
Table 1.
Distribution of GOG-210 tumor histology and grade (for endometrioid tumors only) according to enrollment period and G6 (group of six expert pathologists) review status.
| Enrollment period |
G6 review status |
Total |
|||
|---|---|---|---|---|---|
| Tumor histology | Unrestricted | Restricted | Yes | No | |
| Endometrioid | 2,741 | 916 | 1,420 | 2,237 | 3,657 |
| Endometrioid, grade 1 | 1,407 | 345 | 403 | 1,349 | 1,752 |
| Endometrioid, grade 2 | 985 | 267 | 419 | 833 | 1,252 |
| Endometrioid, grade 3/not graded | 349 | 304 | 598 | 55 | 653 |
|
| |||||
| Serous | 423 | 511 | 914 | 20 | 934 |
| Pure serous | 273 | 390 | 644 | 19 | 663 |
| Mixed serous/endometrioid | 91 | 47 | 138 | 0 | 138 |
| Indeterminate serous/endometrioid | 59 | 74 | 132 | 1 | 133 |
|
| |||||
| Clear cell | 129 | 182 | 308 | 3 | 311 |
| Pure clear cell | 54 | 82 | 133 | 3 | 136 |
| Mixed clear cell/endometrioid | 40 | 30 | 70 | 0 | 70 |
| Indeterminate clear cell/endometrioid | 15 | 17 | 32 | 0 | 32 |
| Mixed clear cell/serous | 17 | 35 | 52 | 0 | 52 |
| Indeterminate clear cell/serous | 3 | 18 | 21 | 0 | 21 |
|
| |||||
| All others | 422 | 542 | 924 | 40 | 964 |
|
| |||||
| Total | 3,715 | 2,151 | 3,566 | 2,300 | 5,866 |
Subsequent analyses were carried out using pure clear cell carcinoma (CC) as the reference group to determine the significance of the CC-EM-M, CC-SER-M, CC-EM-I, and CC-SER-I subtypes. Clinicopathologic characteristics of these groups were generally similar (Table 2). Patients in the CC-EM mixed and indeterminate categories were younger than the others (P=0.0276 for overall effect). Patients with these tumor types did not significantly differ from the reference with regard to body mass index; race; ethnicity; myometrial invasion; lymphovascular invasion; involvement of the cervix, adnexa, lymph nodes, peritoneum, or omentum; FIGO stage; or planned use of adjuvant therapy.
Table 2.
Distribution of baseline characteristics according to clear cell histology subtype for GOG-210 patients, regardless of enrollment period. “Unadjusted” P-values are nominal. “Adjusted” P-values reflect a Bonferroni correction.
| Characteristic | CC |
CC-EM-M |
CC-EM-I |
CC-SER-M |
CC-SER-I |
Total |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | Unadjusted | Adjusted | |
| Age (years) | 0.0012 | 0.0276 | ||||||||||||
| Mean | 68 | 64 | 63 | 70 | 65 | 66 | ||||||||
| St. Dev. | 10 | 11 | 9 | 9 | 10 | 10 | ||||||||
| Median | 67 | 63 | 61 | 71 | 66 | 66 | ||||||||
| Range | 41–89 | 34–90 | 46–82 | 51–91 | 45–87 | 34–91 | ||||||||
| Age < 40 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.3 | ||
| 40 ≤ Age < 50 | 6 | 4.4 | 6 | 8.6 | 1 | 3.1 | 0 | 0.0 | 1 | 4.8 | 14 | 4.5 | ||
| 50 ≤ Age < 60 | 25 | 18.4 | 18 | 25.7 | 13 | 40.6 | 9 | 17.3 | 5 | 23.8 | 70 | 22.5 | ||
| 60 ≤ Age < 70 | 53 | 39.0 | 27 | 38.6 | 12 | 37.5 | 15 | 28.8 | 11 | 52.4 | 118 | 37.9 | ||
| 70 ≤ Age < 80 | 38 | 27.9 | 13 | 18.6 | 4 | 12.5 | 22 | 42.3 | 3 | 14.3 | 80 | 25.7 | ||
| Age ≥ 80 | 14 | 10.3 | 5 | 7.1 | 2 | 6.3 | 6 | 11.5 | 1 | 4.8 | 28 | 9.0 | ||
|
| ||||||||||||||
| BMI (kg/m2) | 0.5629 | 1.0000 | ||||||||||||
| Mean | 30 | 33 | 29 | 30 | 31 | 31 | ||||||||
| St. Dev. | 8 | 12 | 7 | 6 | 8 | 9 | ||||||||
| Median | 29 | 31 | 30 | 30 | 29 | 30 | ||||||||
| Range | 19–53 | 15–78 | 17–47 | 17–47 | 20–49 | 15–78 | ||||||||
| BMI < 18.5 | 0 | 0.0 | 2 | 2.9 | 2 | 6.3 | 2 | 3.8 | 0 | 0.0 | 6 | 1.9 | ||
| 18.5 ≤ BMI < 25 | 41 | 30.1 | 13 | 18.6 | 7 | 21.9 | 10 | 19.2 | 5 | 23.8 | 76 | 24.4 | ||
| 25 ≤ BMI < 30 | 35 | 25.7 | 16 | 22.9 | 9 | 28.1 | 15 | 28.8 | 7 | 33.3 | 82 | 26.4 | ||
| 30 ≤ BMI < 35 | 26 | 19.1 | 17 | 24.3 | 9 | 28.1 | 17 | 32.7 | 4 | 19.0 | 73 | 23.5 | ||
| BMI ≥ 35 | 34 | 25.0 | 22 | 31.4 | 5 | 15.6 | 8 | 15.4 | 5 | 23.8 | 74 | 23.8 | ||
|
| ||||||||||||||
| Race | 0.2929 | 1.0000 | ||||||||||||
| Asian | 3 | 2.2 | 2 | 2.9 | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 | 6 | 1.9 | ||
| Black/African American | 23 | 16.9 | 9 | 12.9 | 6 | 18.8 | 9 | 17.3 | 6 | 28.6 | 53 | 17.0 | ||
| Amerindian/Alaskan | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 3.8 | 0 | 0.0 | 2 | 0.6 | ||
| White | 109 | 80.1 | 58 | 82.9 | 26 | 81.3 | 40 | 76.9 | 15 | 71.4 | 248 | 79.7 | ||
| NA/NR | 1 | 0.7 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.6 | ||
|
| ||||||||||||||
| Ethnicity | 0.1380 | 1.0000 | ||||||||||||
| Hispanic | 7 | 5.1 | 0 | 0.0 | 0 | 0.0 | 2 | 3.8 | 2 | 9.5 | 11 | 3.5 | ||
| Non-Hispanic | 113 | 83.1 | 61 | 87.1 | 29 | 90.6 | 43 | 82.7 | 18 | 85.7 | 264 | 84.9 | ||
| NA/NR | 16 | 11.8 | 9 | 12.9 | 3 | 9.4 | 7 | 13.5 | 1 | 4.8 | 36 | 11.6 | ||
|
| ||||||||||||||
| Myoinvasion | 0.0818 | 1.0000 | ||||||||||||
| None | 47 | 34.6 | 11 | 15.7 | 7 | 21.9 | 15 | 28.8 | 6 | 28.6 | 86 | 27.7 | ||
| Inner Half | 50 | 36.8 | 26 | 37.1 | 17 | 53.1 | 19 | 36.5 | 6 | 28.6 | 118 | 37.9 | ||
| Outer Half | 31 | 22.8 | 30 | 42.9 | 5 | 15.6 | 14 | 26.9 | 7 | 33.3 | 87 | 28.0 | ||
| Serosa | 3 | 2.2 | 2 | 2.9 | 2 | 6.3 | 1 | 1.9 | 1 | 4.8 | 9 | 2.9 | ||
| NA/NR | 5 | 3.7 | 1 | 1.4 | 1 | 3.1 | 3 | 5.8 | 1 | 4.8 | 11 | 3.5 | ||
|
| ||||||||||||||
| Lymphovascular invasion | 0.0211 | 0.4220 | ||||||||||||
| No | 93 | 68.4 | 36 | 51.4 | 24 | 75.0 | 31 | 59.6 | 15 | 71.4 | 199 | 64.0 | ||
| Yes | 37 | 27.2 | 34 | 48.6 | 7 | 21.9 | 20 | 38.5 | 5 | 23.8 | 103 | 33.1 | ||
| NA/NR | 6 | 4.4 | 0 | 0.0 | 1 | 3.1 | 1 | 1.9 | 1 | 4.8 | 9 | 2.9 | ||
|
| ||||||||||||||
| Cervical invasion | 0.2563 | 1.0000 | ||||||||||||
| No | 98 | 72.1 | 46 | 65.7 | 20 | 62.5 | 31 | 59.6 | 14 | 66.7 | 209 | 67.2 | ||
| Glandular (G) | 4 | 2.9 | 1 | 1.4 | 2 | 6.3 | 6 | 11.5 | 1 | 4.8 | 14 | 4.5 | ||
| Stromal (S) | 8 | 5.9 | 2 | 2.9 | 1 | 3.1 | 5 | 9.6 | 0 | 0.0 | 16 | 5.1 | ||
| Both G and S | 22 | 16.2 | 19 | 27.1 | 7 | 21.9 | 8 | 15.4 | 5 | 23.8 | 61 | 19.6 | ||
| Indeterminate | 1 | 0.7 | 2 | 2.9 | 1 | 3.1 | 1 | 1.9 | 0 | 0.0 | 5 | 1.6 | ||
| NA/NR | 3 | 2.2 | 0 | 0.0 | 1 | 3.1 | 1 | 1.9 | 1 | 4.8 | 6 | 1.9 | ||
|
| ||||||||||||||
| Adnexal involvement | 0.3243 | 1.0000 | ||||||||||||
| No | 119 | 87.5 | 59 | 84.3 | 29 | 90.6 | 42 | 80.8 | 16 | 76.2 | 265 | 85.2 | ||
| Yes | 15 | 11.0 | 10 | 14.3 | 2 | 6.3 | 9 | 17.3 | 5 | 23.8 | 41 | 13.2 | ||
| NA/NR | 2 | 1.5 | 1 | 1.4 | 1 | 3.1 | 1 | 1.9 | 0 | 0.0 | 5 | 1.6 | ||
|
| ||||||||||||||
| Nodal involvement | 0.0365 | 0.6935 | ||||||||||||
| No | 107 | 78.7 | 47 | 67.1 | 26 | 81.3 | 36 | 69.2 | 13 | 61.9 | 229 | 73.6 | ||
| Yes | 21 | 15.4 | 20 | 28.6 | 3 | 9.4 | 14 | 26.9 | 7 | 33.3 | 65 | 20.9 | ||
| NA/NR | 8 | 5.9 | 3 | 4.3 | 3 | 9.4 | 2 | 3.8 | 1 | 4.8 | 17 | 5.5 | ||
|
| ||||||||||||||
| Peritoneal involvement | 0.1145 | 1.0000 | ||||||||||||
| No | 71 | 52.2 | 37 | 52.9 | 17 | 53.1 | 18 | 34.6 | 9 | 42.9 | 152 | 48.9 | ||
| Yes | 9 | 6.6 | 7 | 10.0 | 1 | 3.1 | 8 | 15.4 | 2 | 9.5 | 27 | 8.7 | ||
| NA/NR | 56 | 41.2 | 26 | 37.1 | 14 | 43.8 | 26 | 50.0 | 10 | 47.6 | 132 | 42.4 | ||
|
| ||||||||||||||
| Omental involvement | 0.3778 | 1.0000 | ||||||||||||
| No | 94 | 69.1 | 51 | 72.9 | 25 | 78.1 | 33 | 63.5 | 16 | 76.2 | 219 | 70.4 | ||
| Yes | 9 | 6.6 | 5 | 7.1 | 0 | 0.0 | 5 | 9.6 | 3 | 14.3 | 22 | 7.1 | ||
| NA/NR | 33 | 24.3 | 14 | 20.0 | 7 | 21.9 | 14 | 26.9 | 2 | 9.5 | 70 | 22.5 | ||
|
| ||||||||||||||
| FIGO stage | 0.5436 | 1.0000 | ||||||||||||
| I | 78 | 57.4 | 32 | 45.7 | 18 | 56.3 | 24 | 46.2 | 10 | 47.6 | 162 | 52.1 | ||
| II | 19 | 14.0 | 9 | 12.9 | 6 | 18.8 | 6 | 11.5 | 2 | 9.5 | 42 | 13.5 | ||
| III | 30 | 22.1 | 24 | 34.3 | 8 | 25.0 | 16 | 30.8 | 6 | 28.6 | 84 | 27.0 | ||
| IV | 9 | 6.6 | 5 | 7.1 | 0 | 0.0 | 6 | 11.5 | 3 | 14.3 | 23 | 7.4 | ||
|
| ||||||||||||||
| Planned adjuvant therapy | 0.4062 | 1.0000 | ||||||||||||
| CT/RT/CT+RT/Other | 90 | 66.2 | 53 | 75.7 | 20 | 62.5 | 39 | 75.0 | 17 | 81.0 | 219 | 70.4 | ||
| None/NA/NR | 46 | 33.8 | 17 | 24.3 | 12 | 37.5 | 13 | 25.0 | 4 | 19.0 | 92 | 29.6 | ||
BMI, body mass index; CT, chemotherapy; FIGO, International Federation of Gynecology and Obstetrics; NA/NR, not available/not reported; RT, radiotherapy
Follow-up data were examined by using a log-rank test to determine whether these tumor types had differences in biologic behavior or outcome. The overall median follow-up time for vital status was 100.7 months (120.4 months for the unrestricted period and 93.3 months for the restricted period). Survival analyses were performed initially by using a univariate model (Table 3). At the nominal 0.0045 significance level after adjustment, there was no difference in PFS between the five CCC subtypes. Pairwise analysis showed that patients with CC-EM-I had a lower event rate than CC (HR 0.521, 95% CI 0.241–0.997) and CC-SER-I (HR 0.383, 95% CI 0.156–0.906). Results from additional log-rank tests indicated that PFS was statistically significantly shorter with incrementally deeper myometrial invasion, lymphovascular invasion, cervical stromal invasion, adnexal involvement, nodal involvement, omental involvement, and incremental FIGO stage. Similar results were found for OS, including a lower event rate in CC-EM-I versus other categories (specifically CC-SER-I with HR 0.289, 95% CI 0.108–0.721, and CC-SER-M with HR 0.413, 95% CI 0.163–0.928). Given that the log-rank test result did not support that the overall PFS distribution was statistically significantly different among the clear cell subtypes, multiple regression survival analysis was not pursued.
Table 3.
Univariate analysis of progression-free survival and overall survival in clear cell carcinoma patients. “Unadjusted” P-values are nominal. “Adjusted” P-values reflect a Bonferroni correction.
| Characteristic | Comparison | Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted P-value1 | Adjusted P-value | Hazard Ratio2 | 95% Confidence Interval | Unadjusted P-value1 | Adjusted P-value | Hazard Ratio2 | 95% Confidence Interval | ||
| Enrollment period | Restricted vs Unrestricted | 0.2517 | 1.0000 | 1.227 | 0.867–1.752 | 0.2258 | 1.0000 | 1.259 | 0.8699–1.8393 |
|
| |||||||||
| Clear cell subtype | CC-EM-I vs CC | 0.2152 | 1.0000 | 0.521 | 0.241–0.997 | 0.0835 | 1.0000 | 0.498 | 0.206–1.024 |
|
|
|||||||||
| CC-EM-M vs CC | 0.827 | 0.517–1.288 | 0.891 | 0.540–1.428 | |||||
|
|
|||||||||
| CC-SER-I vs CC | 1.361 | 0.697–2.441 | 1.721 | 0.875–3.118 | |||||
|
|
|||||||||
| CC-SER-M vs CC | 1.035 | 0.627–1.650 | 1.205 | 0.710–1.973 | |||||
|
|
|||||||||
| CC-EM-I vs CC-EM-M | 0.630 | 0.280–1.290 | 0.559 | 0.222–1.231 | |||||
|
|
|||||||||
| CC-EM-I vs CC-SER-I | 0.383 | 0.156–0.906 | 0.289 | 0.108–0.721 | |||||
|
|
|||||||||
| CC-EM-I vs CC-SER-M | 0.504 | 0.221–1.054 | 0.413 | 0.163–0.928 | |||||
|
|
|||||||||
| CC-EM-M vs CC-SER-I | 0.608 | 0.314–1.246 | 0.518 | 0.263–1.074 | |||||
|
|
|||||||||
| CC-EM-M vs CC-SER-M | 0.799 | 0.458–1.406 | 0.740 | 0.411–1.341 | |||||
|
|
|||||||||
| CC-SER-I vs CC-SER-M | 1.315 | 0.633–2.596 | 1.428 | 0.681–2.858 | |||||
|
| |||||||||
| Age (years) | High (>median) vs low (≤median) | 0.3232 | 1.0000 | 1.189 | 0.844–1.681 | 0.1969 | 1.0000 | 1.272 | 0.883–1.840 |
|
| |||||||||
| BMI (kg/m2) | 18.5≥BMI<25 vs 25≥BMI<30 | 0.0050 | 0.1100 | 0.997 | 0.584–1.693 | 0.1686 | 1.0000 | 0.944 | 0.547–1.619 |
|
|
|||||||||
| 18.5≤BMI<25 vs 30≤BMI<35 | 0.876 | 0.515–1.481 | 0.948 | 0.545–1.647 | |||||
|
|
|||||||||
| 25≤BMI<30 vs 30≤BMI< 35 | 0.879 | 0.526–1.468 | 1.005 | 0.588–1.723 | |||||
|
|
|||||||||
| BMI≥35 vs 18.5≤BMI<25 | 2.002 | 1.241–3.295 | 1.630 | 0.979–2.753 | |||||
|
|
|||||||||
| BMI≥35 vs 25≤BMI<30 | 1.996 | 1.255–3.222 | 1.538 | 0.937–2.547 | |||||
|
|
|||||||||
| BMI≥35 vs 30 ≤ BMI<35 | 1.754 | 1.107–2.818 | 1.545 | 0.933–2.592 | |||||
|
| |||||||||
| Race | Black/African American vs White | 0.0382 | 0.6935 | 1.554 | 1.003–2.330 | 0.0080 | 0.1687 | 1.783 | 1.135–2.710 |
|
| |||||||||
| Myoinvasion | Inner half vs None | <0.0001 | <0.0001 | 1.585 | 0.957–2.700 | <0.0001 | <0.0001 | 1.741 | 1.008–3.121 |
|
|
|||||||||
| Inner half vs Outer half/serosa | 0.407 | 0.272–0.601 | 0.412 | 0.270–0.621 | |||||
|
|
|||||||||
| None vs Outer half/serosa | 0.257 | 0.154–0.412 | 0.237 | 0.135–0.395 | |||||
|
| |||||||||
| Lymphovascular invasion | No vs Yes | <0.0001 | <0.0001 | 0.442 | 0.311–0.629 | <0.0001 | 0.0002 | 0.435 | 0.300–0.634 |
|
| |||||||||
| Cervical invasion | No/Glandular vs Stromal (S)/Both | <0.0001 | <0.0001 | 0.426 | 0.298–0.615 | <0.0001 | <0.0001 | 0.384 | 0.264–0.564 |
|
| |||||||||
| Adnexal involvement | No vs Yes | <0.0001 | <0.0001 | 0.372 | 0.246–0.581 | <0.0001 | <0.0001 | 0.305 | 0.200–0.480 |
|
| |||||||||
| Nodal involvement | No vs Yes | <0.0001 | <0.0001 | 0.307 | 0.211–0.451 | <0.0001 | <0.0001 | 0.358 | 0.241–0.538 |
|
| |||||||||
| Omental involvement | No vs Yes | <0.0001 | <0.0001 | 0.136 | 0.082–0.237 | <0.0001 | <0.0001 | 0.114 | 0.068–0.199 |
|
| |||||||||
| FIGO stage | I vs II | <0.0001 | <0.0001 | 0.347 | 0.209–0.588 | <0.0001 | <0.0001 | 0.291 | 0.171–0.503 |
| I vs III | 0.276 | 0.178–0.422 | 0.303 | 0.190–0.479 | |||||
| I vs IV | 0.056 | 0.032–0.100 | 0.043 | 0.024–0.082 | |||||
| II vs III | 0.794 | 0.479–1.280 | 1.042 | 0.617–1.716 | |||||
| II vs IV | 0.161 | 0.087–0.300 | 0.149 | 0.078–0.288 | |||||
| III vs IV | 0.203 | 0.120–0.353 | 0.143 | 0.081–0.262 | |||||
Log-rank test,
Estimated by Cox proportional hazards model.
BMI, body mass index; CC, pure clear cell carcinoma; CC-EM-M, mixed clear cell and endometrioid carcinoma; CC-SER-M, mixed clear cell and serous carcinoma; CC-EM-I, indeterminate clear cell vs. endometrioid carcinoma; CC-SER-I, indeterminate clear cell vs. serous carcinoma
To further explore the possible prognostic significance of the various CCC subtypes, Kaplan-Meier curves were drawn to depict PFS (Figure 1A) and OS (Figure 1B) of patients with clear cell subtypes. For comparison, the survival of patients from GOG-210 with pure grade 3 endometrioid adenocarcinoma (EM-grade 3) and pure serous carcinoma (SER) was also plotted. Patients in the CC-EM-I group had longer PFS and OS than those in any other group, including EM-grade 3. Patients with CC-SER-M and CC-SER-I had the shortest PFS and OS of any clear cell subtype, comparable to pure SER. Kaplan-Meier curves of data from only early-stage patients (FIGO stages I and II) (Figure 2A–B) revealed that patients with CC-EM-I had the most favorable outcomes. Other groups were comparable to one another, though patients with CC-SER-M or CC-SER-I had shorter OS and PFS than the other groups. However, this study was not powered to test the statistical significance of these findings.
Figure 1.
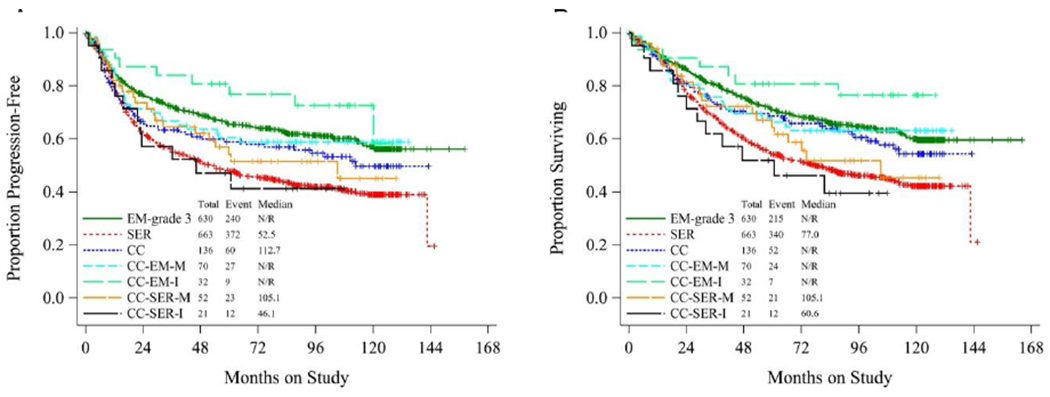
Kaplan-Meier curves depicting (A) progression-free survival and (B) overall survival of patients with clear cell carcinoma subtypes. CC, pure clear cell carcinoma; CC-EM-M, mixed clear cell and endometrioid carcinoma; CC-SER-M, mixed clear cell and serous carcinoma; CC-EM-I, indeterminate clear cell vs. endometrioid carcinoma; CC-SER-I, indeterminate clear cell vs. serous carcinoma. In both panels, survival of patients with grade 3 pure endometrioid carcinoma (EM) and pure serous carcinoma (SER) are plotted for comparison.
Figure 2.
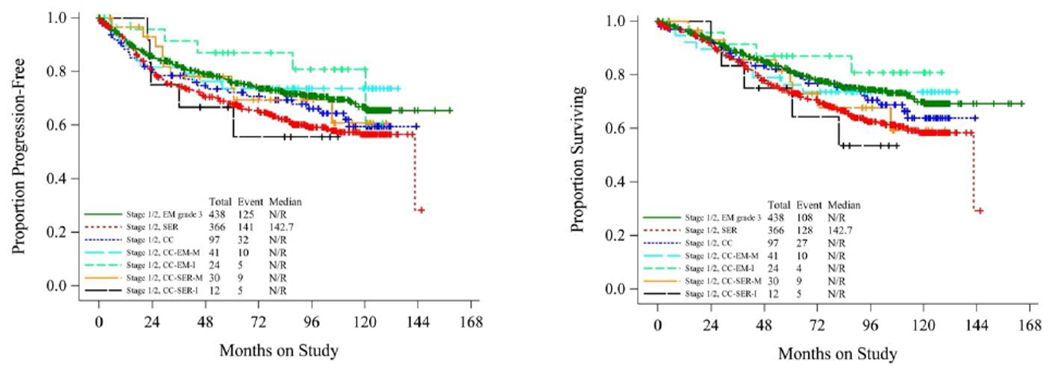
Kaplan-Meier curves depicting (A) progression-free survival and (B) overall survival of patients diagnosed at FIGO stage I or II with clear cell carcinoma subtypes. CC, pure clear cell carcinoma; CC-EM-M, mixed clear cell and endometrioid carcinoma; CC-SER-M, mixed clear cell and serous carcinoma; CC-EM-I, indeterminate clear cell vs. endometrioid carcinoma; CC-SER-I, indeterminate clear cell vs. serous carcinoma. In both panels, survival of patients with grade 3 pure endometrioid carcinoma (EM) and pure serous carcinoma (SER) are plotted for comparison.
DISCUSSION
This study takes advantage of clinicopathologic data and histologic slides from GOG- 210, a large series of endometrial cancer cases with homogeneous initial treatment. We specifically focus here on CCC of the endometrium in pure form, with an admixed endometrioid or serous component, or with features that appear indeterminate/ambiguous between CCC and either endometrioid or serous cancer. Few reports have dealt with the subtypes we define here, although our data show that they occur often within CCC cases. Out of 311 total CCC cases, 175 (56.3%) were CC-EM-M, CC-EM-I, CC-SER-M, or CC-SER-I, and only 136 (43.7%) were pure CC. Although the decision to assign a mixed or indeterminate category is inevitably subjective, mixed and indeterminate forms may be more common than pure forms, so it is important to understand their behavior.
With regard to baseline characteristics, the only statistically significant difference between the groups selected for review was a minor difference in age at presentation. Planned adjuvant therapy did not differ on a binary basis between the groups, but we did not have more detailed data on the adjuvant regimens in each group. Baseline analyses also revealed few significant differences between subtypes of serous carcinomas in GOG-8032, in results previously published [13].
Survival analyses showed that several prognostic factors that are pertinent to endometrioid carcinoma are also significant for CCC and its subtypes. In addition, these analyses demonstrated that patients in the indeterminate CC-EM-I group had a survival advantage over those with CC (PFS only), CC-SER-I (PFS and OS), and CC-SER-M (OS only) tumors. Although this correlation was not significant after adjustment for multiple hypothesis testing, an effect of this size would be clinically meaningful, if confirmed. The category CC-EM-I in this study consisted of tumors that a group of expert pathologists could not confidently assign as clear cell or endometrioid using morphologic features. The apparent prognostic benefit of CC-EM-I could occur because some of these tumors are truly low-grade endometrioid tumors with features such as secretory change, cytoplasmic glycogenation, or Arias-Stella–like reaction [19], that mimic clear cell morphology. Immunostains (HNF-1β, Napsin A, ER, CTH, and ASS1) have been reported to be useful in resolving the differential for tumors with “nonspecific clear cell changes” (analogous to our “indeterminate” category), as they consistently reveal a profile typical of endometrioid adenocarcinoma. Such stains were not part of our protocol but could be recommended to pathologists confronted with such a tumor. However, the stains seem to have limited utility in mixed carcinomas, which can fail to show a specific interpretable immunoprofile [20]. Moreover, these stains are not available in all or even most laboratories, and pathologists may lack experience with them.
This study has several strengths. The cohort was large, comprising 311 cases of a rare entity, clear cell carcinoma. The vast majority of cases were reviewed by a group of six senior gynecologic pathologists using uniform diagnostic criteria. Additionally, lengthy follow-up data were available. Finally, the database was maintained by the GOG Statistical and Data Center using statistical best practices.
Several weaknesses of this study should be recognized. Cell type assignments were made based on examination of representative slides selected by the submitting institutions. Components not present on these slides would not have been seen by the central pathology reviewers. In addition, this protocol was activated in 2003, before widespread adoption of immunohistochemistry for classification of endometrial cancer. The study did not call for any systematic use of immunostains that may be helpful in the diagnosis of clear cell carcinoma. Therefore, H&E-stained slides were used for diagnosis. Immunostain results were provided for some cases, but their existence and their impact on tumor classification was not documented. Given the study design, there could be disagreement about the histologic assignment of any particular case, but given the experience level of the G6 pathologists, systematic error in the classification process was unlikely. Another limitation is that the relative percentage of each component was not documented and could not be determined because only representative slides were submitted for review. The data are thus not informative as to whether the percent admixture of various components predicts clinicopathologic characteristics or survival.
Patients on GOG-210 received uniform initial surgical treatment, but subsequent treatment was not controlled. Our ability to comment on the biologic behavior of the tumors is therefore limited. At baseline, planned adjuvant therapy (yes/no) did not differ between groups (Table 2). All of the histologic types studied here would have been considered high-risk, so one would not expect there to have been systematic differences in treatment.
The design of GOG-210 allowed only patients with residual carcinoma at hysterectomy to be enrolled because those patients had resection material available for central pathology review. The study may therefore be biased towards patients with a larger burden of disease, whereas patients with focal or low-volume tumor completely ablated by the initial sampling are not represented.
The study did not include molecular correlates, although certain mutational profiles are now known to be characteristic of various endometrial cancer types. Thus, the potential role of somatic variants in substantiating tumor classification cannot be evaluated in our data. In future work, it would be possible to perform genetic profiling of banked tumor tissue to address this topic. Molecular profiling suggests that there are subsets of CCC with molecular similarity to either serous or endometrioid adenocarcinoma [4]. Therefore, there may be molecular correlates of the indeterminate histologic groups identified in our study.
There is great interest in determining whether ProMisE-like molecular classifiers have prognostic value in clear cell tumors. The ProMisE algorithm adapts insights from The Cancer Genome Atlas to classify endometrioid and serous tumors into four categories with distinct biologic behavior and treatment susceptibility [21]. Initial findings suggest that, within the overall group of CCC, the Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) categories of p53 abnormal, mismatch repair deficient, and POLE-mutated have the same implications as they do for the endometrioid and serous tumors in which these categories were initially defined. However, CCC in the so-called p53 wild-type/“no specific molecular profile” (NSMP) category differ in behavior from non-clear cell NSMP tumors [22], with CCC/NSMP showing worse prognosis than endometrioid/NSMP. Therefore, ProMisE-like classification is unlikely to completely replace histologic classification for CCC.
To conclude, we report a prospective clinicopathologic analysis of 311 patients with CCC of the endometrium. Specialized pathology review was used to identify a subset of tumors that were either mixed (CCC mixed with endometrioid or serous carcinoma) or indeterminate (homogenous tumors having histologic features intermediate between CCC and either endometrioid or serous carcinoma). No difference in prognosis was identified between pure CCC and the mixed groups. One interpretation of this result is that any component of unequivocal CCC confers high-risk status on an endometrial carcinoma. We found evidence that the CC-EM-I group—patients with tumors considered indeterminate between CC and endometrioid adenocarcinoma—had a better prognosis than other subsets, without statistical significance. It is possible that some of these tumors were in fact low-grade endometrioid adenocarcinomas, which would account for the patients’ good prognosis, although even experienced pathologists saw in them a possible clear cell component. Pathologists are cautioned that cancers that are overall endometrioid, with features that are only suggestive of CCC, may not have the same prognosis as tumors that are definitely pure or mixed CCC. It may be valuable to continue to record the various diagnostic categories described in this study so that their significance can be further defined.
Three hundred eleven endometrial clear cell carcinomas were identified within the GOG-210 cohort.
Expert pathologic review identified some cases as pure clear cell carcinoma and others as mixed or indeterminate.
Clear cell tumors with a definite serous or endometrioid component had outcome similar to pure clear cell carcinoma.
Indeterminate clear cell versus endometrioid tumors had more favorable outcomes.
ACKNOWLEDGEMENTS
The authors thank Kay Park, MD, for her invaluable contributions to the expert pathology review and Debbie Frank, PhD, for editing help.
This study was supported by the following National Cancer Institute grants to NRG Oncology: U10CA180822 (NRG Oncology SDMC), U10CA180868 (NRG Oncology Operations) and U24A196067 (NRG Specimen Bank). Research reported in this publication was supported, in part, by a Cancer Center Support Grant from the NIH/NCI (P30 CA008748).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Memorial Sloan Kettering Cancer Center, Case Western Reserve University, University of Oklahoma Health Sciences Center, University of Chicago, Abington Memorial Hospital–Asplundh Cancer Pavilion, Washington University School of Medicine, Mayo Clinic, University of Iowa Hospitals and Clinics, Women and Infants Hospital, Maine Medical Center–Scarborough Campus, Sutter Cancer Research Consortium, and Metro-Minnesota CCOP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NCT#: NCT00340808
REFERENCES
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics, 2022, CA Cancer J Clin. 72 (2022) 7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization, ed., Female Genital Tumours, 5th ed, International Agency for Research on Cancer, Lyon, 2020. [Google Scholar]
- [3].Olawaiye AB, Boruta DM, Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review, Gynecol Oncol. 113 (2009) 277–283. 10.1016/j.ygyno.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [4].Le Gallo M, Rudd ML, Urick ME, Hansen NF, Zhang S, NISC Comparative Sequencing Program, Lozy F, Sgroi DC, Vidal Bel A, Matias-Guiu X, Broaddus RR, Lu KH, Levine DA, Mutch DG, Goodfellow PJ, Salvesen HB, Mullikin JC, Bell DW, Somatic mutation profiles of clear cell endometrial tumors revealed by whole exome and targeted gene sequencing, Cancer. 123 (2017) 3261–3268. 10.1002/cncr.30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].An H-J, Logani S, Isacson C, Ellenson LH, Molecular characterization of uterine clear cell carcinoma, Mod Pathol. 17 (2004) 530–537. 10.1038/modpathol.3800057. [DOI] [PubMed] [Google Scholar]
- [6].Hoang LN, McConechy MK, Meng B, McIntyre JB, Ewanowich C, Gilks CB, Huntsman DG, Köbel M, Lee C-H, Targeted mutation analysis of endometrial clear cell carcinoma, Histopathology. 66 (2015) 664–674. 10.1111/his.12581. [DOI] [PubMed] [Google Scholar]
- [7].DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, Mohanty AS, Cheng DT, Berger MF, Soslow RA, Weigelt B, The genetic landscape of endometrial clear cell carcinomas, J Pathol. 243 (2017) 230–241. 10.1002/path.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A, Fyles A, Baron M-H, Jürgenliemk-Schulz IM, Kitchener HC, Nijman HW, Wilson G, Brooks S, Carinelli S, Provencher D, Hanzen C, Lutgens LCHW, Smit VTHBM, Singh N, Do V, D’Amico R, Nout RA, Feeney A, Verhoeven-Adema KW, Putter H, Creutzberg CL, PORTEC study group, Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial, Lancet Oncol. 19 (2018) 295–309. 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McMeekin DS, Filiaci VL, Thigpen JT, Gallion HH, Fleming GF, Rodgers WH, Gynecologic Oncology Group study, The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: a Gynecologic Oncology Group study, Gynecol Oncol. 106 (2007) 16–22. 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- [10].Bokhman JV, Two pathogenetic types of endometrial carcinoma, Gynecol Oncol. 15 (1983) 10–17. 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- [11].Alektiar KM, McKee A, Lin O, Venkatraman E, Zelefsky MJ, McKee B, Hoskins WJ, Barakat RR, Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer?, Int J Radiat Oncol Biol Phys. 54 (2002) 79–85. 10.1016/s0360-3016(02)02913-9. [DOI] [PubMed] [Google Scholar]
- [12].Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P, Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium, Gynecol Oncol. 95 (2004) 593–596. 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- [13].Hagemann IS, Deng W, Zaino RJ, Powell MA, Gunderson C, Cosgrove C, Mathews C, Pearl ML, Waggoner S, Ghebre R, Lele S, Guntupalli S, Secord AA, Ioffe O, Park K, Rasty G, Singh M, Soslow R, Creasman W, Mutch DG, The presence of an endometrioid component does not alter the clinicopathologic profile or survival of patients with uterine serous cancer: A gynecologic oncology group (GOG/NRG) study of 934 women, Gynecol Oncol. 160 (2021) 660–668. 10.1016/j.ygyno.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Quddus MR, Sung CJ, Zhang C, Lawrence WD, Minor serous and clear cell components adversely affect prognosis in “‘mixed-type’” endometrial carcinomas: a clinicopathologic study of 36 stage-I cases, Reprod Sci. 17 (2010) 673–678. 10.1177/1933719110368433. [DOI] [PubMed] [Google Scholar]
- [15].Li W, Li L, Wu M, Lang J, Bi Y, The Prognosis of Stage IA Mixed Endometrial Carcinoma, Am J Clin Pathol. 152 (2019) 616–624. 10.1093/ajcp/aqz083. [DOI] [PubMed] [Google Scholar]
- [16].Crum CP, Lee KR, Diagnostic Gynecologic and Obstetric Pathology, Elsevier Saunders, Philadelphia, 2006. [Google Scholar]
- [17].Holm S, A simple sequentially rejective multiple test procedure, Scandinavian Journal of Statistics. 6 (1979) 65–70. [Google Scholar]
- [18].Cox DR, Regression Models and Life-Tables, Journal of the Royal Statistical Society. Series B (Methodological). 34 (n.d.) 187–220. [Google Scholar]
- [19].Lucas E, Carrick KS, Low grade endometrial endometrioid adenocarcinoma: A review and update with emphasis on morphologic variants, mimics, immunohistochemical and molecular features, Semin Diagn Pathol. 39 (2022) 159–175. 10.1053/j.semdp.2022.02.002. [DOI] [PubMed] [Google Scholar]
- [20].Ji JX, Cochrane DR, Tessier-Cloutier B, Leung S, Cheng AS, Chow C, Gilks B, Huntsman DG, Hoang LN, Use of Immunohistochemical Markers (HNF-1β, Napsin A, ER, CTH, and ASS1) to Distinguish Endometrial Clear Cell Carcinoma From Its Morphologic Mimics Including Arias-Stella Reaction, Int J Gynecol Pathol. 39 (2020) 344–353. 10.1097/PGP.0000000000000609. [DOI] [PubMed] [Google Scholar]
- [21].Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, Britton H, Kommoss F, Grevenkamp F, Karnezis A, Yang W, Lum A, Krämer B, Taran F, Staebler A, Lax S, Brucker SY, Huntsman DG, Gilks CB, McAlpine JN, Talhouk A, Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series, Ann Oncol. 29 (2018) 1180–1188. 10.1093/annonc/mdy058. [DOI] [PubMed] [Google Scholar]
- [22].Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, Storness-Bliss C, Farnell D, Huang L, Shum K, Lum A, Senz J, Lee C-H, Gilks CB, Hoang L, McAlpine JN, Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification, Gynecol Oncol. 158 (2020) 3–11. 10.1016/j.ygyno.2020.04.043. [DOI] [PubMed] [Google Scholar]


