Abstract
Introduction
The willingness to pay per quality-adjusted life year gained (WTP/Q) is commonly used to determine whether an intervention is cost-effective in health technology assessment. This study aimed to evaluate the WTP/Q for different disease scenarios in a Chinese population.
Methods
The study employed a quadruple-bounded dichotomous choice contingent valuation method to estimate the WTP/Q in the general public. The estimation was conducted across chronic, terminal and rare disease scenarios. Face-to-face interviews were conducted in a Chinese general population recruited from Jiangsu province using a convenience sampling method. Interval regression analysis was performed to determine the relationship between respondents’ demographic and socioeconomic conditions and WTP/Q. Sensitivity analyses of removing protest responses and open question analyses were conducted.
Results
A total of 896 individuals participated in the study. The WTP/Q thresholds were 128 000 Chinese renminbi (RMB) ($36 364) for chronic diseases, 149 500 RMB ($42 472) for rare diseases and 140 800 RMB ($40 000) for terminal diseases, equivalent to 1.76, 2.06 and 1.94 times the gross domestic product per capita in China, respectively. The starting bid value had a positive influence on participants’ WTP/Q. Additionally, residing in an urban area (p<0.01), and higher household expenditure (p<0.01), educational attainment (p<0.02) and quality of life (p<0.02) were significantly associated with higher WTP/Q. Sensitivity analyses demonstrated the robustness of the results.
Conclusion
This study implies that tailored or varied rather than a single cost-effectiveness threshold could better reflect community preferences for the value of a healthy year. Our estimates hold significance in informing reimbursement decision-making in health technology assessment in China.
Keywords: Health economics, Health systems, Health services research, Cross-sectional survey
WHAT IS ALREADY KNOWN ON THIS TOPIC
The demand-side cost-effectiveness threshold in the form of willingness to pay per quality-adjusted life year gained (WTP/Q) is commonly used to determine the cost-effectiveness of evaluated interventions.
WHAT THIS STUDY ADDS
This study revealed the WTP/Q threshold of 1.76, 2.06 and 1.94 times the gross domestic product per capita in China for chronic diseases, rare diseases and terminal diseases in a Chinese general population. The WTP/Q thresholds also varied by the study participants’ socioeconomic status.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings of this study suggested that context-specific rather than universal cost-effectiveness thresholds may provide a more accurate reflection of the public’s WTP for a quality-adjusted life year. These estimates may be helpful in guiding reimbursement decision-making for health technologies in China.
Introduction
Health economic evaluation has become a popular tool for health policymakers to identify interventions with the best value for money, among which cost-effectiveness analysis (CEA) is one of the most commonly used approaches.1 CEA measures the incremental cost and health outcome between two interventions, and calculates the incremental cost-effectiveness ratio (ICER).2 In practice, a predetermined value, also known as the cost-effectiveness threshold (CET), is used to decide whether the assessed intervention is cost-effective. An intervention is deemed as cost-effective when the ICER is no greater than the CET when it is compared with the alternative.3 4 CET is defined as the maximum monetary value per positive health outcome gained or negative health outcome averted.5 In economic evaluation, quality-adjusted life years (QALYs) or disability-adjusted life years (DALYs) are commonly used health outcome metrics because they each incorporate some account of survival and morbidity and thus provide a measure of outcome that is able to be compared across diseases.
While CEAs are widely conducted worldwide, a CET is not always clearly defined at the country level.6 A recent systematic review found that 17 countries have officially recognised CETs.7 Among these countries, England (£20 000–30 000 per QALY),8 Thailand (160 000 Thai baht per QALY),9 Ireland (€45 000 per QALY)10 and Norway (500 000 Norwegian krone)10 have explicit CETs. For most of the countries which lack formal thresholds, indicative CETs gauged by gross domestic product (GDP) per capita are often used alternatives,11 especially in low-income and middle-income countries.12 However, GDP per capita-based approaches to CETs have been criticised due to a lack of theoretical and methodological justification.7 Considering these limitations, the WHO and UK’s National Institute for Health and Care Excellence (NICE) have expressed concerns about the use of this fixed GDP-based CET.13 It has been argued instead that country-specific CET based on relevant data is vital to better inform resource allocation and decision-making.11 14 15
In general, there are two different empirical approaches for generating CETs—supply-side or demand-side estimation. Supply-side CET represents the opportunity cost of diverting funding from the current marginal best buy and thus investment in the proposed new treatment needs to have a cost per QALY gained that is lower than that benchmark. Demand-side CET is a measure through individual willingness to pay (WTP) valuations for the health improvement (such as a QALY gained) and is expressed in monetary terms.12 In contrast to the supply-side approach, this WTP-based method is seen to reflect social preferences for health,16 and thus has a strong grounding in welfare economic principles.17 It has been investigated in a number of countries and has played some role in informing health policy decisions.4
As equity concerns in economic evaluations receive more attention from scholars and policymakers, differentiated CETs have also been proposed to inform health resource allocation decisions. An empirical study has shown that valuations based on WTP produce different CETs for different diseases in Iran.18 Moreover, health technology assessment agencies in the UK and Australia have set higher CETs for very rare diseases or end-of-life treatments.13 19 In addition, WTP/Q is not always constant and could be influenced by respondents’ characteristics such as age, educational attainment and employment.20 This implies that when estimating WTP/Q, it is important to take into consideration the existence of varied WTP thresholds across different diseases.
In China, the National Healthcare Security Administration of China developed the national reimbursement drug list, where medications are listed depending on their effectiveness and cost-effectiveness, and reimbursed entirely or partly by the public health insurance schemes.21 GDP per capita-based CETs are often used to determine whether a medication is cost-effective.22–32 This approach has been adopted in the China guidelines for pharmacoeconomic evaluations.33 A review of the Chinese economic evaluation studies reported that nearly half of the included studies employed a threshold of three times GDP per capita as the basis to judge the cost-effectiveness of evaluated interventions.34 However, the rationale for using this as the CET in the Chinese setting is lacking. This could lead to potentially inappropriate resource allocation decisions being made based on inconsistent and arbitrary use of thresholds and highlights the need for a China-specific CET. In fact, China has made attempts to establish a threshold in decision context. Studies performed for demand-side estimation found that China’s CET is 1.5 times of GDP per capita per QALY gained or 63% of GDP per capita per DALY averted.6 35 However, estimations of demand-side WTP/Q specific to China are not currently available. This study aims to fill in the research gap by first, measuring the maximum WTP/Q in Chinese general population; second, measuring WTP/Q for different disease scenarios: chronic diseases, rare diseases and terminal diseases; and finally, estimating how these values vary by sociodemographic factors.
Methods
Patient and public involvement
Patients and members of the general public were involved in the questionnaire development and pilot study. Specifically, during the bid setting phase, we sought the perspectives of individuals from the general public to ask their opinions on what would be deemed an appropriate amount for the bid values. Furthermore, in the subsequent pilot study, respondents were not only tasked with completing the questionnaire but were also encouraged to provide feedback on the appropriateness of the bid values, the comprehensibility of the scenario descriptions and their recommendations for necessary amendments to the questionnaire.
Study participants
To investigate the WTP/Q, community residents, including those living in any demographic, health or socioeconomic conditions, were invited to participate in the study. The inclusion criteria for participation were Chinese citizens who can read and write in Chinese and were aged 18 years or older. The exclusion criteria were: (1) people highly dependent on medical care who may be unable to give consent; (2) people with cognitive impairment, an intellectual disability or a mental illness; and (3) people who may be involved in illegal activities. To find the understandability of the questionnaire and the appropriateness of the bid value settings, a pilot study with a small sample (n=20) was conducted. After the interview, participants were invited to provide suggestions for improving the questionnaire. The results of the pilot study did not lead to any modification to the questionnaire. Before the formal interview, inclusion and exclusion criteria were presented to all potential respondents, and the qualification for participation was self-assessed. Those eligible to participate in the study were provided with a written consent form, and only those who were willing to participate in the study proceeded with the survey. We employed a convenience sampling method to identify respondents for the interviews in city-level or district-level hospitals in Jiangsu province. Respondents were a mixed population of healthy participants who underwent general health examinations and patients with chronic diseases. Face-to-face interviews were conducted between January and April 2022. The interviewers were Master students being trained in interview skills.
Contingent valuation method
In this study, we employed the contingent valuation method (CVM) to estimate the WTP/Q. CVM, a stated preference method, is often used to assign a monetary value to a special good or service by presenting respondents with hypothetical scenarios and asking them to make choices.36 It has been widely used worldwide to investigate WTP for health-related benefits.37 38 The National Oceanic and Atmospheric Administration (NOAA) developed guidelines for CVM performance.39 In this study, we used quadruple-bounded dichotomous choice CVM, where respondents were presented with a dichotomous choice to indicate whether they were willing to pay a provided amount for health gains. Subsequently, based on their responses, three additional amounts were presented and corresponding choices were made. The design of hypothetical scenarios in our study is consistent with the NOAA guidelines.
Questionnaire and scenarios
The questionnaire contains three disease scenarios (ie, chronic diseases, rare diseases and terminal diseases), sociodemographic status and quality of life of the study participants. Participants were shown three scenarios successively and provided their WTP/Q thresholds for all the three scenarios. Participants were asked to imagine that they had a disease that fell in each scenario and to consider whether they were willing to pay a predefined initial amount of money for a treatment if it could extend their life by 1 year in perfect health:
Imagine that you have a common non-communicable chronic disease (rare diseases in scenario B and terminal diseases in scenario C). Assume that treatment A is able to extend your life by 1 year. You will be completely healthy in this extended year without any pains. However, the treatment A is not covered by your public health insurance scheme and you do not have any private health insurance to pay for this treatment. All expenses will be paid out of pocket. Suppose that treatment A will cost X Chinese renminbi (RMB). In this case, are you willing to pay for treatment A?
(X is the original bid value.)
Participants who answered ‘yes’ for the first bid value will be asked a follow-up bid value with a higher amount until the participate selected ‘no’ to the bid value, while for those who answered ‘no’ for the first bid, we provided decreasing bid values until they selected ‘yes’. After the dichotomous choices, an open question was asked to respondents for their perceived amount of WTP/Q for each of the scenarios.
To enhance the comprehensibility of the scenarios and minimise potential comprehension bias, we incorporated examples of commonly encountered diseases into each scenario. Specifically, we used hypertension and diabetes as examples for chronic diseases. Terminal stage of malignant tumour was employed as an example for terminal diseases. For rare diseases, we clarified their low incidence rates (lower than 1/10 000) and presented examples such as albinism and haemophilia.
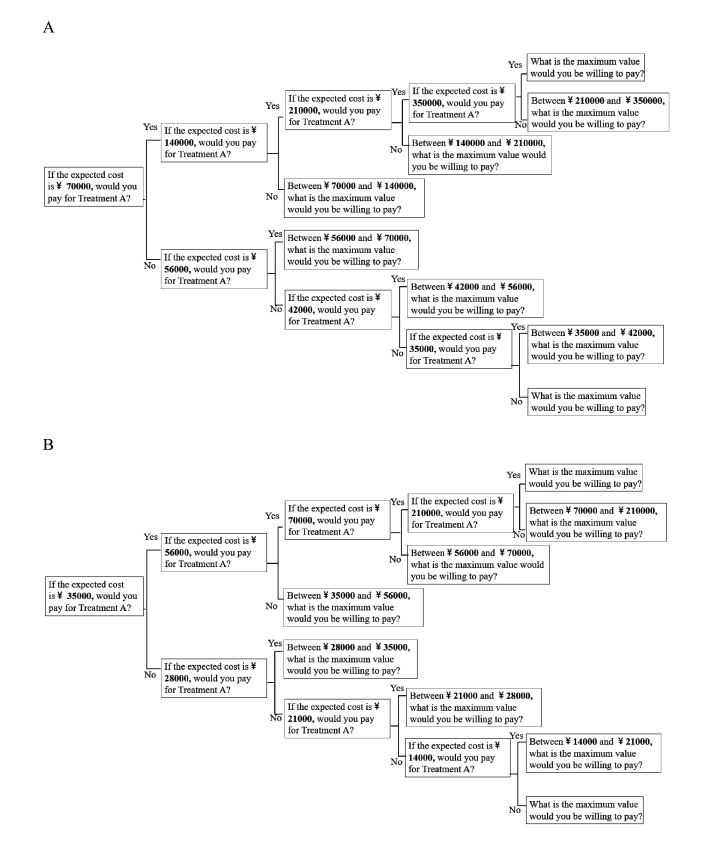
Moreover, to address the anchoring effect, where participants’ valuation varied according to the initial bid values showed to them,36 we included two bid value settings with different initial bids—thus creating two versions of the questionnaires A and B. The bid values of quadruple-bounded dichotomous choice CVM were shown in figure 1. For questionnaire A, the opening bid value was 70 000 RMB—approximately China’s GDP per capita in 2020 (72 447 RMB).40 41 The limit bid was set at five times GDP per capita (350 000 RMB) (figure 1A). In questionnaire B, the first value was set at 0.5 times of GDP per capita (35 000 RMB) in China in 2020 and the limit bid was set at three times of GDP per capita (21 000 RMB) (figure 1B). The two versions of questionnaires were randomly distributed to the participants.
Figure 1.
Bid values of quadruple-bounded dichotomous choice contingent valuation method used in this study. (A) Bid values for questionnaire A (with starting bid value 70 000 RMB) and (B) bid values for questionnaire B (with starting bid value 35 000 RMB). At each bidding node, participants were asked to imagine that they had a type of disease and consider whether they were willing to pay for a treatment out of pocket if it could extend their life by 1 full year. Figure 1 only showed bid values for scenario A but the bid value settings for three scenarios are the same. RMB, Chinese renminbi.
Following their valuations, participants were asked to nominate the reason for their valuation with choices including: (a) this is my perceived value of 1 year of my life in full health (rational valuation); (b) this is the amount that I can afford (budget limitation); (c) I do not think I will have this disease; and (d) I think the treatment should be covered by health insurance. As the protest answers were defined where participants refuse to engage in the hypothesised CVM scenario or refuse to state their WTP/Q,42 the latter two options were identified as protest responses and were deleted in sensitivity analyses.
The second part is socioeconomic and demographic status of participants, including their age, sex, educational attainment (1 primary school and below; 2 middle school; 3 high school; 4 university and above), employment (1 employed; 2 retired; 3 unemployed), location of residence (1 urban; 2 rural and urban–rural areas), economic status and social health insurance. Of note, economic status was measured using the household consumption expenditure43 and was categorised into quintile groups. Status of social health insurance was categorised into Urban Employee Basic Medical Insurance and Urban–Rural Residents Basic Medical Insurance (URRBMI) which was merged from the former New Rural Cooperative Medical Schemes and Urban Residents Basic Medical Insurance. These two types of health insurance combined cover 96% of the Chinese population.44
The third part of the questionnaire was to evaluate the study participants’ health-related quality of life using the five-level EuroQol five-dimensional questionnaire (EQ-5D-5L). The responses were converted to health state utilities using the EQ-5D-5L value set for China.45 Considering that a large proportion of the Chinese general population reported full health with a utility of 1,46 the health status was divided into two groups: the perfect health group whose utility equalled to 1 and non-perfect health group whose utility was less than 1. The questionnaire for this study was shown in online supplemental material 1.
bmjgh-2023-013070supp001.pdf (5.2MB, pdf)
Statistical analysis
Descriptive analysis was used to summarise the sociodemographic characteristics of the study participants. Difference tests including Student’s t-test, Χ2 test and Kruskal-Wallis test were conducted to evaluate the sociodemographic differences across two groups that used different versions of questionnaires. A two-tailed p value of 0.05 indicated statistical significance.
Considering that only interval outcomes instead of the exact values were obtained from dichotomous choice CVM, interval regression analysis suitable for modelling interval outcomes was performed to estimate WTP/Q and identify related factors using the following formula:
where is the coefficient value of covariates, , and is the error term which is normally distributed. indicates the interval outcome (, ) determined by participants’ responses to the bid values, and its distribution will be .
We assumed that the study participants’ WTP was higher than 0. Using X to represent the one-time GDP per capita in China in 2020, the likelihood function for the WTP/Q estimation with the first bidding of 70 000 RMB is as follows47:
The approach to estimate the WTP/Q for those who were assigned with questionnaire B was similar to that for questionnaire A, except that the bid values were modified accordingly. Finally, protest responses and valid responses were identified according to the reasons of WTP valuation from participants, and sensitivity analyses were performed by removing protest responses to test robustness of the results with all study participants. Open-ended questions regarding respondents’ perceived amounts of WTP/Q were also examined as a part of the sensitivity analyses to explore WTP/Q preferences across different diseases. The results were presented in RMB and converted into US dollars using purchasing power parities (US$1=3.52 RMB).40 48
Results
Table 1 presents the sociodemographic characteristics of the study participants. A total of 896 study participants completed the questionnaires. Most of the study participants were aged below 45 years (78%), were married (61%), and had high school education or above (77%). The average per annum household expenditure was 140 438 RMB. There were 55% participants living in rural or urban–rural areas. Half of the respondents were covered by the URRBMI, while one-third of them also had a complementary commercial insurance. There were 578 respondents (65%) who reported perfect health.
Table 1.
Socioeconomic and demographic characteristics of study participants
| Variable | Total | Q_A N=447 |
Q_B N=449 |
Difference test | ||||
| n | % | n | % | |||||
| Sex | Male | 452 | 50.45 | 213 | 47.65 | 239 | 53.23 | p=0.095 |
| Female | 444 | 49.55 | 234 | 52.35 | 210 | 46.77 | ||
| Age | ≤25 | 250 | 27.9 | 155 | 34.68 | 95 | 21.16 | p<0.001 |
| 26–35 | 295 | 32.92 | 122 | 27.29 | 173 | 38.53 | ||
| 36–45 | 151 | 16.85 | 76 | 17.00 | 75 | 16.70 | ||
| 46–55 | 125 | 13.95 | 62 | 13.87 | 63 | 14.03 | ||
| ≥55 | 75 | 8.37 | 32 | 7.16 | 43 | 9.58 | ||
| Household consumption expenditure (1000 RMB) |
140.4±18.6 | 146.5±187.5 | 134.4±184.1 | p=0.167 | ||||
| Marital status | Unmarried | 324 | 36.16 | 178 | 39.82 | 153 | 34.08 | p=0.305 |
| Married | 546 | 60.94 | 257 | 57.49 | 281 | 62.58 | ||
| Widowed | 9 | 1.00 | 6 | 1.34 | 4 | 0.89 | ||
| Divorced | 15 | 1.67 | 5 | 1.12 | 9 | 2.00 | ||
| Other | 2 | 0.22 | 1 | 0.22 | 2 | 0.45 | ||
| Educational level | Primary school and below | 73 | 8.15 | 19 | 4.25 | 54 | 12.03 | p<0.001 |
| Middle school | 130 | 14.51 | 58 | 12.98 | 72 | 16.04 | ||
| High school | 159 | 17.75 | 74 | 16.55 | 85 | 18.93 | ||
| University and above | 534 | 59.60 | 296 | 66.22 | 238 | 53.01 | ||
| Employment status | Employed | 624 | 69.64 | 302 | 67.56 | 322 | 71.71 | p=0.372 |
| Retired | 30 | 3.35 | 17 | 3.80 | 13 | 2.90 | ||
| Unemployed | 242 | 27.01 | 128 | 28.64 | 114 | 25.39 | ||
| Location of residence | Urban | 399 | 44.53 | 228 | 51.01 | 171 | 38.08 | p<0.001 |
| Rural and urban–rural areas | 497 | 55.47 | 219 | 49.00 | 278 | 61.92 | ||
| Number of household members | 1 | 52 | 5.80 | 19 | 4.25 | 33 | 7.35 | p=0.524 |
| 2 | 96 | 10.71 | 35 | 7.83 | 61 | 13.59 | ||
| 3 | 279 | 31.14 | 161 | 36.02 | 118 | 26.28 | ||
| 4 | 219 | 24.44 | 114 | 25.50 | 105 | 23.39 | ||
| ≥5 | 250 | 27.90 | 118 | 26.40 | 132 | 29.40 | ||
| SHI | UEBMI | 403 | 44.98 | 190 | 42.51 | 213 | 47.44 | p=0.053 |
| URRBMI | 462 | 51.56 | 246 | 55.03 | 216 | 48.11 | ||
| None | 31 | 3.46 | 11 | 2.46 | 20 | 4.45 | ||
| Commercial insurance | Yes | 273 | 30.67 | 138 | 31.08 | 135 | 30.27 | p=0.793 |
| No | 617 | 69.33 | 306 | 68.92 | 311 | 69.73 | ||
| EQ-5D-5L utility45 | Perfect health | 578 | 64.51 | 293 | 65.55 | 285 | 63.47 | p=0.517 |
| Non-perfect health | 318 | 35.49 | 154 | 34.45 | 164 | 36.53 | ||
Perfect health: EQ-5D-5L utility=1; non-perfect health: EQ-5D-5L utility <1.
EQ-5D-5L, five-level EuroQol five-dimensional questionnaire; Q_A, questionnaire A; Q_B, questionnaire B; RMB, Chinese renminbi; SHI, social health insurance; UEBMI, Urban Employee Basic Medical Insurance; URRBMI, Urban–Rural Residents Basic Medical Insurance.
Compared with those who were assigned with questionnaire A, study participants with questionnaire B were older (p<0.001), differed by educational characteristics—for example, less likely to have completed university (p<0.001), and more likely to reside in urban areas (p<0.001). Respondents’ bid path responses of the CVM were shown in online supplemental material 2.
bmjgh-2023-013070supp002.pdf (32.6KB, pdf)
Results of interval regression analysis are shown in table 2. Predicted mean WTP/Q was 128 000 RMB (approximately $36 364; 95% CI 124 900 to 131 100 RMB), which was equivalent to 1.76 times of GDP per capita in China for chronic diseases (scenario A). The respective WTP/Q was 149 500 RMB (approximately $42 472; 95% CI 146 400 to 152 700 RMB) and 140 800 RMB (approximately $40 000; 95% CI 136 800 to 144 700 RMB) for rare diseases and terminal diseases, which was equivalent to 2.06 and 1.94 times of GDP per capita, respectively.
Table 2.
Interval regression results for WTP per QALY gained in different scenarios
| Variables | Chronic diseases | Rare diseases | Terminal diseases | ||||
| β | P value | β | P value | β | P value | ||
| Questionnaire | A | ||||||
| B | −5.38 | 0.00 | −2.67 | 0.00 | −3.68 | 0.00 | |
| Sex | Male | ||||||
| Female | −1.17 | 0.10 | −1.18 | 0.16 | −1.35 | 0.13 | |
| Marital status | Unmarried | ||||||
| Married | 0.51 | 0.68 | −0.17 | 0.91 | 1.75 | 0.26 | |
| Widowed | −1.04 | 0.79 | 5.45 | 0.24 | 8.31 | 0.09 | |
| Divorced | −5.95 | 0.05 | −6.56 | 0.05 | −4.08 | 0.26 | |
| Other | 2.99 | 0.69 | −2.44 | 0.84 | 66.82 | 0.97 | |
| Location of residence | Urban | ||||||
| Rural and urban–rural areas | −2.62 | 0.00 | −3.82 | 0.00 | −3.96 | 0.00 | |
| Household consumption expenditure | Low | ||||||
| Low-middle | −1.02 | 0.36 | −0.79 | 0.54 | −1.86 | 0.18 | |
| Middle | 2.01 | 0.05 | 1.32 | 0.26 | 1.12 | 0.38 | |
| High-middle | 1.03 | 0.33 | 1.45 | 0.24 | 1.74 | 0.19 | |
| High | 5.17 | 0.00 | 4.39 | 0.00 | 4.19 | 0.01 | |
| SHI | UEBMI | ||||||
| URRBMI | −0.20 | 0.82 | −1.33 | 0.18 | −1.21 | 0.26 | |
| None | 1.04 | 0.61 | −0.48 | 0.83 | −0.49 | 0.84 | |
| Commercial insurance | Yes | ||||||
| No | 0.48 | 0.55 | −0.79 | 0.40 | −0.83 | 0.41 | |
| Educational level | Primary school and below | ||||||
| Middle school | 1.01 | 0.53 | 1.35 | 0.46 | −0.08 | 0.97 | |
| High school | 1.27 | 0.45 | 3.73 | 0.05 | 2.25 | 0.28 | |
| College and above | 4.09 | 0.02 | 6.94 | 0.00 | 5.54 | 0.01 | |
| Age | ≤25 | ||||||
| 26–35 | 0.15 | 0.92 | 1.32 | 0.41 | −0.81 | 0.64 | |
| 36–45 | −0.13 | 0.94 | 3.10 | 0.12 | −1.67 | 0.43 | |
| 46–55 | 1.19 | 0.51 | 3.02 | 0.15 | 1.07 | 0.64 | |
| ≥55 | −1.12 | 0.61 | 1.58 | 0.53 | −0.24 | 0.93 | |
| Employment status | Employed | ||||||
| Retired | −1.84 | 0.40 | −1.36 | 0.59 | −1.82 | 0.51 | |
| Unemployed | 0.65 | 0.54 | 0.66 | 0.58 | 0.61 | 0.64 | |
| EQ-5D-5L utility | Perfect health | ||||||
| Non-perfect health | −1.81 | 0.02 | −1.05 | 0.23 | −0.39 | 0.68 | |
| Constant | 13.46 | 0.00 | 13.08 | 0.00 | 14.66 | 0.00 | |
Perfect health: EQ-5D-5L utility=1; non-perfect health: EQ-5D-5L utility <1.
EQ-5D-5L, five-level EuroQol five-dimensional questionnaire; QALY, quality-adjusted life year; SHI, social health insurance; UEBMI, Urban Employee Basic Medical Insurance; URRBMI, Urban–Rural Residents Basic Medical Insurance; WTP, willingness to pay.
Respondents living in urban areas were willing to pay higher across all scenarios (p<0.01). WTP/Q was higher in those with a higher household expenditure (p<0.01). There was a positive association between the study participants’ school educational attainment and their WTP/Q. People with an education background of university or above had significantly higher WTP/Q than those with primary school education and below (p<0.02) after controlling for their economic status. Of note, in scenario A for general chronic diseases, study participants’ health-related quality of life was an additional associated factor, those who reported perfect health were willing to pay 181 000 RMB (approximately $51 420) more than those who reported non-perfect health.
Mean WTP/Q was lower in respondents who were assigned with the questionnaire starting with a lower initial bid value across all disease scenarios (p<0.01). For those with questionnaire A, their mean WTP/Q was 155 100 RMB (approximately $44 063; 95% CI 151 900 to 158 300 RMB), 164 400 (approximately $46 705; 95% CI 160 800 to 168 100 RMB) and 157 900 RMB (approximately $44 858; 95% CI 154 000 to 161 700 RMB), while that for people using questionnaire B was 94 800 RMB (approximately $26 932; 95% CI 92 800 to 96 800 RMB), 129 300 RMB (approximately $36 733; 95% CI 126 600 to 132 100 RMB) and 119 700 RMB (approximately $34 006; 95% CI 114 900 to 124 600 RMB) for chronic disease, rare disease and terminal disease scenarios, respectively.
The sensitivity analyses showed that after removing protest responses, the mean WTP/Q was consistently higher than that in the base case analysis. The corresponding WTP/Q was 132 000 RMB (approximately $37 500; 1.82 times of GDP per capita; 95% CI 128 900 to 135 100 RMB) for chronic diseases, 152 700 RMB (approximately $43 381; 2.10 times of GDP per capita; 95% CI 149 400 to 156 000 RMB) for rare diseases and 143 600 RMB (approximately $40 795; 1.98 times of GDP per capita; 95% CI 139 500 to 147 700 RMB) for terminal diseases, respectively. Moreover, socioeconomic variables and specific groups related to WTP/Q were the same as those without removing protest responses (table 3).
Table 3.
Interval regression for WTP per QALY gained in disease scenarios after excluding protest responses
| Variables | Chronic diseases | Rare diseases | Terminal diseases | ||||
| β | P value | β | P value | β | P value | ||
| Questionnaire | A | ||||||
| B | −5.47 | 0.00 | −2.76 | 0.00 | −3.77 | 0.00 | |
| Sex | Male | ||||||
| Female | −1.25 | 0.09 | −1.43 | 0.09 | −1.69 | 0.06 | |
| Marital status | Unmarried | ||||||
| Married | 0.34 | 0.79 | −0.15 | 0.92 | 1.67 | 0.29 | |
| Widowed | −1.20 | 0.76 | 5.56 | 0.23 | 8.29 | 0.10 | |
| Divorced | −3.77 | 0.22 | −5.48 | 0.12 | −3.33 | 0.38 | |
| Other | 2.57 | 0.74 | 11.04 | 0.23 | 71.22 | 0.95 | |
| Location of residence | Urban | ||||||
| Rural and urban–rural areas | −2.53 | 0.00 | −3.85 | 0.00 | −3.89 | 0.00 | |
| Household consumption expenditure | Low | ||||||
| Low-middle | −1.16 | 0.31 | −0.75 | 0.57 | −1.93 | 0.17 | |
| Middle | 2.15 | 0.04 | 1.59 | 0.18 | 1.25 | 0.33 | |
| High-middle | 1.30 | 0.23 | 1.80 | 0.15 | 1.78 | 0.19 | |
| High | 5.83 | 0.00 | 5.12 | 0.00 | 4.78 | 0.00 | |
| SHI | UEBMI | ||||||
| URRBMI | −0.14 | 0.88 | −1.27 | 0.21 | −1.06 | 0.34 | |
| None | 1.36 | 0.51 | −0.67 | 0.77 | −0.61 | 0.80 | |
| Commercial insurance | Yes | ||||||
| No | 0.44 | 0.59 | −1.03 | 0.28 | −1.10 | 0.28 | |
| Educational level | Primary school and below | ||||||
| Middle school | 1.15 | 0.48 | 1.51 | 0.41 | 0.01 | 1.00 | |
| High school | 1.03 | 0.54 | 3.80 | 0.05 | 2.45 | 0.25 | |
| College and above | 3.61 | 0.04 | 6.82 | 0.00 | 5.53 | 0.01 | |
| Age | ≤25 | ||||||
| 26–35 | 0.15 | 0.92 | 1.32 | 0.42 | −0.68 | 0.70 | |
| 36–45 | 0.47 | 0.79 | 3.17 | 0.12 | −1.33 | 0.54 | |
| 46–55 | 0.82 | 0.66 | 2.79 | 0.19 | 1.09 | 0.64 | |
| ≥55 | −1.69 | 0.44 | 1.24 | 0.62 | −0.33 | 0.90 | |
| Employment status | Employed | ||||||
| Retired | −1.75 | 0.43 | −1.33 | 0.60 | −1.76 | 0.52 | |
| Unemployed | 0.57 | 0.59 | 0.54 | 0.66 | 0.46 | 0.73 | |
| EQ-5D-5L utility | Perfect health | ||||||
| Non-perfect health | −1.92 | 0.01 | −1.30 | 0.14 | −0.73 | 0.44 | |
| Constant | 14.16 | 0.00 | 13.61 | 0.00 | 15.18 | 0.00 | |
Perfect health: EQ-5D-5L utility=1; non-perfect health: EQ-5D-5L utility <1.
EQ-5D-5L, five-level EuroQol five-dimensional questionnaire; QALY, quality-adjusted life year; SHI, social health insurance; UEBMI, Urban Employee Basic Medical Insurance; URRBMI, Urban–Rural Residents Basic Medical Insurance; WTP, willingness to pay.
The results of participants’ perceived amounts of WTP/Q also revealed that participants had the highest WTP for rare diseases, followed by terminal diseases. The average perceived WTP amount for chronic diseases was 161 600 RMB (approximately $45 909; 95% CI 146 600 to 176 600 RMB), while for rare diseases and terminal diseases, it was 195 100 RMB (approximately $55 426; 95% CI 176 400 to 213 800 RMB) and 189 000 RMB (approximately $53 693; 95% CI 170 600 to 207 400 RMB), respectively.
Discussion
This study estimated the WTP for one QALY among the Chinese general population under three disease scenarios. Using CVM, we found that Chinese WTP/Q varied significantly across kinds of diseases, which was 128 000 RMB (approximately $36 364; 1.76 times of GDP per capita), 149 500 RMB (approximately $42 472; 2.06 times of GDP per capita) and 140 800 RMB (approximately $40 000; 1.94 times of GDP per capita) for general chronic diseases, rare diseases and terminal diseases, respectively. In each disease scenario, participants living in urban areas, having more educational attainment or living in better economic status were willing to pay higher for each disease scenario.
The 1.76–2.06 times of GDP per capita estimated in this study represents the WTP preference of health improvement among the general population. Results are comparable with previous studies in other countries or regions, and the reported WTP/Q in the Netherlands (1.61–2.51 times GDP per capita), South Korea (1.24–1.48 times GDP per capita), Taiwan, China (1.25–1.35 times GDP per capita) and Finland (1.81 times GDP per capita)49–51 agreed with our study. China has also made several attempts on WTP/Q estimation in the last decade. A study conducted in 2009 reported a lower WTP/Q than ours (0.72 times GDP per capita).52 Two potential reasons account for the differences. On the one hand, the previous study measured WTP for health improvement from current health state to perfect health, while in our study, we posed the scenario of moving from different illness to total health. The health state for general people is usually better than patients therefore requiring less cost to achieve perfect health, leading to lower WTP. This explanation is supported by studies in Thailand.53 54 On the other hand, demand for health and healthcare among the Chinese general population has increased in the last decade, leading to higher WTP for health improvement,55 and also implied the significance of updated WTP/Q studies. Another recent estimation using similar methods to ours reported a WTP of 1.75 times GDP per capita and supported our findings.56 Our study revealed a 12 800 RMB (approximately $3636) higher WTP/Q for terminal diseases than general chronic diseases. Our results are consistent with earlier studies in Japan, Thailand and the USA which also indicated WTP/Q varied across diseases,53 57 58 while findings from Iran and Japan reported higher WTP/Q in serious diseases than other conditions.18 50 59 These findings are consistent with the health technology assessment agencies when they determine the cost-effectiveness of treatments for different disease scenarios. For example, NICE in the UK has established a standard threshold of £20 000–30 000 per QALY for most common diseases. This threshold can increase to £50 000 and £300 000 per QALY gained for end-of-life treatments and very rare diseases, respectively.13 Australia has also implemented a Life Saving Drugs Program to fund essential medicines for individuals with rare and life-threatening diseases, even if these medicines failed to meet cost-effectiveness criteria for inclusion in the Pharmaceutical Benefits Scheme.19 While various studies may present varying estimates of WTP/Q across different countries, a common trend emerges: WTP/Q for terminal or rare diseases consistently exceeds that for common chronic diseases.13 18 59 60 One potential reason might be differential disease burden. Terminal diseases such as cancer usually cause severe consequences including painful symptoms and short survival time. The fact that treatments for terminal diseases are often more costly could also partly explain the high WTP. For example, cancer has been reported to have the greatest impact on family economic living standard, while stroke has the highest risk of causing poverty.61 The issue is also challenging patients and the healthcare system in other countries and, as a response, NICE increased the threshold from £20 000–30 000 for general technologies to £50 000 for end-of-life interventions in 2009.62 Another explanation could be the present biased preference rationale extracted from behavioural economics: people tend to disproportionately place more weight on immediate compared with future concerns.63 In the scenario of a terminal disease, dying immediately could be more frightening for most individuals than dying later for chronic diseases. Therefore, they would place a higher value on extending their lives for 1 more year immediately than they would for 1 more year occurring many years later. This results in a higher WTP for terminal diseases. Another interesting finding is that the highest WTP/Q was found for rare diseases, 16.80% and 6.18% higher than chronic diseases and terminal diseases, respectively. Often being caused by genetic disorders, rare diseases are characterised by severe symptoms through the entire life and causing shortened survival time.64 The price of interventions is also disproportionately high because the cost of treatment development could only be recouped from the small patient groups.65 For example, a systematic review reported an annual cost of almost €300 000 per patient for rare disease treatment.66 The required long-term care of patients further increased both the disease burden and economic burden,67 leading to high WTP. It suggests that rare diseases should be particularly considered in resource allocation decisions.
Similar with existing evidence, socioeconomic status was also found to be related to WTP/Q in this study. The positive association of economic status and WTP/Q has been widely reported. Rich individuals would be able to spend more on health improvement and value their health more than the poor. A study found that 1% higher income was related to 0.6% increase of WTP/Q.68 Additionally, more health knowledge and better healthcare access ability of individuals with higher educational level and those living in urban areas account for their relationship with higher WTP/Q.69 The WTP/Q difference across socioeconomic status indicates distributional issue in healthcare resource allocation, especially for socioeconomically vulnerable subpopulation, and implies flexible threshold in decision-making.
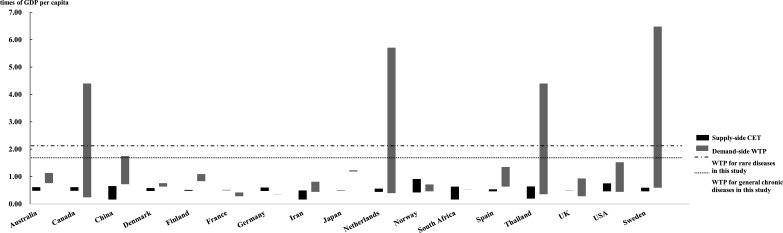
Another commonly used approach to estimate a CET is from the supply side. In China, supply-side estimation reported a CET of 1.50 times GDP per capita every QALY gained or 0.63 times GDP per capita for every DALY averted,6 35 while that from demand side is 0.77–2.06 times GDP per capita for a QALY gained. This is in line with results from other countries or regions. From existing estimates, we found that in many countries including Australia, Denmark, Japan, the Netherlands, Finland, Spain and Sweden, thresholds estimated from supply side are lower than that from demand side which are commonly measured by WTP (figure 2 and online supplemental material 3). Differences in these values could be attributed to the notion that supply-side threshold estimates are established under budget constraints, while demand-side WTP thresholds are not explicitly so; they tend to measure individual health preference and reflect the social net benefits of medical treatment.12 70 Although the notion of budget-constrained maximisation in principle should more realistically reflect social preferences, it has been argued that lower thresholds limit the incentives in innovation needed to drive innovation in the long run.35
Figure 2.
Supply-side CET and demand-side WTP/Q in different countries. This figure was used for comparison of supply-side CET and demand-side WTP/Q in different countries. Considering that most countries had more than one estimate, this figure summarised all the results found. The bottom of each bar represents the maximum value in each context while the top of the bar shows the minimum value. Full details could be found in online supplemental material 3. CET, cost-effectiveness threshold; GDP, gross domestic product; WTP/Q, willingness to pay per quality-adjusted life year gained.
bmjgh-2023-013070supp003.pdf (91.5KB, pdf)
Findings of this study that people’s WTP/Q changes with types of diseases and socioeconomic status strengthened our belief that more factors should be taken into consideration when determining CET in a context, and this applies in most countries around the world. Studies in other countries have also stated the impropriety of applying one fixed threshold in all cases,38 and agreed to incorporate conditional health preferences on societal decisions.71 On the other hand, an explicit condition-specific threshold category may cause discrimination against a specific group of population.53 A plausible solution could be an indistinct threshold with explicit reasonable range of adjustments based on previous decision-making practice, country’s healthcare budget and population health preference. Approaches like multicriteria decision analysis framework, which aims to evaluate the value of an intervention by incorporating other factors that could have impact on value estimation,72 73 could be helpful in CET determination.
This study generated new evidence of WTP/Q for different diseases in the Chinese general population and potentially enables differential thresholds to be used for decision-making. However, limitations of this study should be acknowledged. First, only broad groups of disease rather than specific diseases were presented. Although interviewers were trained to explain the differences and listed some examples for each kind of diseases, participants may have had difficulty in understanding the hypothesised conditions.56 Second, it has been stated that double-bounded CVM, though is believed better than single-question investigation, may cause risk of participants’ resistance by asking them follow-up questions.37 Our study employed quadruple-bounded method hence also faces the same problem and the resulting potential bias. It is also important to note that due to the use of a convenience sample from a single province, the results might not fully represent the WTP/Q for the entire Chinese population. Nonetheless, the correlations observed between individuals’ demographic and socioeconomic status and their WTP/Q could have potential applicability to other subpopulations in China, contributing to predicting WTP/Q within the broader Chinese general population. Further studies are expected to address the limitations.
Conclusions
This study estimated a WTP of 1.76–2.06 times GDP per capita for 1 additional healthy year among the Chinese general population, a value that is consistent with similar studies done in China and internationally. As the WTP/Q thresholds vary across disease scenarios and across socioeconomic groups, there is an argument for tailored rather than generalised CETs to better reflect community preferences. Our estimates are important in enabling the expansion in use of health technology assessments and economic evaluations to inform decision-making and ultimately enable China to achieve universal health coverage with limited resources.
Footnotes
Handling editor: John Lee
Correction notice: This article has been corrected since it published Online to reflect the correct affiliations for author Lei Si.
Contributors: LS, SJ and MC conceived and designed the study. LS, MC and LX designed the interview questionnaire. SJ, BA, LS, KH, MC and LX reviewed and revised the questionnaire. MC and LX were responsible for conducting the pilot study, performing face-to-face interviews and collecting data. LX did the statistical analysis and drafted the initial version of the manuscript. LS, YJ, SJ and LX contributed to data interpretation. LS supervised the study. LX, LS and MC are responsible for the overall content as the guarantors. All authors critically reviewed the manuscript and approved the final version prior to submission and resubmission.
Funding: This study was funded by the China Medical Board (19-346). LX is supported by an Australian Government Research Training Program Scholarship and a Western Sydney University Research Theme Grant.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are available upon academic research request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Human Research Ethics & Clinical Trials Governance, UNSW Sydney (HC210700). Participants gave informed consent to participate in the study before taking part.
References
- 1.Neumann PJ, Ganiats TG, Russell LB, et al. Cost-effectiveness in health and medicine. In: Cost-effectiveness in health and medicine. Oxford University Press, 2016. 10.1093/acprof:oso/9780190492939.001.0001 [DOI] [Google Scholar]
- 2.Sampson C, Zamora B, Watson S, et al. Supply-side cost-effectiveness thresholds: questions for evidence-based policy. Appl Health Econ Health Policy 2022;20:651–67. 10.1007/s40258-022-00730-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobinac A, Van Exel NJA, Rutten FFH, et al. Willingness to pay for a quality-adjusted life-year: the individual perspective. Value Health 2010;13:1046–55. 10.1111/j.1524-4733.2010.00781.x [DOI] [PubMed] [Google Scholar]
- 4.Moradi N, Woldemichael A, Malekian P, et al. An exploratory study to estimate cost-effectiveness threshold value for life saving treatments in Western Iran. Cost Eff Resour Alloc 2020;18:47. 10.1186/s12962-020-00241-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nimdet K, Chaiyakunapruk N, Vichansavakul K, et al. A systematic review of studies eliciting willingness-to-pay per quality-adjusted life year: does it justify CE threshold PLoS One 2015;10:e0122760. 10.1371/journal.pone.0122760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochalek J, Wang H, Gu Y, et al. Informing a cost-effectiveness threshold for health technology assessment in China: A marginal productivity approach. Pharmacoeconomics 2020;38:1319–31. 10.1007/s40273-020-00954-y [DOI] [PubMed] [Google Scholar]
- 7.Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? clashing opinions and an absence of data: a systematic review. Glob Health Action 2018;11:1447828. 10.1080/16549716.2018.1447828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–44. 10.2165/00019053-200826090-00004 [DOI] [PubMed] [Google Scholar]
- 9.Isaranuwatchai W, Nakamura R, Wee HL, et al. What are the impacts of increasing cost-effectiveness threshold? a protocol on an empirical study based on economic evaluations conducted in Thailand. PLoS One 2022;17:e0274944. 10.1371/journal.pone.0274944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Mahony JF, Coughlan D. The Irish cost-effectiveness threshold: does it support rational rationing or might it lead to unintended harm to Ireland’s health system? Pharmacoeconomics 2016;34:5–11. 10.1007/s40273-015-0336-1 [DOI] [PubMed] [Google Scholar]
- 11.Bertram MY, Lauer JA, De Joncheere K, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94:925–30. 10.2471/BLT.15.164418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health 2016;19:929–35. 10.1016/j.jval.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulden M. Recent amendments to NICE’s value-based assessment of health Technologies: implicitly inequitable Expert Rev Pharmacoecon Outcomes Res 2017;17:239–42. 10.1080/14737167.2017.1330152 [DOI] [PubMed] [Google Scholar]
- 14.Robinson LA, Hammitt JK, Chang AY, et al. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan 2017;32:141–5. 10.1093/heapol/czw096 [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer R, Rochau U, Saverno K, et al. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. J Comp Eff Res 2015;4:485–504. 10.2217/cer.15.38 [DOI] [PubMed] [Google Scholar]
- 16.Sund B, Svensson M. Estimating a constant WTP for a QALY-a mission impossible Eur J Health Econ 2018;19:871–80. 10.1007/s10198-017-0929-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanovskiy M, Levy ON, Shaki YY, et al. Cost-effectiveness threshold for Healthcare: justification and Quantification. Inquiry 2022;59:469580221081438. 10.1177/00469580221081438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moradi N, Rashidian A, Nosratnejad S, et al. Willingness to pay for one quality-adjusted life year in Iran. Cost Eff Resour Alloc 2019;17:4. 10.1186/s12962-019-0172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Australian Government Department of Health and Aged Care . Cost of medicines. n.d. Available: https://www.health.gov.au/topics/medicines/cost
- 20.Nielsen JS, Gyrd-Hansen D, Kjaer T. Sample restrictions and the Elicitation of a constant willingness to pay per quality adjusted life year. Health Econ 2021;30:923–31. 10.1002/hec.4236 [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Zhang L, Hu M, et al. Use of health technology assessment in drug reimbursement decisions in China. BMJ 2023;381:e068915. 10.1136/bmj-2021-068915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan J, Zheng W, Liu W, et al. Cost-effectiveness of intensive versus standard blood pressure treatment in older patients with hypertension in China. Hypertension 2022;79:2631–41. 10.1161/HYPERTENSIONAHA.122.20051 [DOI] [PubMed] [Google Scholar]
- 23.Guo J, Zhang H, Zhang H, et al. Cost-effectiveness of Pneumococcal vaccines among adults aged 65 years and older in China: A comparative study. Vaccine 2023;41:716–23. 10.1016/j.vaccine.2022.12.004 [DOI] [PubMed] [Google Scholar]
- 24.Chayab L, Konstantelos N, Leighl NB, et al. A systematic review of the cost-effectiveness analyses of Anaplastic lymphoma kinase (ALK) inhibitors in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). Pharmacoeconomics 2023;41:945–80. 10.1007/s40273-023-01279-2 [DOI] [PubMed] [Google Scholar]
- 25.Gong Y, Yao X, Peng J, et al. Cost-effectiveness and health impacts of different influenza vaccination strategies for children in China. Am J Prev Med 2023;65:155–64.:S0749-3797(23)00035-1. 10.1016/j.amepre.2023.01.028 [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Jiang S, Ahumada-Canale A, et al. Breast cancer screening should embrace precision medicine: evidence by reviewing economic evaluations in China. Adv Ther 2023;40:1393–417. 10.1007/s12325-023-02450-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S, Kruger E, Tan SC, et al. Cost-effectiveness analysis of Folfox4 and sorafenib for the treatment of advanced hepatocellular carcinoma in China. Cost Eff Resour Alloc 2018;16:29. 10.1186/s12962-018-0112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Legood R, Sadique Z, et al. Cost-effectiveness of risk-based breast cancer screening programme, China. Bull World Health Organ 2018;96:568–77. 10.2471/BLT.18.207944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Li Y, Zheng X. Cost-effectiveness of the combination of Immunotherapy and chemotherapy for extensive-stage small-cell lung cancer: a systematic review. BMC Health Serv Res 2023;23:691. 10.1186/s12913-023-09727-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia R, Zeng H, Liu W, et al. Estimated cost-effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high-risk areas in China. JAMA Netw Open 2021;4:e2121403. 10.1001/jamanetworkopen.2021.21403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Ming J, Qu S, et al. Cost-effectiveness of flash glucose monitoring for the management of patients with type 1 and patients with type 2 diabetes in China. Diabetes Ther 2021;12:3079–92. 10.1007/s13300-021-01166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou Z, Fairley CK, Ong JJ, et al. Domestic HPV vaccine price and economic returns for Cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health 2020;8:e1335–44.:S2214-109X(20)30277-1. 10.1016/S2214-109X(20)30277-1 [DOI] [PubMed] [Google Scholar]
- 33.Liu GG. China Guidelines for Pharmacoeconomic Evaluations. Beijing China: China Market Press, 2020. [Google Scholar]
- 34.Butt T, Liu GG, Kim DD, et al. Taking stock of cost-effectiveness analysis of Healthcare in China. BMJ Glob Health 2019;4:e001418. 10.1136/bmjgh-2019-001418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai D, Shi S, Jiang S, et al. Estimation of the cost-effective threshold of a quality-adjusted life year in China based on the value of statistical life. Eur J Health Econ 2022;23:607–15. 10.1007/s10198-021-01384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angell B, Cullen P, Laba T, et al. What is the value of a driver licence? A contingent valuation study of Australian adults. Transportation Research Part A: Policy and Practice 2018;108:25–34. 10.1016/j.tra.2017.12.010 [DOI] [Google Scholar]
- 37.Cawley J. Contingent valuation analysis of willingness to pay to reduce childhood obesity. Econ Hum Biol 2008;6:281–92. 10.1016/j.ehb.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 38.Lim YW, Shafie AA, Chua GN, et al. Determination of cost-effectiveness threshold for health care interventions in Malaysia. Value Health 2017;20:1131–8.:S1098-3015(17)30202-4. 10.1016/j.jval.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 39.Arrow K, Solow R, Portney PR, et al. Report of the NOAA panel on contingent valuation. Fed Regist 1993;58:4601–14. [Google Scholar]
- 40.The Campbell and Cochrane Economics Methods Group . EPPI-centre cost converter [available from. Available: https://eppi.ioe.ac.uk/costconversion/default.aspx [Accessed 30 Oct 2022].
- 41.THE WORLD BANK . GDP per capita (current US$) - China, Available: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=CN [Accessed 30 Oct 2022].
- 42.Pennington M, Gomes M, Donaldson C. Handling protest responses in contingent valuation surveys. Med Decis Making 2017;37:623–34. 10.1177/0272989X17691771 [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Atun R, Oldenburg B, et al. Physical Multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population-based panel data. Lancet Glob Health 2020;8:e840–9.:S2214-109X(20)30127-3. 10.1016/S2214-109X(20)30127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Bureau of Statistics of China . Statistical Communiqué of the People’s Republic of China on the 2021 National Economic and Social Development, . 2021Available: http://www.stats.gov.cn/tjsj/zxfb/202102/t20210227_1814154.html [Accessed 2 Nov 2022].
- 45.Luo N, Liu G, Li M, et al. Estimating an EQ-5D-5L value set for China. Value Health 2017;20:662–9.:S1098-3015(16)34125-0. 10.1016/j.jval.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 46.Sun S, Chen J, Johannesson M, et al. Population health status in China: EQ-5D results, by age, sex and socio-economic status, from the national health services survey 2008. Qual Life Res 2011;20:309–20. 10.1007/s11136-010-9762-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson L, Spanbauer M, Button P. How valuable are national parks? evidence from a proposed National Park expansion in Alaska. J Park Recreat Admi 2019;37. 10.18666/JPRA-2019-8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray AM, Clarke PM, Wolstenholme JL, et al. Applied methods of cost-effectiveness analysis in Healthcare: OUP. Oxford, 2010. [Google Scholar]
- 49.Bobinac A, van Exel NJA, Rutten FFH, et al. Valuing QALY gains by applying a societal perspective. Health Econ 2013;22:1272–81. 10.1002/hec.2879 [DOI] [PubMed] [Google Scholar]
- 50.Shiroiwa T, Sung Y-K, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness Health Econ 2010;19:422–37. 10.1002/hec.1481 [DOI] [PubMed] [Google Scholar]
- 51.J. Soini E. Contingent valuation of eight new treatments: what is the clinician’s and politician’s willingness to pay TOALTMEDJ 2012;4:1–11. 10.2174/1876391X01204010001 [DOI] [Google Scholar]
- 52.Zhao F-L, Yue M, Yang H, et al. Willingness to pay per quality-adjusted life year: is one threshold enough for decision-making?: results from a study in patients with chronic Prostatitis. Med Care 2011;49:267–72. 10.1097/MLR.0b013e31820192cd [DOI] [PubMed] [Google Scholar]
- 53.Thavorncharoensap M, Teerawattananon Y, Natanant S, et al. Estimating the willingness to pay for a quality-adjusted life year in Thailand: does the context of health gain matter? Clinicoecon Outcomes Res 2013;5:29–36. 10.2147/CEOR.S38062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thongprasert S, Crawford B, Sakulbumrungsil R, et al. Willingness to pay for lung cancer treatment: patient versus general public values. Int J Technol Assess Health Care 2015;31:264–70. 10.1017/S0266462315000409 [DOI] [PubMed] [Google Scholar]
- 55.Yip W, Fu H, Chen AT, et al. 10 years of health-care reform in China: progress and gaps in universal health coverage. Lancet 2019;394:1192–204. 10.1016/S0140-6736(19)32136-1 [DOI] [PubMed] [Google Scholar]
- 56.Ye Z, Abduhilil R, Huang J, et al. Willingness to pay for one additional quality adjusted life year: A population based survey from China. Appl Health Econ Health Policy 2022;20:893–904. 10.1007/s40258-022-00750-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byrne MM, O’Malley K, Suarez-Almazor ME. Willingness to pay per quality-adjusted life year in a study of knee osteoarthritis. Med Decis Making 2005;25:655–66. 10.1177/0272989X05282638 [DOI] [PubMed] [Google Scholar]
- 58.Lieu TA, Ray GT, Ortega-Sanchez IR, et al. Willingness to pay for a QALY based on community member and patient preferences for temporary health States associated with herpes Zoster. Pharmacoeconomics 2009;27:1005–16. 10.2165/11314000-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 59.Shiroiwa T, Igarashi A, Fukuda T, et al. WTP for a QALY and health States: more money for Severer health States Cost Eff Resour Alloc 2013;11:22. 10.1186/1478-7547-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olofsson S, Gerdtham U-G, Hultkrantz L, et al. Measuring the end-of-life premium in cancer using individual ex ante willingness to pay. Eur J Health Econ 2018;19:807–20. 10.1007/s10198-017-0922-6 [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Yang H. Does elderly chronic disease hinder the Sustainability of borderline poor families' wellbeing: an investigation from catastrophic health expenditure in China. Int J Public Health 2022;67:1605030. 10.3389/ijph.2022.1605030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bovenberg J, Penton H, Buyukkaramikli N. 10 years of end-of-life criteria in the United Kingdom. Value Health 2021;24:691–8.:S1098-3015(21)00037-1. 10.1016/j.jval.2020.11.015 [DOI] [PubMed] [Google Scholar]
- 63.O’Donoghue T, Rabin M. Doing it now or later. American Economic Review 1999;89:103–24. 10.1257/aer.89.1.103 [DOI] [Google Scholar]
- 64.Chu SY, Weng CY. Introduction to genetic/rare disease and the application of genetic counseling. Hu Li Za Zhi 2017;64:11–7.:JN.000063. 10.6224/JN.000063 [DOI] [PubMed] [Google Scholar]
- 65.Moro D, Schlander M, Telser H, et al. Evaluating discrete choice experiment willingness to pay [DCE-WTP] analysis and relative social willingness to pay [RS-WTP] analysis in a health technology assessment of a treatment for an ultra-rare childhood disease [CLN2]. Expert Rev Pharmacoecon Outcomes Res 2022;22:581–98. 10.1080/14737167.2022.2014324 [DOI] [PubMed] [Google Scholar]
- 66.Schlander M, Dintsios CM, Gandjour A. Budgetary impact and cost drivers of drugs for rare and Ultrarare diseases. Value Health 2018;21:525–31.:S1098-3015(17)33623-9. 10.1016/j.jval.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 67.Dwyer AA, Uveges MK, Dockray S, et al. Exploring rare disease patient attitudes and beliefs regarding genetic testing: implications for person-centered care. J Pers Med 2022;12:477. 10.3390/jpm12030477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kouakou CRC, Poder TG. Willingness to pay for a quality-adjusted life year: a systematic review with meta-regression. Eur J Health Econ 2022;23:277–99. 10.1007/s10198-021-01364-3 [DOI] [PubMed] [Google Scholar]
- 69.JieAnNaMu Xu X, You H, et al. Inequalities in health-related quality of life and the contribution from socioeconomic status: evidence from Tibet, China. BMC Public Health 2020;20:630. 10.1186/s12889-020-08790-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vallejo-Torres L, García-Lorenzo B, Castilla I, et al. On the estimation of the cost-effectiveness threshold: Why, what, how Value Health 2016;19:558–66.:S1098-3015(16)00069-3. 10.1016/j.jval.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 71.Brouwer W, van Exel J, Baker R, et al. The new myth: the social value of the QALY. Pharmacoeconomics 2008;26:1–4. 10.2165/00019053-200826010-00001 [DOI] [PubMed] [Google Scholar]
- 72.Baltussen R, Marsh K, Thokala P, et al. Multicriteria decision analysis to support health technology assessment agencies: benefits, limitations, and the way forward. Value Health 2019;22:1283–8.:S1098-3015(19)32358-7. 10.1016/j.jval.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 73.Dionne F, Mitton C. Is Multicriteria decision analysis a resource allocation framework. Value Health 2020;23:1400–1. 10.1016/j.jval.2020.02.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-013070supp001.pdf (5.2MB, pdf)
bmjgh-2023-013070supp002.pdf (32.6KB, pdf)
bmjgh-2023-013070supp003.pdf (91.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are available upon academic research request.