Abstract
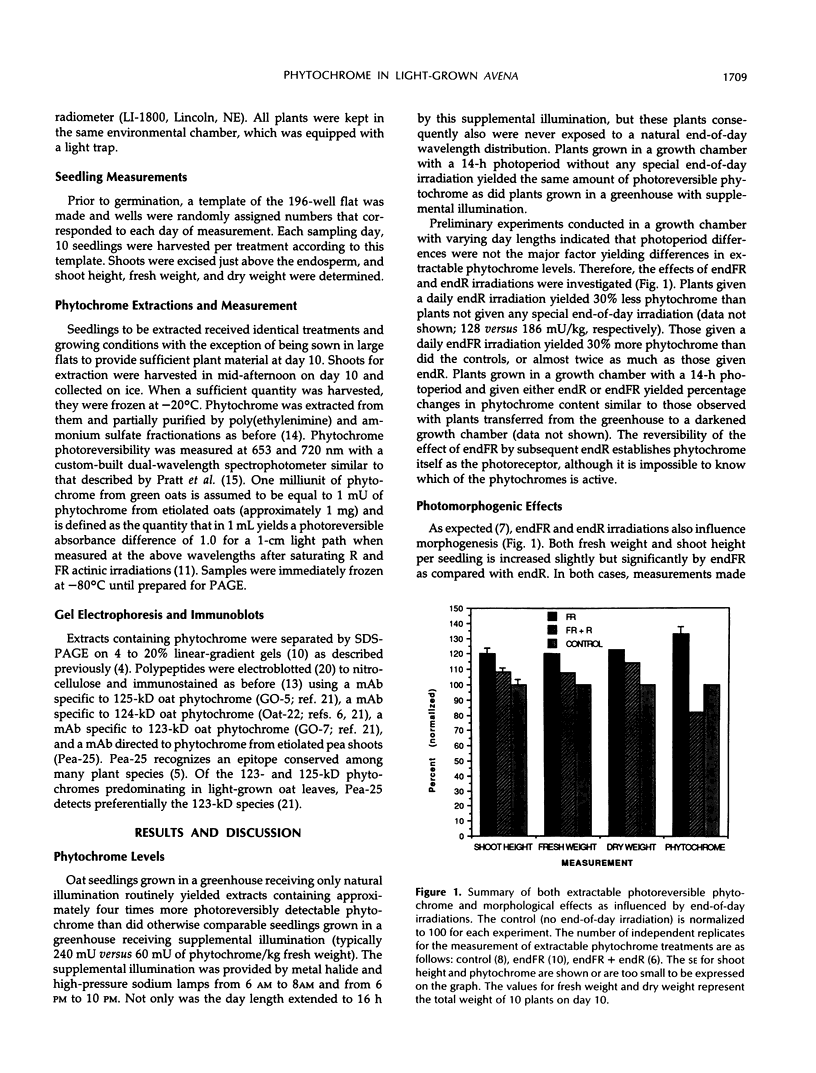
The effect of 15-minute end-of-day irradiations on photoreversible phytochrome levels in light-grown oat (Avena sativa L., cv Garry) seedlings was investigated. Oat seedlings were grown in a cycle of 8 hours of natural daylight and 16 hours of complete darkness, from sowing until harvest at day 10. The level of extractable, photoreversible phytochrome per unit fresh weight was 60% higher after end-of-day far-red irradiation than after either end-of-day red irradiation or end-of-day far-red followed by end-of-day red. Seedlings irradiated with end-of-day far-red also exhibited a small but significant increase in shoot height and fresh weight per seedling. Extracts of seedlings given each of these end-of-day treatments were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotted, and immunostained with monoclonal antibodies specific to different phytochromes. Regardless of end-of-day light treatment, phytochrome that is abundant in etiolated tissue was below the limit of detection, indicating that one or more of the phytochromes predominating in green tissue changes in abundance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordonnier M. M., Greppin H., Pratt L. H. Identification of a highly conserved domain on phytochrome from angiosperms to algae. Plant Physiol. 1986 Apr;80(4):982–987. doi: 10.1104/pp.80.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperbauer M. J. Spectral Distribution of Light in a Tobacco Canopy and Effects of End-of-Day Light Quality on Growth and Development. Plant Physiol. 1971 Jun;47(6):775–778. doi: 10.1104/pp.47.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G. H., Pratt L. H. Phytochrome destruction: an apparent requirement for protein synthesis in the induction of the destruction mechanism. Plant Physiol. 1973 Oct;52(4):309–311. doi: 10.1104/pp.52.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sharrock R. A., Quail P. H. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989 Nov;3(11):1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]



