Abstract
Introduction
Women with a history of gestational diabetes mellitus (GDM) are at high risk of developing type 2 diabetes, while the exact mechanisms underlying its pathophysiology are still unclear. We investigated the association of glucagon-like peptide-1 (GLP-1) response to oral glucose with parameters of glycemic control in women with previous GDM in the prospective PPSDiab (Prediction, Prevention, and Subclassification of Type 2 Diabetes) study.
Research design and methods
Glucose metabolism parameters and GLP-1 secretion were analyzed during oral glucose tolerance test (OGTT) in women with previous GDM (n=129) and women with a history of normal glucose tolerance (n=67) during pregnancy (controls). First- and second-phase insulin and GLP-1 secretion in relation to plasma glucose (PG) levels were assessed, and development of pre-diabetes was analyzed after 5-year follow-up among women with previous GDM and a normal glycemic state at baseline (n=58).
Results
The area under the curve (AUC during the OGTT 0–120 min) of PG and insulin but not GLP-1 differed significantly between post-GDM women and controls. However, women with previous GDM had a significantly decreased GLP-1 response in relation to PG and plasma insulin during the second phase of the OGTT. After a follow-up of 5 years, 19.0% post-GDM women with a normal glycemic state at the baseline visit developed abnormal glucose metabolism. The total, first- and second-phase AUC GLP-1/PG and GLP-1/insulin ratios were not associated with development of abnormal glucose tolerance.
Conclusions
Women with previous GDM showed a reduced GLP-1 response in relation to PG and insulin concentrations indicating early abnormalities in glucose metabolism. However, the altered GLP-1 response to oral glucose did not predict progression to pre-diabetes and type 2 diabetes in the first 5 years after GDM.
Keywords: Gestational Diabetes Mellitus, GLP-1, Incretin, Cohort Studies
WHAT IS ALREADY KNOWN ON THIS TOPIC
Glucagon-like peptide-1 (GLP-1) enhances glucose-stimulated insulin secretion. The GLP-1 effect is reduced in patients with type 2 diabetes. Only few studies have investigated GLP-1 secretory response in women after gestational diabetes mellitus (GDM) reporting contrasting results.
WHAT THIS STUDY ADDS
We found decreased GLP-1 concentrations to oral glucose in relation to insulin and glucose in post-GDM women as compared with control subjects after a normal pregnancy. Our 5-year follow-up data suggest that the GLP-1 response may not play a role as independent predictor for the development of pathologic glucose metabolism.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Alterations in GLP-1 may be involved in the disturbance of glucose metabolisms in an early pre-diabetic state in young women. Further studies and longer follow-up are required to investigate the mechanisms involved in defect GLP-1 secretion/potential GLP-1 resistance and therapeutic options derived from these findings.
Introduction
During pregnancy, insulin resistance is gradually increasing leading to a higher insulin demand to maintain physiologic glucose homeostasis.1 Women with defects in compensatory mechanism are predisposed to develop gestational diabetes mellitus (GDM) but usually return to normal glucose tolerance (NGT) after delivery. Thus, pregnancy can be seen as a natural challenge to identify a high-risk group of otherwise healthy women at a very early phase of pre-diabetes, which may be very helpful to characterize early underlying mechanisms involved in the pathogenesis of type 2 diabetes.2 3
The incretin glucagon-like peptide-1 (GLP-1) potentiates glucose-mediated insulin secretion after oral food ingestion. Subjects with abnormal glucose tolerance or type 2 diabetes exhibit an impaired incretin effect,4–6 which is thought to be involved in diabetes development. However, GLP-1 secretion has been shown to vary; in fact, to be normal,7 8 reduced,9 10 or increased11 in women with GDM.
Controversial results were also reported postpartum. A slightly reduced GLP-1 response to oral glucose load was observed in normoglycemic post-GDM women,9 12 whereas other studies showed no difference13 14 or detected decreased GLP-1 secretion in post-GDM women having developed a pre-diabetic metabolic state.15
At present, longitudinal data from women after GDM are completely lacking with respect to the association of the GLP-1 response and development of pre-diabetes or overt type 2 diabetes. Therefore, the aims of the present study were (1) to examine GLP-1 secretion after a glucose load in post-GDM women and controls and (2) to determine the predictive value of GLP-1 and other indices associated with incretin secretion with parameters of glycemic control in women with previous GDM after a 5-year follow-up period.
Research design and methods
Study cohort
The prospective, monocentric observational cohort study PPSDiab (“Prediction, Prevention, and Subclassification of Type 2 Diabetes”) included women with GDM during their last pregnancy (post-GDM group) and women following a normoglycemic pregnancy (control group or non-GDM group) in a 2:1 ratio. The sample size was calculated to detect differences in the development of pre-diabetes/type 2 diabetes during follow-up with a power (1−β) of 80% and an α level of 0.05. The study protocol, inclusion and exclusion criteria have been described in detail elsewhere.16
Eligible women were premenopausal and within 3 to 16 months after a singleton or twin pregnancy with live birth(s). In accordance with the German national guidelines, GDM was diagnosed by a screening 75 g oral glucose tolerance test (OGTT) with the cut-off values according to the International Association of the Diabetes and Pregnancy Study Groups (plasma glucose (PG): fasting ≥5.1 mmol/L (92 mg/dL), 1 hour ≥10.0 mmol/L (180 mg/dL), and 2 hours ≥8.5 mmol/L (153 mg/dL). Women with a normoglycemic pregnancy were included in the control group.17
At the 5-year follow-up, 78% still participated in the study. In the present study, plasma samples for the detection of GLP-1 levels from 5-point OGTT at the first visit after delivery were available from 196 women, 129 women with a history of GDM, and 67 controls after a normoglycemic pregnancy. Among 129 women with a history of GDM, 44 had a disturbed glucose tolerance and 85 participants had a normal glucose homeostasis at the baseline visit. None of these women was treated with antidiabetic drugs. After 5 years, 58 participants with GDM history and a normal glycemic state at baseline visit underwent the follow-up 75 g OGTT with plasma samples and were included in the follow-up analysis. In 27 post-GDM women, only OGTTs without plasma samples were available or subjects were lost to follow-up.
Study visits
The baseline visit (V1) of the PPSDiab study took place 3–16 months after the index pregnancy, while the 5-year follow-up visit (V3) was conducted 58–66 months postpartum. The 5-year follow-up visit was permitted to occur later in case of an additional pregnancy or if the early postpartum phase (6 months postpartum) overlapped with the time range for this visit; it could as well be postponed for personal reasons or an acute illness of the participants (n=47; median (Q1–Q3) = 70 (68 –74) months postpartum).
The participants in the post-GDM group attended yearly in-person visits with an OGTT between the baseline and the 5-year follow-up visit. The diagnosis of diabetes ended the study participation of the affected women. For the remaining participants, PPSDiab is still ongoing.
Anthropometric measurements
Body mass was measured by a bioelectrical impedance analysis (BIA) scale (Tanita BC-418; Tanita Corporation, Tokyo, Japan) in kilograms. Height and waist circumference were measured to the nearest cm using a tape measure. Body mass index (BMI) was calculated from body weight in kg divided by height in meter squared at study enrollment. We defined overweight/obesity as a BMI ≥25 kg/m2 and normal weight as a BMI <25 kg/m2. Systolic and diastolic blood pressure readings were obtained from all participants in a sitting position (both arms, two repeated measurements, the average value was recorded from the arm with the higher systolic value).
Oral glucose tolerance test
An OGTT with a five-point (0, 30, 60, 90, and 120 min after 75 g glucose intake) measurement of PG and serum insulin was conducted as previously described.16 PG and serum insulin levels were measured in mg/dL and µU/mL, respectively. The American Diabetes Association criteria were used for the diagnosis of pathologic glucose metabolism (impaired fasting glucose (IFG), impaired glucose tolerance (IGT), IFG+IGT, and type 2 diabetes mellitus (T2DM)), as defined based on PG levels at 0 and 120 min of 75 g OGTT.18
Biochemical measurements
All blood samples, except for postload glucose and insulin during OGTT, were determined after an overnight fast (12 hours). PG was measured by a hexokinase method (Glucose HK Gen.3; Roche Diagnostics, Mannheim, Germany), serum insulin by a chemiluminescent immunoassay (DiaSorin LIASON Systems, Saluggia, Italy), and plasma Glycated hemoglobin (HbA1c) by high-performance liquid chromatography (VARIANT II TURBO HbA1c Kit, Bio-Rad Laboratories, Hercules, California, USA).
GLP-1 levels were assessed using frozen (−80°C) plasma samples collected in tubes containing proteinase inhibitors (BD P800; BD Biosciences, San Jose, California, USA). Since the focus of the study was to estimate changes in GLP-1 secretion total GLP-1 but not inact GLP-1 was measured.19 Total GLP-1 concentrations (pmol/L) were detected by the Northern Lights Mercodia Total GLP-1 NL-ELISA assay in accordance with the manufacturer’s instructions.
To assess glucose clearance as well as insulin and GLP-1 secretion during OGTT, the area under the curve (AUC) for glucose, insulin, and GLP-1 (AUC 0–30 min, 30–60 min, 60–90 min, 90–120 min, 30–120 min and total 0–120 min) was determined using the trapezoidal rule. The interval 0–30 min refers to the first phase and the interval 30–120 min to the second phase of insulin response, respectively. We calculated the ratios between plasma GLP-1 and PG (GLP-1/PG), plasma insulin (GLP-1/Ins), insulin to glucose (Ins/PG) and AUC GLP-1/PG, AUC GLP-1/Ins, AUC Ins/PG, respectively. These variables provide a measure for GLP-1 and insulin secretion in relation to PG during an OGTT and a parameter to estimate the change of insulin secretion relative to the change of circulating GLP-1.
Statistical analyses
All metric and normally distributed variables were expressed as mean±SD. Non-normally distributed variables were presented as median (first quartile; third quartile). Comparison of normally distributed variables between groups was performed by two-sample t-test. Comparison of non-normally distributed variables between groups was performed by Mann-Whitney U test. To assess the associations between post-GDM status and AUC of GLP-1/PG or AUC of GLP-1/Ins, multivariate logistic regression analysis adjusted for age and BMI was performed. To assess the associations between pathologic glucose metabolism at 5-year follow-up and AUC of GLP-1/PG and AUC of GLP-1/Ins, univariate logistic regression analysis was applied. A two-sided p value <0.05 was considered significant. Statistical analysis was performed using R (V.4.2.1) and RStudio (V.2022.02.3+492) software.
Results
Baseline characteristics
A total of 196 participants (129 women with a history of GDM and 67 controls) underwent a baseline OGTT after delivery with plasma samples for GLP-1 measurement. The groups were similar regarding age and time to index pregnancy. Post-GDM women had a larger waist circumference, higher BMI, higher fasting plasma glucose, and HbA1c levels than controls. At enrollment, 36.2% of the total study participants were overweight/obese. Baseline characteristics by the status of GDM are presented in table 1. In the first OGTT after delivery, 44 (34.1%) post-GDM women and 4 (6.0%) controls presented a pathologic glucose metabolism. More specifically, 22 (11.2%) women had impaired fasting glycemia (IFG), 20 (10.2%) women had IGT, 6 (3.1%) women had both IFG+IGT, and no one had overt T2DM.
Table 1.
Baseline characteristics of women in the PPSDiab study, at the time of the baseline visit (3–16 months after the index pregnancy), by GDM status stratification (total n=196)
| Group | Post-GDM | Control | P value |
| No. of subjects (%) | 129 | 67 | |
| Clinical characteristics | |||
| Insulin therapy during pregnancy (%) | 72 (36.7) | 0 | |
| Glucose metabolism (%) | |||
| NGT | 85 (65.9) | 63 (94.0) | <0.001 |
| IFG | 20 (15.5) | 2 (3.0) | <0.01 |
| IGT | 18 (14.0) | 2 (3.0) | <0.05 |
| IFG+IGT | 6 (4.7) | 0 | <0.096 |
| T2DM | 0 | 0 | |
| Age (years) (mean±SD) | 35.5 (± 4.0) | 35.4 (± 4.2) | 0.968 |
| BMI (kg/m²) (median (Q1–Q3)) | 23.6 (21.4–27.8) | 22.1 (20.8–25.2) | 0.009 |
| Waist circumference (cm) (median (Q1–Q3)) | 79.0 (74.0–86.0) | 75.0 (71.0–81.5) | 0.002 |
| Systolic blood pressure (mm Hg) (median (Q1–Q3)) | 119.0 (111.0–125.0) | 111.0 (104.0–119.5) | <0.001 |
| Diastolic blood pressure (mm Hg) (median (Q1–Q3)) | 74.0 (69.0–79.0) | 69.0 (64.0–77.0) | <0.001 |
Bold values are statistically significant
BMI, body mass index; GDM, gestational diabetes mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; PPSDiab, Prediction, Prevention, and Subclassification of Type 2 Diabetes; T2DM, type 2 diabetes mellitus.
Association of GLP-1 secretion with glucose and insulin levels in post-GDM women and controls at baseline visit
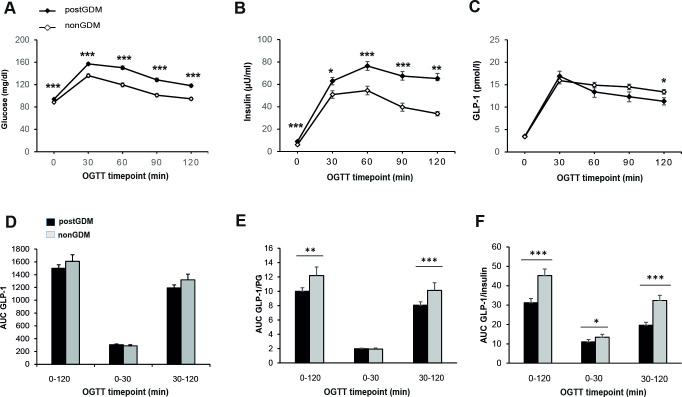
PG levels at all time points during OGTT and AUC glucose (16 257±268.6 mg/dL*minutes vs 13454±276.6 mg/dL*minutes, p<0.001) as well as plasma insulin and AUC insulin were significantly higher in the post-GDM group (figure 1A,B). Plasma values of GLP-1 during the OGTT did not significantly differ between the two groups at 0, 30, 60, and 90 min (figure 1C), while GLP-1 levels at 120 min of OGTT were significantly lower in women with previous GDM (post-GDM: 10.3 (7.8–13.4) pmol/L vs control: 12.0 (9.2–15.6) pmol/L, p<0.05). Calculation of AUCs revealed no differences in the GLP-1 response between the two groups, neither in the first (GLP-1 0–30 min) nor in the second OGTT phase (GLP-1 30–120 min), nor during the entire OGTT (GLP-1 0–120 min) (figure 1D).
Figure 1.
Time course of glucose, insulin, and GLP-1 during 75 g OGTT in post-GDM (n=129) versus non-GDM (n=67) at baseline visit. Concentration of plasma glucose (A), plasma insulin (B), plasma total GLP-1 (C), and area under the curve (AUC) for GLP-1 (D), AUC GLP-1/plasma glucose ratio (E), and AUC GLP-1/insulin ratio (F). Data are shown as mean+SEM during OGTT at baseline visit. *p<0.05, **p<0.01, ***p<0.001, calculated by Mann-Whitney U test. GDM, gestational diabetes mellitus; GLP-1, glucagon-like peptide-1; OGTT, oral glucose tolerance test.
An increase of PG during OGTT provides a stimulus to insulin secretion via glucose and GLP-1, a mechanism that can be impaired in patients with pathologic glucose tolerance or diabetes mellitus.20 To analyze the interaction between higher glucose levels and GLP-1, we calculated GLP-1 levels in relation to PG and insulin. There was a correlation between plasma GLP-1 and glucose and plasma GLP-1 and insulin concentrations at 30 min after a glucose load (online supplemental table 1). We found that the post-GDM women demonstrated significantly lower GLP-1 response in relation to elevated glucose levels during the entire OGTT (0–120 min) and the second OGTT phase (30–120 min), and significantly lower GLP-1 response in relation to elevated insulin levels during the entire OGTT, the first (0–30 min) and the second OGTT phase (30–120 min) in relation to elevated insulin levels in post-GDM women (figure 1E,F).
bmjdrc-2023-003706supp002.pdf (50.6KB, pdf)
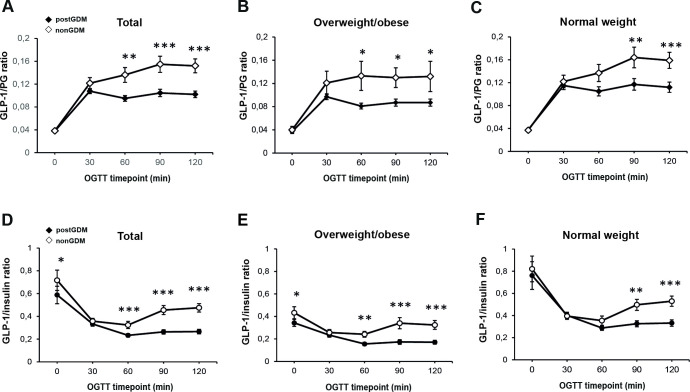
Next, we hypothesized that there may be different pathophysiologic mechanisms in women with overweight/obesity versus post-GDM women with normal weight. After stratifying the cohort by post-GDM status, lower GLP-1/PG and GLP-1/Ins ratios throughout all the OGTT time points were observed in women with overweight/obesity than in women with normal weight, with and without previous GDM (online supplemental figure 1). Importantly, GLP-1/PG ratios were significantly lower in the post-GDM group than in the control group both among women with overweight/obesity and normal weight at the late OGTT phase (90 and 120 min) (figure 2B,C). The comparison between post-GDM and control subjects also revealed that in the second OGTT phase GLP-1/Ins ratios (figure 2E,F) were significantly lower in the post-GDM group, in both weight groups.
Figure 2.
Indices of GLP-1 levels in relation to plasma glucose and insulin during OGTT in women with overweight/obesity and with normal weight for the comparison between post-GDM and non-GDM women. GLP-1 levels in relation to plasma glucose and GLP-1 to insulin levels in all women (A, D), overweight/obese (B, E), and lean (C, F) individuals at baseline visit. Data are shown as mean±SEM during OGTT at baseline visit. *p<0.05, **p<0.01, ***p<0.001, calculated by Mann-Whitney U test. GDM, gestational diabetes mellitus; GLP-1, glucagon-like peptide-1; OGTT, oral glucose tolerance test.
bmjdrc-2023-003706supp001.pdf (91.8KB, pdf)
Multivariate logistic regression analysis, considering GDM status as a dependent variable and AUC GLP-1/PG, AUC GLP-1/Ins, or AUC Ins/PG as independent variables, showed that the total and the second-phase AUC GLP-1/PG as well as AUC GLP-1/Ins were inversely associated with post-GDM status after adjustment for age and BMI (table 2). In contrast, no significant differences in first- and second-phase Ins/PG ratios were observed between investigated groups (table 2).
Table 2.
Association of the total, first- and second-phase area under the curve (AUC) GLP-1/PG, AUC GLP-1/Ins, and Ins/PG ratios with post-GDM status after adjustment for age and BMI
| OR | 95% CI | P value | |
| GLP-1/PG | |||
| AUC total GLP/PG | 0.940 | (0.895 to 0.981) | 0.0078 |
| AUC 0–30 GLP/PG | 0.850 | (0.635 to 1.138) | 0.2702 |
| AUC 30–120 GLP/PG | 0.928 | (0.877 to 0.974) | 0.0052 |
| GLP-1/Ins | |||
| AUC total GLP/insulin | 0.984 | (0.971 to 0.997) | 0.0155 |
| AUC 0–30 GLP/insulin | 0.999 | (0.635 to 1.138) | 0.2702 |
| AUC 30–120 GLP/insulin | 0.972 | (0.953 to 0.989) | 0.0025 |
| Ins/PG | |||
| AUC total insulin/PG | 1.012 | (0.996 to 1.031) | 0.1727 |
| AUC 0–30 insulin/PG | 0.995 | (0.899 to 1.108) | 0.9194 |
| AUC 30–120 insulin/PG | 1.015 | (0.997 to 1.037) | 0.1392 |
OR and 95% CI for post-GDM were calculated based on multivariate logistic regression models adjusted for age and BMI (n=196).
AUC, area under the curve; BMI, body mass index; GDM, gestational diabetes mellitus; GLP-1, glucagon-like peptide-1; Ins, plasma insulin; OGTT, oral glucose tolerance test; PG, plasma glucose.
Association of plasma GLP-1 levels at the first postpartum OGTT with pathologic glucose metabolism at 5-year follow-up
Intraindividual fluctuations of glucose tolerance in the PPSDiab study during 5 years post-GDM have been described recently.21 To analyze whether GLP-1 indices are predictive for development of pre-diabetes/diabetes during follow-up, we examined the incidence of pathologic glucose metabolism at 5-year follow-up among post-GDM women with a normal OGTT at the first visit after pregnancy (n=58). The incidence of pathologic glucose metabolism was 19.0% (11 of 58). More specifically, 3 (5.2%) women had IFG, 5 (8.6%) women had IGT, 2 (3.5%) women had both IFG+IGT, and 1 (1.7%) woman was diagnosed with T2DM at the 5-year OGTT. In univariate logistic regression analyses, AUC GLP-1/PG, AUC GLP-1/Ins, as well as Ins/PG ratios, were not significantly associated with development of abnormal glucose metabolism 5 years after the index pregnancy (table 3).
Table 3.
Association of AUC GLP-1/PG, AUC GLP-1/Ins, or AUC Ins/PG with pathologic glucose metabolism at 5-year follow-up
| OR | 95% CI | P value | |
| AUC total GLP/PG | 0.978 | (0.880 to 1.086) | 0.673 |
| AUC 30–120 GLP/PG | 0.986 | (0.882 to 1.101) | 0.798 |
| AUC 0–30 GLP/PG | 0.532 | (0.229 to 1.240) | 0.144 |
| AUC 30–60 GLP/PG | 0.841 | (0.532 to 1.329) | 0.458 |
| AUC 60–90 GLP/PG | 0.981 | (0.729 to 1.319) | 0.897 |
| AUC 90–120 GLP/PG | 0.996 | (0.767 to 1.294) | 0.977 |
| AUC total GLP/Ins | 0.992 | (0.964 to 1.021) | 0.587 |
| AUC 30–120 GLP/Ins | 0.992 | (0.957 to 1.030) | 0.684 |
| AUC 0–30 GLP/Ins | 0.964 | (0.881 to 1.055) | 0.424 |
| AUC 30–60 GLP/Ins | 0.978 | (0.872 to 1.098) | 0.710 |
| AUC 60–90 GLP/Ins | 0.968 | (0.863 to 1.085) | 0.575 |
| AUC 90–120 GLP/Ins | 0.988 | (0.900 to 1.084) | 0.793 |
| AUC total insulin/PG | 1.013 | (0.984 to 1.043) | 0.379 |
| AUC 30–120 insulin/PG | 1.017 | (0.985 to 1.051) | 0.303 |
| AUC 0–30 insulin/PG | 1.020 | (0.839 to 1.239) | 0.843 |
| AUC 30–60 insulin/PG | 1.030 | (0.926 to 1.146) | 0.588 |
| AUC 60–90 insulin/PG | 1.053 | (0.960 to 1.155) | 0.273 |
| AUC 90–120 insulin/PG | 1.060 | (0.970 to 1.158) | 0.200 |
OR and 95% CI for pathologic glucose metabolism at 5-year follow-up were calculated based on univariate logistic regression models (n=58 post-GDM women with normal glucose metabolism at baseline visit).
AUC, area under the curve; BMI, body mass index; GDM, gestational diabetes mellitus; Ins, plasma insulin; PG, plasma glucose.
Discussion
In the present study, we analyzed the GLP-1 response during a 75 g OGTT in a cohort of women with and without previous gestational diabetes. Circulating plasma GLP-1 levels after glucose challenge were significantly reduced in post-GDM women at the late OGTT phase. The difference was more pronounced when the GLP-1 secretion was analyzed in relation to PG and plasma insulin levels, suggesting an inadequately low second-phase GLP-1 secretory response in the post-GDM cohort, which may contribute to the altered control of hyperglycemia at an early pre-diabetic phase.
Our data are in agreement with previous studies reporting on reduced GLP-1 levels and incretin resistance in subjects with IGT and/or impaired fasting glucose.5 6 22 However, other studies reported no difference in GLP-1 secretion in pre-diabetes and type 2 diabetes.23 Only a few studies have investigated GLP-1 secretory response in women after GDM and reported contrasting results. In line with our findings, decreased GLP-1 secretion during OGTT and lower second-phase GLP-1/glucose ratios were observed in post-GDM normoglycemic women9 and in post-GDM women who had developed pre-diabetes.15 In a smaller study, a preserved GLP-1 response to oral glucose was described in normoglycemic women after GDM.13 These discrepancies may be explained by differences in the phenotype of study participants, for example, the degree of obesity and insulin resistance, the average time after the index pregnancy (2–3 months to several years), and the assays used to measure total or intact GLP-1 plasma concentration.
The decreased GLP-1/PG ratio in the post-GDM group may be partly explained by a weak stimulation of GLP-1 secretion suggesting a defect in the incretin hormone secretion pathway or an insensitivity of beta cells to GLP-1. Potential GLP-1 resistance of beta cells is unlikely because we observed lower GLP-1/Ins ratios indicating relatively high insulin concentrations despite low GLP-1 levels during OGTT in the post-GDM group.
It is known that fasting and stimulated GLP-1 plasma concentrations are dependent on age and BMI.6 23 We confirmed these findings by the detection of lower GLP-1 plasma levels in women with overweight/obesity with and without a history of GDM. The association of the late GLP-1 response with GDM status remained significant after adjustment for age and BMI. Of note, a substantial proportion of our post-GDM cohort had a normal weight (58.9%), including women without the typical obesity-related phenotype associated with development of type 2 diabetes. We observed alteration in GLP-1 secretion not only in women with overweight/obesity but also in post-GDM women with normal weight, suggesting a disturbance of GLP-1 signaling independent of the metabolic syndrome. Further studies are required to determine the underlying pathophysiologic changes of the altered GLP-1 release in women with previous GDM.
Next, we investigated whether the reduced GLP-1 response during the first postpartum OGTT can predict the development of pathologic glucose metabolism during follow-up. Post-GDM women have about 10-fold increased risk for type 2 diabetes as compared with women after a normoglycemic pregnancy (18.9% vs 2.0% within 9 years).24 In line with these findings, we here report at the baseline visit on disturbed glucose tolerance in 31.1% of subjects after GDM and 6% of controls after a normoglycemic pregnancy. At 5-year follow-up, 19% converted from normoglycemia to disturbed glucose tolerance. This subgroup with incident disturbed glucose tolerance was selected to analyze the association of variables of GLP-1 secretion with progression to abnormal glucose metabolism. In our follow-up analysis using the univariate logistic regression model, we did not observe a significant association between any parameter of GLP-1 secretory response or Ins/PG ratio with development of pathologic glucose metabolism. Thus, GLP-1 might not be a predictor for a rapid development of pre-diabetes or type 2 diabetes after GDM. Therefore, the altered GLP-1 response might not be an indicator but a consequence of a disturbed glucose metabolism.
The strengths of our study include the uniformly recruited cohort consisting of young women shortly after index pregnancy who have very few concomitant diseases or confounding medication. This is the first prospective study on the predictive value of GLP-1 secretory response to oral glucose and the development of abnormal glucose metabolism during follow-up after a previous GDM. The main limitations of our study are the limited number of participants and the relatively short follow-up period of 5 years. Further clinical studies are necessary to clarify the relationships between the associations reported here and the future development of pathologic glucose metabolism including type 2 diabetes.
Conclusions
Women after GDM, who are at increased risk for development of type 2 diabetes, showed a significantly decreased second-phase GLP-1 secretion in relation to PG and plasma insulin. Alterations in GLP-1 release may contribute to glucose dysregulation in a very early pre-diabetic state. However, our longitudinal data suggest that the GLP-1 response to glucose does not seem to play a role as independent predictor for the development of pathologic glucose metabolism within 5 years.
Acknowledgments
The authors thank all participants in the PPSDiab study who made the study possible. The authors especially thank Javier Mauricio Villamizar Cujar and Mariia Hartzendorf for their excellent technical assistance.
Footnotes
Contributors: Conceptualization: UF, AL, and JS. Investigation and data analysis: EP, KB, SH, HH, CT, UF, AL, and JS. Writing—review and editing: all. Supervision: UF and JS. Funding acquisition: AL and JS. JS is the guarantor of this work. This work was not published previously.
Funding: This study was funded by LMU Klinikum and the German Center for Diabetes Research, Helmholtz Zentrum München. The funders had no involvement in the study design, the collection, analysis and interpretation of the data and the decision to submit the paper for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data from the PPSDiab study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The protocol was approved by the ethical review committee of the Ludwig-Maximilian-University (study ID 300-11), and the study was conducted in accordance with the declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1.Powe CE, Huston Presley LP, Locascio JJ, et al. Augmented insulin secretory response in early pregnancy. Diabetologia 2019;62:1445–52. 10.1007/s00125-019-4881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minooee S, Ramezani Tehrani F, Rahmati M, et al. Diabetes incidence and influencing factors in women with and without gestational diabetes mellitus: a 15 year population-based follow-up cohort study. Diabetes Res Clin Pract 2017;128:24–31. 10.1016/j.diabres.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 3.Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361. 10.1136/bmj.m1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauck M, Stöckmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986;29:46–52. 10.1007/BF02427280 [DOI] [PubMed] [Google Scholar]
- 5.Laakso M, Zilinskaite J, Hansen T, et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008;51:502–11. 10.1007/s00125-007-0899-2 [DOI] [PubMed] [Google Scholar]
- 6.Færch K, Torekov SS, Vistisen D, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes 2015;64:2513–25. 10.2337/db14-1751 [DOI] [PubMed] [Google Scholar]
- 7.Cypryk K, Vilsbøll T, Nadel I, et al. Normal secretion of the Incretin hormones glucose-dependent Insulinotropic polypeptide and glucagon-like peptide-1 during gestational diabetes mellitus. Gynecol Endocrinol 2007;23:58–62. 10.1080/09513590601137004 [DOI] [PubMed] [Google Scholar]
- 8.Krystynik O, Karasek D, Kahle M, et al. Non-altered incretin secretion in women with impaired fasting plasma glucose in the early stage of pregnancy: a case control study. Diabetol Metab Syndr 2023;15:12. 10.1186/s13098-023-00981-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lencioni C, Resi V, Romero F, et al. Glucagon-like peptide-1 secretion in women with gestational diabetes mellitus during and after pregnancy. J Endocrinol Invest 2011;34:e287–90. 10.3275/7799 [DOI] [PubMed] [Google Scholar]
- 10.Sukumar N, Bagias C, Goljan I, et al. Reduced GLP-1 secretion at 30 minutes after a 75-G oral glucose load is observed in gestational diabetes mellitus: a prospective cohort study. Diabetes 2018;67:2650–6. 10.2337/db18-0254 [DOI] [PubMed] [Google Scholar]
- 11.Fritsche L, Hummel J, Wagner R, et al. The German gestational diabetes study (PREG), a prospective Multicentre cohort study: rationale, methodology and design. BMJ Open 2022;12:e058268. 10.1136/bmjopen-2021-058268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forbes S, Moonan M, Robinson S, et al. Impaired circulating glucagon-like peptide-1 response to oral glucose in women with previous gestational diabetes. Clin Endocrinol (Oxf) 2005;62:51–5. 10.1111/j.1365-2265.2004.02172.x [DOI] [PubMed] [Google Scholar]
- 13.Yu SH, Cho B, Lee Y, et al. Insulin secretion and incretin hormone concentration in women with previous gestational diabetes mellitus. Diabetes Metab J 2011;35:58–64. 10.4093/dmj.2011.35.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier JJ, Gallwitz B, Askenas M, et al. Secretion of incretin hormones and the Insulinotropic effect of gastric inhibitory polypeptide in women with a history of gestational diabetes. Diabetologia 2005;48:1872–81. 10.1007/s00125-005-1863-7 [DOI] [PubMed] [Google Scholar]
- 15.Foghsgaard S, Vedtofte L, Andreasen C, et al. Women with prior gestational diabetes mellitus and prediabetes are characterised by a decreased incretin effect. Diabetologia 2017;60:1344–53. 10.1007/s00125-017-4265-8 [DOI] [PubMed] [Google Scholar]
- 16.Rottenkolber M, Ferrari U, Holland L, et al. The diabetes risk phenotype of young women with recent gestational diabetes. J Clin Endocrinol Metab 2015;100:E910–8. 10.1210/jc.2014-3898 [DOI] [PubMed] [Google Scholar]
- 17.Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ElSayed NA, Aleppo G, Aroda VR, et al. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care 2023;46:S19–40. 10.2337/dc23-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holst JJ, Albrechtsen NJW, Rosenkilde MM, et al. Physiology of the Incretin hormones, GIP and GLP ‐1—regulation of release and posttranslational modifications. Compr Physiol 2019:1339–81. 10.1002/cphy.c180013 [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Tang X, Cao H, et al. Impaired secretion of total glucagon-like peptide-1 in people with impaired fasting glucose combined impaired glucose tolerance. Int J Med Sci 2012;9:574–81. 10.7150/ijms.4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haschka SJ, Gar C, Sacco V, et al. Pre-diabetes, diabetes and fluctuations of glucose tolerance after gestational diabetes mellitus: 5-year follow-up of a contemporary, prospective study in Germany. BMJ Open Diabetes Res Care 2022;10:e002621. 10.1136/bmjdrc-2021-002621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008;57:1340–8. 10.2337/db07-1315 [DOI] [PubMed] [Google Scholar]
- 23.Nauck MA, Müller TD. Incretin hormones and type 2 diabetes. Diabetologia 2023;66:1780–95. 10.1007/s00125-023-05956-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feig DS, Zinman B, Wang X, et al. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ 2008;179:229–34. 10.1503/cmaj.080012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2023-003706supp002.pdf (50.6KB, pdf)
bmjdrc-2023-003706supp001.pdf (91.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data from the PPSDiab study are available from the corresponding author upon reasonable request.