Abstract
The unique germfree, colostrum-deprived, immunologically “virgin” piglet model was used to evaluate the ability of lactoferrin (LF) to protect against lethal shock induced by intravenously administered endotoxin. Piglets were fed LF or bovine serum albumin (BSA) prior to challenge with intravenous Escherichia coli lipopolysaccharide (LPS), and temperature, clinical symptoms, and mortality were tracked for 48 h following LPS administration. Prefeeding with LF resulted in a significant decrease in piglet mortality compared to feeding with BSA (16.7 versus 73.7% mortality, P < 0.001). Protection against the LPS challenge by LF was also correlated with both resistance to induction of hypothermia by endotoxin and an overall increase in wellness, as quantified by a toxicity score developed for these studies. In vitro studies using a flow cytometric assay system demonstrated that LPS binding to porcine monocytes was inhibited by LF in a dose-dependent fashion, suggesting that the mechanism of LF action in vivo may be inhibition of LPS binding to monocytes/macrophages and, in turn, prevention of induction of monocyte/macrophage-derived inflammatory-toxic cytokines.
Despite the development of potent, broad-spectrum antibiotics, septic shock remains both the most frequent cause of death of intensive care patients and the 13th leading cause of death overall in the United States (20). Death from septic shock is thought to be a consequence of the effect of monocyte-derived cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), and IL-6, which are induced in response to bacterial endotoxin (lipopolysaccharide [LPS]). A number of approaches that block the action of endotoxin on target cells have been evaluated as potential therapies for septic shock, including anti-LPS monoclonal antibodies (MAbs) and anti-TNF-α antibody (4, 31). The results of these evaluations have been mixed at best. Recently, a number of reports have suggested that the naturally occurring protein lactoferrin (LF) may be a therapeutic candidate for septic shock (1, 14).
LF is a 77-kDa iron binding glycoprotein found in high levels in various secretions, e.g., milk and pancreatic juice (25), and is resistant to acids and several proteases (19). Because LF is very stable, it can be used in in vivo experiments to investigate its protective effects against lethal shock due to endotoxin. In vitro studies have demonstrated both bacteriostatic (6, 18, 28) and bactericidal (2, 3, 8, 9) activities of LF for gram-negative organisms via two possible mechanisms. One is to chelate iron ions, which are essential for bacterial growth (5, 6), and the other is to destabilize the outer membrane of gram-negative organisms (8). Recently, it was reported that LF exhibits binding activity for the lipid A portion of LPS, which may serve to inhibit monocyte activation and cytokine production by interfering with the access of endotoxin to its cell surface receptor (1).
In the current study, we have tested the ability of oral LF to modulate lethal endotoxin shock in vivo. As a model system, we have employed the unique, germfree (GF), colostrum-deprived, immunologically “virgin” Minnesota miniature piglet (13) and oral feeding of LF that will allow introduction of intact LF into the blood circulation via gastrointestinal absorption (16). The use of this unique model system allows the study of the response to true primary toxicity of endotoxin (the lipid A portion of LPS) in the absence of any secondary toxicity due to the acquisition of hypersensitivity by the host to some portion of the LPS preparation, namely, O and R polysaccharides and contaminating peptides. The primary and secondary toxicities are interdependent, depending on the immunological state of the host. Therefore, the biological activity of the endotoxin will vary not only because of the heterogenicity of the preparation but also because of its dependency on the immunological reactions of the host to the various portions of the endotoxin (10–12, 27). (i) In immunologically virgin animals, which have neither hypersensitivity nor acquired immunity, biological activity is due entirely to the primary toxicity of the lipid A portion of the endotoxin. (ii) So-called normal animals may have been exposed to the environment (microbes, endotoxins) and will develop hypersensitivity, as well as acquired immunity, to various degrees and will have various degrees of susceptibility. Here, both the primary and secondary toxicities interdependently enhance their activities, and interaction due to various low degrees of acquired immunity (the anti-lipid A portion of endotoxin) is also possible. (iii) Sensitized animals have a high degree of hypersensitivity and a low degree of immunity. Here, primary toxicity and secondary toxicity will be greatly enhanced interdependently, and the animals become extremely susceptible to the endotoxin. (iv) In immunized animals (so-called tolerant animals), in which the degree of hypersensitivity is not important because the primary toxicity is blocked by antiendotoxin antibody, secondary toxicity is not enhanced and acts to desensitize the animals. We demonstrated that so-called tolerance is, in fact, specific immunity to the endotoxin (the lipid A portion) due to 19S antibodies which are capable of assisting phagocytes to detoxify the primary toxicity. Antibodies to O and R polysaccharides play a little role in antiendotoxin immunity and may actually contribute to secondary toxicity. Thus, we will be able to examine the protective effects of LF against the primary toxicity of endotoxin (LPS)-induced lethal shock.
MATERIALS AND METHODS
Animals.
GF, colostrum-deprived, immunologically virgin, neonatal Minnesota miniature piglets were aseptically obtained by hysterectomy 0 to 5 days prior to term (the expected date of confinement [EDC]). The body weights of newborn GF piglets ranged from 450 to 650 g. Randomly selected littermates were maintained in GF isolators and fed a milk-free soy protein formula, Nursoy (Wyeth Laboratories, Inc., Philadelphia, Pa.). Young adult, specific-pathogen-free Minnesota miniature swine were maintained in a barrier-sustained facility with filtered air (HEPA filter) and fed with an autoclaved diet and chlorinated water.
All GF piglet littermates were divided into three groups. Group A, the LF-LPS group, was fed with 2,000 mg of sterile LF (Tatua Biologics, Morrinsville, New Zealand) in 10 ml of phosphate-buffered saline (PBS) by gastric tube at 0, 8, and 20 h after birth and fed with 20 mg of sterile LF per ml of Nursoy (diluted 1:2 with distilled water) every 4 h after birth. Group B, the bovine serum albumin (BSA)-LPS group, was fed with 2,000 mg of sterile BSA (A2153, fraction V, 96% pure; Sigma Chemical Co., St. Louis, Mo.) in 10 ml of PBS by gastric tube at 0, 8, and 20 h after birth and fed with 20 mg of sterile BSA per ml of Nursoy (diluted 1:2 with distilled water) every 4 h after birth. Group C, the control group, was fed with only 10 ml of sterile PBS (pH 7.2) by gastric tube at 0, 8, and 20 h after birth and maintained with sterile Nursoy (diluted 1:2 with distilled water) given every 4 h after birth. Both groups A and B were injected with 750 or 850 μg of Escherichia coli O55:B5 LPS (L-2637, lot 123H4024; Sigma Chemical Co.) per kg of body weight into the jugular vein at 23 h after birth (3 h after the last tube feeding of LF or BSA), while group C was injected with only 1 ml of PBS. Rectal temperatures were measured, and clinical symptoms (degree of weakness, degree of food intake, and death) were observed at 0, 1, 2, 3, 6, 9, 12, 20, 24, 28, 32, 36, 44, and 48 h after LPS injection.
Bovine LF.
Bovine LF was purchased from Tatua Biologics (batch TB194048) and was >95% pure, as judged by polyacrylamide gel electrophoresis. Fluorescein isothiocyanate (FITC)-labelled E. coli O55:B5 LPS (catalog no. F7632) was purchased from Sigma Chemical Co. Anti-CD14 MAb My23.5 was kindly supplied by Michael Fanger, Dartmouth Medical School, and goat anti-mouse immunoglobulin G-FITC was purchased from Southern Biotech (Birmingham, Ala.). RPMI 1640 with HEPES was the product of BioWhittaker, Walkersville, Md.
PBM isolation.
Porcine peripheral blood mononuclear cells (PBMs) were isolated from whole blood of young adult, specific-pathogen-free swine by density gradient centrifugation on Histopaque (Sigma Chemical Co.). Whole blood was diluted 1:3 with Dulbecco’s PBS (Ca2+ and Mg2+ free) and layered on 10 ml of Histopaque in a 50-ml conical tube (maximum of 25 ml of diluted blood/tube). Gradients were centrifuged at 400 × g and room temperature for 35 min, and the PBM layer was harvested by aspiration with a Pasteur pipette. The pooled PBMs were washed twice with 12 ml of cold RPMI 1640 and resuspended in 10 to 12 ml of cold RPMI 1640. Cell yield was determined by hemocytometer counting, using trypan blue to determine the viability of the cells.
Flow cytometric analysis of LPS binding to PBMs.
A flow cytometric assay was developed to assess the effect of LF on the binding of LPS to porcine PBMs. PBMs isolated as described above were resuspended at 5 × 106/ml in cold RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated (56°C, 30 min) autologous serum. A 200-μl volume of the cell suspension was mixed with 100 μl of LF in RPMI 1640 at the indicated concentration or with RPMI 1640 alone and then preincubated on ice for 15 min. Following this incubation, 10 μl of 200-μg/ml LPS-FITC (E. coli O55:B5; Sigma) was added (6.45-μg/ml final concentration) and the mixture was incubated for an additional 60 min at 4°C. The incubation was terminated by addition of 1 ml of cold assay buffer (PBS–2% fetal bovine serum–0.1% azide), followed by centrifugation. The cell supernatant was discarded, and the cells were washed twice with 1 ml of cold assay buffer and then resuspended in 500 μl of cold assay buffer. Cells were kept on ice until flow cytometer analysis. In studies on the role of monocyte CD14 antigen in LPS binding in this model system, anti-CD14 MAb My23.5 was substituted for the LF in the above-described protocol at a final concentration of 2 μg/ml, and the cells were preincubated with the MAb for 30 min on ice prior to the addition of LPS-FITC.
Flow cytometric analysis of LPS-FITC binding was done on a Becton Dickinson FACScan by using logarithmic amplification of the FITC fluorescence signal, and the data were acquired on a total of 10,000 PBMs/sample. For routine analysis of LPS-FITC binding to monocytes, dot plots of forward light scatter versus 90° light scatter were used to differentiate lymphocytes and monocytes, based on the elevated 90° light scatter of the latter. Flow cytometric analysis of cells stained with the anti-CD14 MAb, followed by anti-mouse immunoglobulin G-FITC, demonstrated that, typically, >80% of the cells falling within the monocyte light scatter gate reacted with this monocyte-specific MAb (data not shown). For analysis, a cutoff was set on the negative control population, such that less than 1% of the total monocytes were scored as positive for LPS-FITC binding, and the percentage of cells binding LPS-FITC was determined for the experimental samples. The percent inhibition of LPS-FITC binding to monocytes was calculated by using the following formula: % inhibition of LPS-FITC binding = [1 − (% positive monocytes with inhibitor − % positive monocytes in negative control)/(% positive monocytes without inhibitor − % positive monocytes in negative control)] × 100.
RESULTS
Effect of LF on lethal endotoxin shock.
All GF piglets were divided into three groups. Those in group A (LF-LPS) were fed with LF to observe the protective effect against a lethal endotoxin shock. For comparison, those in group B (BSA-LPS) were fed with BSA, which is known to have no effect on endotoxin lethality (data not shown). Those in group C (control) were fed with PBS alone as an additional control. All GF piglets were fed by gastric tube to administer measured amounts of LF, BSA, and PBS. Each group was randomly assigned from each sex after division of littermates into males and females. The dosages (LD75) of LPS injected intravenously (i.v.) were 750 μg/kg for GF piglets obtained 3 to 5 days prior to EDC and those with less than 500 g of body weight obtained 0 to 2 days prior to EDC and 850 μg/kg for GF piglets with over 500 g of body weight obtained 0 to 2 days prior to EDC.
The results in Table 1 demonstrate that there was a marked difference (16.7 versus 73.7%) in the mortality induced by endotoxin between the LF-LPS and BSA-LPS groups (P < 0.001). In both the LF-LPS and BSA-LPS groups, more than 30% of the deaths (1 of 3 in the LF-LPS group, 5 of 14 in the BSA-LPS group) took place within 12 h, more than 60% of the deaths (2 of 3 in the LF-LPS group, 9 of 14 in the BSA-LPS group) took place within 24 h, and more than 90% of the deaths (3 of 3 in the LF-LPS group, 13 of 14 in the BSA-LPS group) took place within 36 h.
TABLE 1.
Mortality rates of control, LF-LPS-treated, and BSA-LPS-treated piglets
| Group | No. of piglets | No. dead/total (% dead)a
|
|||
|---|---|---|---|---|---|
| 12 hb | 24 h | 36 h | 48 h | ||
| A (LF-LPS) | 18 | 1/18 (5.6) | 2/18 (11.1) | 3/18 (16.7) | 3/18 (16.7) |
| B (BSA-LPS) | 19 | 5/19 (26.3) | 9/19 (47.4) | 13/19 (68.4) | 14/19 (73.7) |
| C (control) | 8 | 0/8 (0.0) | 0/8 (0.0) | 0/8 (0.0) | 0/8 (0.0) |
The log rank and Wilcoxon tests were employed to compare the survival distribution of groups A and B. Both methods yield highly significantly different results (P < 0.001).
Time after LPS administration.
Changes in rectal temperature and toxicity score.
Rectal temperatures were measured by an electronic digital thermometer. For objective evaluation of the clinical symptoms, a toxicity scoring system was developed (Table 2). In this scoring system, a high score indicates strong endotoxin toxicity.
TABLE 2.
Toxicity scoring systema
| Score | Degree of:
|
||
|---|---|---|---|
| Hypothermia (°F)b | Weakness | Food intake | |
| 0 | >98 | Strongly active | Trough empty |
| 1 | 97–98 | Slightly weak | Trough 2/3 empty |
| 2 | 96–97 | Weak | Trough 1/2 empty |
| 3 | 95–96 | Not standing | Trough 1/3 empty |
| 4 | 94–95 | Lethargic | Trough full |
| 5 | <94 | Dying | |
A toxicity score of 15 corresponded to death.
Rectal temperatures are shown.
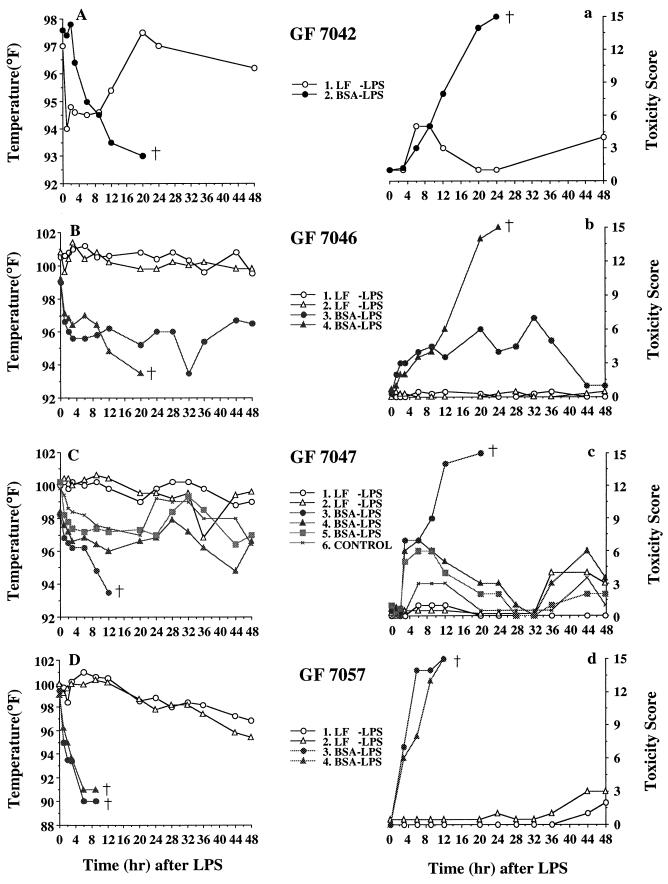
The GF7042 littermates consisted of only two piglets, one treated with LF-LPS and the other treated with BSA-LPS. The LF-LPS piglet showed initial hypothermia followed by recovery, and the BSA-LPS piglet showed no such recovery and died after 24 h (Fig. 1A). The BSA-LPS piglet showed increasing toxicity until the piglets died 24 h after injection, but the LF-LPS piglet maintained a lower toxicity score (Fig. 1a).
FIG. 1.
Effect of LF on the rectal temperatures (A, B, C, D) and toxicity scores (a, b, c, d) of GF piglets injected i.v. with LPS. LF or BSA (2,000 mg) was fed by gastric tube every 8 h for 1 day, followed by i.v. injection of LPS at 750 μg/kg, and GF piglets were maintained on 20 mg of LF or BSA per ml of Nursoy diet, and then rectal temperatures and toxicity scores were measured at 0, 1, 2, 3, 6, 9, 12, 20, 24, 28, 32, 36, 44, and 48 h after injection. †, death.
The GF7046 littermates consisted of four piglets. Two were treated with LF-LPS, and the other two were treated with BSA-LPS. The LF-LPS group was able to maintain normal body temperatures, while the BSA-LPS group immediately became hypothermic and one piglet died within 24 h after injection (Fig. 1B). The LF-LPS group showed consistently low toxicity scores, while the BSA-LPS group had increased toxicity scores (Fig. 1b).
The GF7047 littermates, consisting of six piglets, showed patterns of change in rectal temperature and toxicity score very similar to those of GF7046 littermates. In this set of littermates, one animal was included as a control and given 10 ml of sterile PBS by gastric tube and no LPS challenge. There were no differences in rectal temperature and toxicity score between the control group and the LF-LPS group (Fig. 1C and c).
Four GF7057 littermates presented very simple and clear results. Both piglets in the BSA-LPS group showed severe hypothermia with body temperatures dropping to 90°F, resulting in death between 9 and 12 h after LPS administration, whereas both piglets in the LF-LPS group showed mild hyperthermia rather than hypothermia within 12 h after LPS injection. The latter piglets appeared to be unaffected by the injected endotoxin and showed very low toxicity scores, while the former piglets died (toxicity score higher than 12) a short time after LPS injection (Fig. 1D and d).
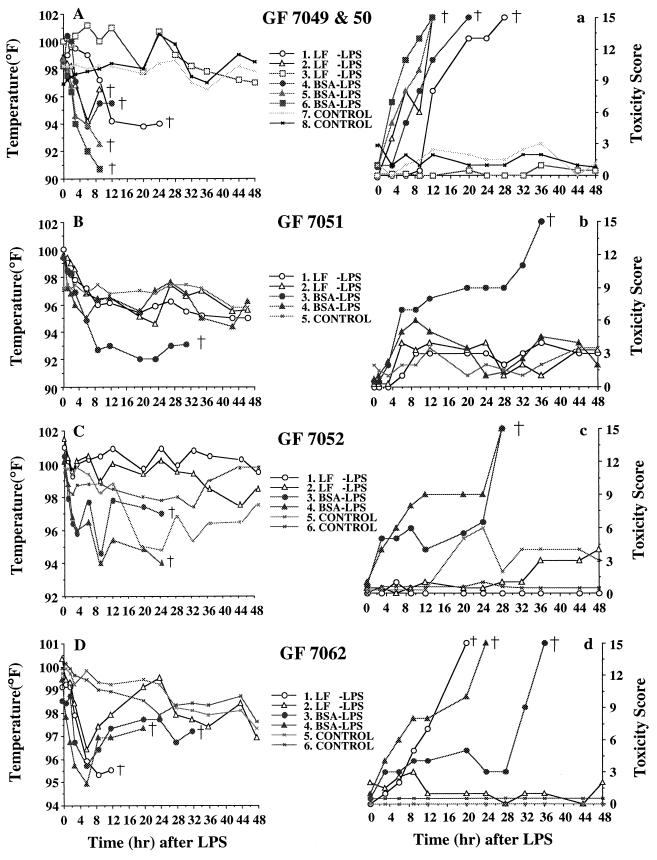
Two GF7049 and two GF7050 littermates from two hysterectomies performed at the same time were randomly divided into three groups (three in the LF-LPS group, three in the BSA-LPS group, and two in the control group). The two former groups were injected with 850 μg of LPS per kg of body weight according to the criteria of dosage determination (body weights were greater than 500 g). The temperatures of all of the piglets in the BSA-LPS group and 2 in the LF-LPS group acutely dropped to or below 94°F. All of these piglets subsequently died. The remaining piglet in the LF-LPS group was healthy and strong and showed no hypothermia, despite LPS injection. The changing toxicity score pattern of these littermates was similar to the body temperature pattern (Fig. 2A and a).
FIG. 2.
Effect of LF on rectal temperatures (A, B, C, D) and toxicity scores (a, b, c, d) of GF piglets injected i.v. with LPS. LF or BSA (2,000 mg) was fed by gastric tube every 8 h for 1 day, followed by i.v. injection of 750 or 850 μg of LPS per kg, and GF piglets were maintained on 20 mg of LF or BSA per ml of Nursoy diet, and then rectal temperatures and toxicity scores were measured at 0, 1, 2, 3, 6, 9, 12, 20, 24, 28, 32, 36, 44, and 48 h after injection. †, death.
The GF7051 littermates were composed of five piglets (two in the LF-LPS group, two in the BSA-LPS group, and one in the control group). All piglets in the BSA-LPS group, except one, which showed severe hypothermia and died within 36 h after injection, showed mild hypothermia down to 96°F and kept their temperatures at that level (Fig. 2B and b).
Six GF7052 littermates were divided randomly into three groups of two piglets. While all of the piglets in the LF-LPS group and one in the control group had relatively constant body temperatures, all in the BSA-LPS group (two piglets) showed a rapid drop in body temperature for the initial 3 h after LPS injection and thereafter showed temperature fluctuations for more than 20 h, eventually dying. Although all in the BSA-LPS group showed relatively low toxicity scores (less than 10), they all died around 24 h after injection (Fig. 2C and c).
The GF7062 littermates were six piglets (two in the LF-LPS group, two in the BSA-LPS group, and two in the control group). All piglets in the LF-LPS and BSA-LPS groups showed an initial acute drop in body temperature, while all in the control group were in the normal range. One in the LF-LPS group recovered from hypothermia, while another died around 12 h after injection. Two in the BSA-LPS group also recovered from hypothermia to a lesser extent than one piglet of the LF-LPS group, but they were unable to drink Nursoy by themselves and had a gradual increase in toxicity score to 10. Eventually, both of them died at 23 and 33 h after LPS injection (Fig. 2D and d).
Characterization of LPS-FITC binding to porcine monocytes.
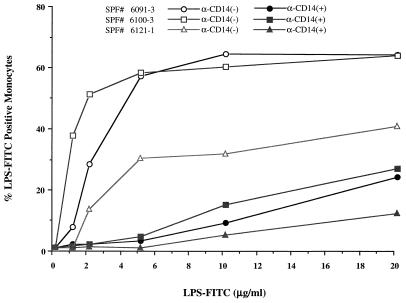
A flow cytometric assay was developed to monitor LPS binding to porcine PBMs. Density gradient-isolated cells were incubated with FITC-labelled E. coli LPS in the presence of autologous serum, and binding to PBMs was characterized by flow cytometry. Only LPS-FITC binding to monocytes was observed, as assessed by light scatter, and no significant binding to cells with light scatter characteristic of lymphocytes was noted (data not shown). Dose-response curves of LPS-FITC concentration versus percent positive cells demonstrated that 40 to 60% of the cells with light scatter characteristics of monocytes were positive for LPS-FITC binding (Fig. 3). To verify that the cells binding LPS-FITC were, in fact, monocytes, cells were preincubated with a MAb to porcine CD14, a monocyte-specific antigen which has been shown to function as a receptor for LPS in humans (29). As the results in Fig. 3 demonstrate, the anti-porcine CD14 MAb was effective in blocking LPS-FITC binding to PBMs, suggesting that all LPS binding occurs on monocytes.
FIG. 3.
Effect of anti-CD14 MAb on LPS binding to porcine monocytes. Percent binding of LPS to porcine monocytes without the anti-CD14 MAb (open symbols) and with the anti-CD14 MAb (2 μg) (closed symbols) is shown. SPF, specific pathogen free.
Effect of LF on LPS binding to porcine monocytes.
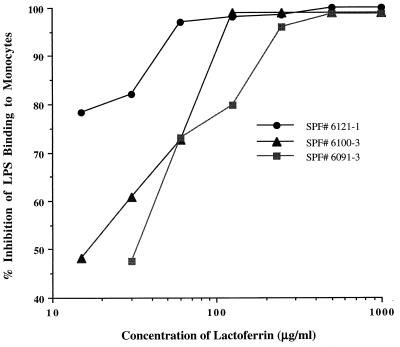
The flow cytometric assay was used to examine the effect of the preincubation of monocytes with LF on LPS-FITC binding. Isolated PBMs were incubated at 4°C for 15 min with various concentrations of LF, and then a fixed concentration of LPS-FITC was added. Cells were analyzed by flow cytometry, and the percent inhibition of LPS-FITC binding to monocytes was determined as described in Materials and Methods. As the results in Fig. 4 demonstrate, preincubation of cells with LF resulted in dose-dependent inhibition of LPS-FITC binding to porcine monocytes. Fifty percent inhibition of LPS binding under these conditions required an approximately 1:1 weight ratio of LF to LPS.
FIG. 4.
Percent inhibition of LPS binding to porcine monocytes by LF. Porcine PBMs (5 × 106/ml) were incubated with various concentrations of LF, and then LPS-FITC binding was analyzed by flow cytometer, and percent inhibition was calculated as described in Materials and Methods. SPF, specific pathogen free.
DISCUSSION
In the present study, the effect of oral LF administration on the response to lethal shock induced by intravenous LPS administration was studied in a GF, colostrum-deprived, immunologically virgin piglet model. Prefeeding of LF was clearly associated with a significant decrease in mortality, specifically, 17% for LF versus 74% for control animals fed BSA (Table 1; P < 0.001). These results represent the first report that oral administration of LF can significantly modify septic shock. In an earlier study using a murine model system, Zagulski et al. (30) reported significant protection from endotoxin shock by i.v. preadministration of LF. In the current model, oral administration of LF is likely associated with absorption of LF via the gastrointestinal tract and systemic dissemination, as GF piglets have been demonstrated to be capable of absorption of macromolecules for the first 3 days after birth (16).
During the course of these studies, all animals were monitored for additional clinical correlates of endotoxin shock, including temperature, food consumption, and activity level (Table 2). In BSA-fed control animals, administration of LPS was followed immediately by the rapid appearance of hypothermia in 100% of the animals and 74% of the animals exhibiting hypothermia subsequently died. In contrast, only 38% of the LF-fed animals exhibited hypothermia and 13% subsequently died (Fig. 1 and 2). The ability of LF to interfere with the induction of hypothermia suggests that its locus of action is one or more of the initial events leading to lethal shock. Endotoxin-induced shock is thought to be at least in part a consequence of LPS activation of monocytes/macrophages, followed by production of cytokines, including TNF-α, IL-1, and IL-6 (23, 24). It has been demonstrated that LF firmly binds to LPS (1, 7) and/or inactivates LPS (26), which inhibits the endotoxin-induced TNF-α and IL-6 responses (14, 15). IL-6 is a major cause of hypothermia (21), but TNF-α is not (21, 22). TNF-α is one of the principal mediators of the lethal effect of endotoxin (4).
A flow cytometric assay system developed to characterize LPS interactions with porcine PBMs clearly demonstrated preferential binding of LPS to CD14-positive PBMs (Fig. 3), as is the case in humans (29). Moreover, LF was able to inhibit LPS binding to porcine PBMs in a dose-dependent fashion (Fig. 4), suggesting that the ability to block in vivo endotoxin shock may be a consequence of inhibition of LPS binding to monocytes. Although monocytes have been reported to possess LF receptors (17), preliminary studies using PBMs have shown that preincubation of PBMs with LF is ineffective in blocking LPS binding if the cells are washed before addition of LPS-FITC. When LF and LPS-FITC were mixed in vitro and added to PBMs immediately or after preincubation for up to 60 min, the degrees of inhibition of LPS binding to PBMs by LF were not significantly different (8a). These observations and the fact that pretreatment of PBMs with an anti-CD14 MAb blocked the binding of LPS support the hypothesis that direct LF interaction with LPS may prevent endotoxin from binding to the cell surface CD14 receptor and other receptors of monocytes/macrophages and that this is followed by reduced TNF-α production, as well as reduced IL-1 and IL-6 production, resulting in a lower mortality rate in GF piglets challenged with parenterally administered endotoxin.
ACKNOWLEDGMENTS
This work was supported in part by Ross Laboratories.
We acknowledge the assistance of Tony del Rosario, DVM; Larry Shannon; and Remus Burchette in procuring animals for this study and Lisa Roberts for technical assistance with in vitro studies.
REFERENCES
- 1.Appelmelk B J, An Y-Q, Geerts M, Thijs B G, de Boer H A, MacLaren D M, de Graaff J, Nuijens J H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold R R, Brewer M, Gauthier J J. Bacterial activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun. 1980;28:893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R R, Russell J E, Champion W J, Gauthier J J. Bactericidal activity of human lactoferrin: influences of physical conditions and metabolic state of the target microorganisms. Infect Immun. 1981;32:655–660. doi: 10.1128/iai.32.2.655-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 5.Britigan B E, Serody J S, Cohen M S. The role of lactoferrin as an anti-inflammatory molecule. Adv Exp Med Biol. 1994;357:143–156. doi: 10.1007/978-1-4615-2548-6_14. [DOI] [PubMed] [Google Scholar]
- 6.Bullen J J, Rogers H J, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 7.Elass-Rochard E, Roseanu A, Logrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison R T, III, Giehl T J. Killing of gram-negative bacteria by lactoferrin and lysozymes. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Farmer, J. L., and L. Roberts. Unpublished data.
- 9.Gutteberg T J, Rokke O, Anderson O, Joergensen T. Early fall of circulating iron and rapid rise of lactoferrin in septicemia and endotoxemia: an early defence mechanism. Scand J Infect Dis. 1989;21:708–715. doi: 10.3109/00365548909021701. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y B, Watson D W. Modification of host responses to bacterial endotoxins. II. Passive transfer of immunity to bacterial endotoxin with fractions containing 19S antibodies. J Exp Med. 1965;121:751–759. doi: 10.1084/jem.121.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y B, Watson D W. Role of antibodies in reactions to gram-negative bacterial endotoxins. Ann N Y Acad Sci. 1966;133:727–745. doi: 10.1111/j.1749-6632.1966.tb52402.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y B, Watson D W. Biologically active endotoxin from salmonella mutants deficient in O- and R-polysaccharides and heptose. J Bacteriol. 1967;94:1320–1326. doi: 10.1128/jb.94.5.1320-1326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y B. Developmental immunity in the piglet. In: Bergsma D, Good R A, Finnstad J, editors. Immunodeficiency in man and animals. Birth defects: original articles series. XI (no. 1) Sunderland, Mass: Sinauer Associates, Inc.; 1975. pp. 549–557. [PubMed] [Google Scholar]
- 14.Machnicki M, Zimecki M, Zagulski T. Lactoferrin regulates the release of tumor necrosis factor alpha and interleukin 6 in vivo. Int J Exp Pathol. 1993;74:433–439. [PMC free article] [PubMed] [Google Scholar]
- 15.Mattsby-Baltzer I, Roseanu A, Motas C, Elverfors J, Engberg I, Hanson L A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr Res. 1996;40:157–262. doi: 10.1203/00006450-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Mehrazar K, Gilman-Sachs A, Kim Y B. Intestinal absorption of immunologically intact macromolecules in germfree colostrum-deprived piglets maintained on total parenteral nutrition. J Parenter Enteral Nutr. 1993;17:8–15. doi: 10.1177/014860719301700108. [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa K, Mantel C, Lu L, Morrison D C, Broxymeyer H E. Lactoferrin-lipopolysaccharide interactions: effect on lactoferrin binding to monocyte/macrophage-differentiated HL-60 cells. J Immunol. 1991;146:723–729. [PubMed] [Google Scholar]
- 18.Naidu S S, Svensson U, Kishore A R, Naidu A S. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1993;37:240–245. doi: 10.1128/aac.37.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitsche, D. August 1993. Use of lactoferrin for treatment of toxic effect of endotoxins. U.S. patent 5,240,909, p. 1–16.
- 20.Parrillo J E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 21.Schotanus K, Holtkamp G M, Van Rooijen N, Tilders F J M, Berkenbosch F. Circulating tumor necrosis factor-α does not mediate endotoxin-induced hypothermia in rats. Am J Physiol. 1995;168:R989–R996. doi: 10.1152/ajpregu.1995.268.4.R989. [DOI] [PubMed] [Google Scholar]
- 22.Tolchard S, Hare A S, Nutt D J, Clarke G. TNFα mimics the endocrine but not the thermoregulatory responses of bacterial lipopolysaccharide (LPS): correlation with FOS-expression in the brain. Neuropharmacology. 1996;35:243–248. doi: 10.1016/0028-3908(96)00002-0. [DOI] [PubMed] [Google Scholar]
- 23.Tracey K J, Cerami A. Cachectin/tumor necrosis factor and other cytokines in infectious disease. Curr Opin Immunol. 1987;1:454–461. doi: 10.1016/0952-7915(88)90026-x. [DOI] [PubMed] [Google Scholar]
- 24.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C-S, Chan W-Y, Kloer H U. Comparative studies on the chemical and immunochemical properties of human milk, human pancreatic juice and bovine milk lactoferrin. Comp Biochem Physiol. 1984;78B:575–580. doi: 10.1016/0305-0491(84)90100-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Pabst K M, Aida Y, Pabst M J. Lipopolysaccharide-inactivating activity of neutrophils is due to lactoferrin. J Leukocyte Biol. 1995;57:865–874. doi: 10.1002/jlb.57.6.865. [DOI] [PubMed] [Google Scholar]
- 27.Watson D W, Kim Y B. Modification of host responses to bacterial endotoxins. I. Specificity of pyrogenic tolerance and the role of hypersensitivity in pyrogenicity, lethality, and skin reactivity. J Exp Med. 1963;118:425–446. doi: 10.1084/jem.118.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 30.Zagulski T, Lipinski P, Zagulska A, Broniek S, Morse C. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br J Exp Pathol. 1989;70:697–704. [PMC free article] [PubMed] [Google Scholar]
- 31.Ziegler E J, Fisher C J, Jr, Sprung C L, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]